Abstract
Background
Laparoscopic Roux-en-Y gastric bypass (LRYGB) is one of the most effective bariatric procedures. Internal hernia (IH) is the commonest long-term complication seen after LRYGB. We analyzed the impact of closure of mesenteric defect at primary surgery on the incidence of IH. We also studied the effectiveness of pre-operative abdominal contrast-enhanced computerized tomography (CECT) in diagnosing IH.
Methods
This is a retrospective cohort study in which we analyzed prospectively the collected data of all patients who underwent LRYGB from 2005 to 2014. All patients post-LRYGB presenting with unexplained abdominal pain with a suspicion of IH were subjected to a CECT abdomen, in which we looked specifically for “whirlpool” sign and “clustering of bowel loops.” All patients underwent diagnostic laparoscopy. We compared the incidence of IH in those who did not undergo mesenteric defect closure (2005–2008, i.e., group A) with those who had the mesenteric defects closed during primary surgery (2009–2014, i.e., group B). We also calculated the sensitivity of abdominal CECT in diagnosing IH pre-operatively.
Results
Among patients who did not undergo closure of any mesenteric defect (group A 2005–2009), 21/600 (3.5 %) developed IH, while 17/976 (1.7 %) patients who underwent mesenteric defect closure (group B 2009–2014) developed IH (p = 0.027). Pre-operative CECT abdomen confirmed the diagnosis of IH in 47.5 % (19/40 patients).
Conclusions
Closing of mesenteric defects after laparoscopic gastric bypass seems to be related to a lower incidence of internal hernia in the follow up. As the sensitivity of abdominal CECT is low, laparoscopic exploration is recommended based on clinical suspicion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence and prevalence of morbid obesity is on the rise. Bariatric surgery is the only treatment option leading to sustained and effective weight loss [1]. Laparoscopic Roux-en-Y gastric bypass (LRYGB) is one of the most frequently performed surgeries for morbid obesity [2]. However, in spite of its effectiveness and relative safety, it is associated with certain short-term and long-term complications [1]. The most common long-term complication following LRYGB is internal hernia (IH) [1]. IH can lead to small bowel obstruction (SBO), as well as bowel strangulation and gangrene. Various modifications in surgical technique have been proposed in an attempt to decrease the incidence of IH, and one of which is the closure of mesenteric defects at the time of primary surgery. The aim of this study is to determine the impact that mesenteric defect closure during LRYGB has on the incidence of IH. We also report the effectiveness of abdominal contrast-enhanced computerized tomography (CECT) in diagnosing IH and review the relevant literature.
Materials and Methods
A database of all patients undergoing bariatric surgery at the specialized Minimal Access Surgery Department of this tertiary care institute has been maintained concurrently using our indigenous software, on a Microsoft Excel worksheet. The present study is a retrospective cohort study, in which prospectively collected data of those patients who underwent LRYGB from 2005 to 2014 was analyzed. Patients included in this study met the criteria for morbid obesity and underwent detailed informed consent procedure, before opting for LRYGB. The patients were operated on by one of five experienced surgeons, using a standardized operative protocol. All five surgeons were equally experienced, with over 10 years of laparoscopic gastro-intestinal surgery experience, and were involved in setting up the Minimal Access Surgery Department at this institute.
The salient features of the surgical steps performed are as follows: All surgeries were performed laparoscopically. The omentum is divided vertically in order to decrease tension on the Roux limb. Initially, the Roux limb is prepared with a biliopancreatic limb of 75 cm (100 cm for BMI > 50) and alimentary limb of 100 cm (150 cm for BMI > 50), with division of the mesentery. The jejuno-jejunostomy (JJ) is created in a side-to-side fashion with a wide opening, by applying 60 mm linear endo-stapler with a white cartridge in both directions, followed by closure of the common enterotomy by a third application of 60 mm linear endo-stapler (white cartridge), i.e., the “triple-stapled technique.” A gastric pouch 20–30 ml size is formed by the serial application of 60 mm linear endo-stapler (blue cartridge). The gastrojejunostomy (GJ) is ante-gastric with ante-colic orientation of Roux limb and is created using 25 mm circular stapler (3.5 mm stapler height), the anvil being inserted orally by anesthetist (OrVil®). After creation of the GJ, an intra-operative methylene blue test (IOMBT) was performed to rule out anastomotic leak. If IOMBT was positive, indicative of a leak from the GJ, additional sutures were taken at suspected sites of discontinuity. All staple lines were examined for bleeding, and 200-mm clips were applied for hemostasis. In case the control of bleeding was not achieved with clips, hemostatic sutures were taken. All ports larger than 10-mm diameter were closed under vision. In the initial period, i.e., from 2005 to 2008, the Petersen defect (i.e., the potential defect between the alimentary Roux limb and the transverse meso-colon) as well as the mesenteric defect at the JJ was not closed—this set of patients is labeled group A. From 2009 to 2014, both these defects were closed from the base of the mesentery to the apex by a continuous non-absorbable suture technique—these patients constitute group B.
Follow-up visits were scheduled at 1, 3, and 6 months and at the end of 1, 3, and 5 years post-surgery. In case of the absence of patient at scheduled follow-up, an attempt was made to contact them telephonically. In addition, all patients were provided with a 24-h emergency help-line number, manned by experienced surgeons, and were actively advised to report alarming symptoms such as abdominal pain, vomiting, and bleeding per rectum.
All patients who presented in the follow-up period with unexplained abdominal pain and a strong suspicion of internal hernia (i.e., history of left-sided abdominal pain, colicky in nature, increased following meals, aggravated by standing erect, and relieved by bending forwards) were subjected to CECT abdomen. The main signs that we looked for on the scan were the following: first, clustering of dilated small bowel pressed against the abdominal wall with no overlying omental fat, leading to central displacement of the colon as well as the displacement of the mesenteric vascular trunk and duodeno-jejunal junction (Fig. 1.), and second, the “whirlpool” sign, i.e., twisting of the mesenteric vessels and bowel due to volvulus of the herniated small bowel (Fig. 2.). All patients in whom IH was suspected were subjected to a diagnostic laparoscopy, irrespective of CT findings.
In this study, we compared the incidence of IH in the two groups of patients, i.e., those without closure of the mesenteric defects (group A) and those with closure of the mesenteric defects (group B). We also studied the effectiveness of radiological investigations, specifically an abdominal CECT scan in making a pre-operative diagnosis of IH. We also considered the outcomes of patients who developed IH and reviewed the relevant literature.
Results
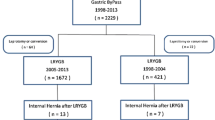
A total of 1576 morbidly obese patients underwent LRYGB at this institute between 2005 and 2014, of which 600 did not undergo closure of either of the mesenteric defects (group A—operated from 2005 to 2008), while 976 were operated in the period 2009–2014 and underwent closure of both the JJ mesenteric defect as well as Petersen’s defect (group B). The demographic data of the entire patient population is presented in Table 1. There were no statistical differences between the two groups, with respect to age, sex, BMI, and comorbidities (i.e., hypertension and diabetes mellitus), at the time of LRYGB.
All 1576 cases were successfully completed laparoscopically. IOMBT was positive in 39/1576 (2.5 %) patients, and none of these patients developed post-operative anastomotic leak. There were no patients with significant intra-operative hemorrhage (significant being defined by conversion to open surgery for hemostasis). Two patients in group A and one in group B developed early post-operative intra-abdominal hemorrhage and underwent diagnostic laparoscopy with the evacuation of the blood clots, control of hemorrhage, and blood transfusions.
Table 2 presents the post-operative complications encountered after LRYGB.
The follow-up rate of our case series was 71 % at 1 year post-surgery and dropped to 62 % at 5 years post-surgery.
The mean age of patients who developed IH was 40.4 years (range, 16–61 years), with an almost equal distribution between sexes (male 19, female 21). The mean duration to presentation was 15.84 months (range, 7–28 months) after surgery, and all of them had over 50 % excess weight loss (EWL), with mean %EWL being 59.2 %. The cases of IH were uniformly distributed over the entire time period, in both groups.
Forty patients underwent diagnostic laparoscopy on the basis of a clinical suspicion of IH. IH was present in 38 patients, while two had adhesive small bowel obstruction. Pre-operative CECT confirmed the diagnosis of IH in 19/40 (47.5 %) while the remaining did not have conclusive radiological features and were diagnosed only at the time of laparoscopy. The sites of occurrence of IH were as follows: JJ mesenteric defect in 29 (16 in group A versus 13 in group B) and Petersen’s defect in 9 (5 in group A versus 4 in group B). The incidence of IH in the non-closure group was 3.5 % (21/600). However, only 17/976 patients in the closure group developed IH, an incidence of 1.7 %. The incidence of IH was found to be doubled in the non-closure group as compared to the group with mesenteric defects closed. On applying chi-square test to a 2 × 2 contingency table, p value is equal to 0.027, which is statistically significant (p < 0.05). Of the 38 patients with IH, 37 underwent successful laparoscopic reduction of the hernia, and closure of both the mesenteric defects. However, one patient had developed gangrene of the Roux limb, extending onto the common limb, and had to undergo open resection of small bowel with reversal of the gastric bypass. There was no mortality in any of the patients with IH in our study.
Discussion
This study shows that the incidence of IH was significantly reduced when the mesenteric defects were closed at the time of primary surgery (3.5 % incidence in non-closure group versus. 1.7 % incidence in closure group, p = 0.027). It was also observed that CECT abdomen is helpful in pre-operative diagnoses of IH in less than half the patients (47.5 %), emphasizing the fact that IH should be strongly suspected when post-LRYGB patients present with unexplained abdominal pain.
The distribution of cases of IH was uniform in both groups over the entire period of time. This may be attributed to the fact that all five surgeons were equally experienced and had entered the field of laparoscopic bariatric surgery after several years of extensive laparoscopic gastro-intestinal surgical experience.
The strengths of our study are the large numbers included, the standardized surgical protocol followed, and two distinct groups available for comparison (i.e., closure versus non-closure). The drawbacks include the fact that it is a retrospective comparative non-randomized study, conducted only at a single-center. Also patients operated on in the latter part of study period may yet present with IH in the future.
The most common long-term complication following LRYGB is the occurrence of IH [1]. LRYGB involves a rearrangement of anatomy of the gastrointestinal tract, resulting in the formation of two or three potential sites at which herniation can occur, depending on the technique employed. Firstly, there is a potential space between the mesentery of two small bowel loops at the JJ. The second site where an IH could occur is the Petersen space, between the Roux limb and the transverse colon, mesocolon, or retroperitoneum. The third possibility is unique to retro-colic Roux limb construction and involves an IH developing in the trans-mesocolic space.
The mean incidence of IH after LRYGB is 2.5 % [3], range 0.5 to 11 % [4]. The incidence increases with longer periods of follow-up, with the mean duration of follow-up prior to the occurrence of IH being approximately 2 years [3]. The figures in our study are similar, −1.7 % incidence in the group undergoing mesenteric defect closure and 3.5 % incidence in the non-closure group, with time to presentation ranging from 7–28 months. The incidence of IH is also higher following the laparoscopic approach as compared to open. This is postulated to be due to the decreased formation of intra-abdominal adhesions after laparoscopic surgery [3]. Another contributing factor to the etiology of IH is rapid weight loss and consequent loss of intra-abdominal fat, resulting in the widening of potential mesenteric spaces [5, 6]. Hence, careful follow-up of bariatric patients’ post-LRYGB is necessary.
Patients with IH post-LRYGB have generally lost more than 50 % of their original excess weight. The pain is often localized to the left hypochondrium and is colicky in nature, increasing after meals. The patient may find it difficult to stand erect and may experience relief on bending forward. The pain may be intermittent, lasting several hours [7], but resolved when the hernia reduces. However, it may also present with vague persistent abdominal discomfort. IH is the most common cause of SBO following LRYGB [6, 8, 9] and can result in complications such as bowel strangulation and gangrene, irrespective of the presence or absence of SBO. Therefore, a clinician must be alert to the possibility of IH when a patient post-LRYGB presents with unexplained abdominal pain.
The diagnosis of IH on a radiological basis is difficult, and imaging may be inconclusive. The radiological investigation of choice is an abdominal CT scan with intra-venous as well as enteral contrast [10]. A number of signs have been described, with the clustering of small bowel loops and swirling of mesentery, i.e., the whirlpool sign, being the most reliable [11]. The latter sign has a sensitivity of 78–100 % and a specificity between 80–90 % [8]. As the symptoms are often vague and the radiological findings may be inconclusive, it is recommended to keep a low threshold for diagnostic laparoscopy in a patient who is post-LRYGB and presents with unexplained abdominal pain [8]. In our study, a pre-operative CECT scan was diagnostic of IH in less than half the patients (19/40, i.e., 47.5 %). Not only does laparoscopy aid in confirming the diagnosis but it also offers the potential for definite therapeutic intervention.
The definitive management consists of a reduction of the herniated bowel loops and closure of all potential mesenteric defects by non-absorbable suture material. In case of gangrene and necrosis, affected bowel will need resection followed by restoration of bowel continuity. In case massive small bowel resection is required, the gastric bypass is reversed so as to avoid potentiating the malabsorption that is expected to occur secondary to short-gut syndrome [12]. The decision of whether to proceed with this laparoscopically or to convert to laparotomy would depend on a number of factors including the general condition of the patient, surgical expertise, and facilities available.
Prevention of IH by various technical modifications is the matter of much discussion in the world of obesity surgery. Undoubtedly, the construction of an ante-colic Roux limb instead of retro-colic has markedly reduce the incidence of IH as it eliminates one potential hernia site, i.e., the trans-mesocolic defect [4]. In fact, a number of case series [4, 8, 13] have found that this trans-mesocolic defect created for the passage of a retro-colic Roux loop is the commonest site of IH. In case of ante-colic Roux-en-Y construction, the most common site seems to be the JJ mesenteric defect [6]. This was seen in our study too, where 29 IHs occurred at the JJ mesenteric defect, and only nine were at the Petersen defect.
Another technical modification of importance is the closure of mesenteric defects so as to eliminate potential sites of herniation. A meta-analysis performed in 2014 [13] showed that the lowest incidence of IH was in ante-colic group with closure of both defects (1 %), followed by ante-colic group with all defects left open, and retro-colic group with closure of mesenteric and meso-colic defects (2 % each). The highest incidence of IH was 3 % and occurred in ante-colic group with closure of only the jejunal defect as also in the retro-colic group with closure of all defects. These findings are consistent with those in our study. The opponents of mesenteric closure state that the step can be technically difficult as well as time-consuming. They cite various complications of mesenteric closure such as tearing of mesentery and bleeding, tension to the GJ or JJ due to suturing, and risk of injury to mesenteric vessels. They also state that the defects may open out after loss of mesenteric fat permitting the formation of IH, despite closure of the defects at the time of initial surgery [8, 14, 15]. In fact, in a small study conducted by Hope et al. [16], it was found that in 15/18 patients, the mesenteric or meso-colic defects had opened up, in spite of closure at the time of primary surgery. Despite this opposition, it seems from our study as well as with various others [13] that the closure of mesenteric defects at the time of primary surgery does decrease, although it does not eliminate, the incidence of IH [17]. Various techniques of closure have been described including the use of staplers and sutures, whether absorbable or non-absorbable, running or continuous. Durability of closure depends on surgeon skill as well as the technique used. Some surgeons report “cutting through” and opening up of defects when non-absorbable sutures are used [16], while on the other hand, some say that absorbable sutures may lead to adhesion formation and SBO [18, 19]. Aghajani used a stapling device to close the defects, the advantage being that it is less time-consuming and easier to perform. However, four patients (0.2 %) required reoperation for bowel obstruction caused by the staples [20].
Other techniques to decrease internal herniation have been described, such as mesenteric irritation, i.e., rubbing against the mesenteric edges till petechiae appear so that adhesions may be induced to be formed, which may in turn ensure that the defects stay closed and thereby avoid IH [21]. Some have suggested that the use of fibrin glue on the staple lines and mesenteric edges may play a role in decreasing IH [22]. Some recommend positioning the distal closed end of the alimentary limb towards the right so that the bowel loop curves towards the left [23]. However, till date, there are no conclusive benefits of any of these modifications.
A significant drawback of a retrospective cohort study is that one cannot estimate the sample size and hence power of the study. IH post-LRYGB has a very low incidence. Hence, we recommend further studies, preferably a prospective two-sided study design, using the same two groups, i.e., with and without closure of mesenteric defects. Presuming an alpha risk of 0.05 and a beta risk of 0.2, and basing the same size calculation on our findings, i.e., assuming the difference between 1.7 and 3.5 % incidence in non-closure and closure group respectively to be statistically significant, 1260 patients have to be included in each group if the dropout rate is 0 %, i.e., follow-up rate 100 %; if this rate raises to 20 %, the number of patients to be included has to be increased to 1575 per group, and if the loss to follow-up rate is 40 % (as it is in many bariatric series), 2100 patients per group have to be included to find the presented differences as statistically significant. Such a study would give the best possible chance for a definitive answer on this topic.
Conclusion
The incidence of IH can be reduced, although not eliminated, by the closure of mesenteric defects at the time of LRYGB. As the symptoms are vague and radiological findings are often inconclusive, a high index of suspicion is required, and one must proceed to diagnostic laparoscopy on the basis of clinical judgment alone, in order to avoid the disastrous complications of strangulation and gangrene.
References
Frrebo MB, Sommer T. Leakage and internal herniation are the most common complications after gastric bypass. Dan Med J. 2014;61(5):A4844.
Lee WJ, Yu PJ, Wang W, et al. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005;242(1):20–8. doi:10.1097/01.sla.0000167762.46568.98.
Iannelli A, Facchiano E, Gugenheim J. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1–7.
Iannelli A, Buratti MS, Novellas S, et al. Internal hernia as a complication of laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17:1283–6.
Schneider C, Cobb W, Scott J, et al. Rapid excess weight loss following laparoscopic gastric bypass leads to increased risk of internal hernia. Surg Endosc. 2011;25(5):1594–8.
Ahmed AR, Rickards G, Husain S, et al. Trends in internal hernia incidence after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(12):1563–6.
Greenstein AJ, O’Rourke RW. Abdominal pain following gastric bypass: suspects and solutions. Am J Surg. 2011;201(6):819–27.
Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13(4):596–600.
Capella RF, Iannace VA, Capella JF. Bowel obstruction after open and laparoscopic Roux-en-Y gastric bypass for morbid obesity. J Am Coll Surg. 2006;203(3):328–35.
Ahmed AR, Rickards G, Johnson J, et al. Radiological findings in symptomatic internal hernias after laparoscopic gastric bypass. Obes Surg. 2009;19(11):1530–5.
Al-Saeed O, Fahmy D, Kombar O, et al. Sixty-four-slice multidetector computerized tomography in the evaluation of transmesenteric internal hernias following Roux-en-Y bariatric surgery. Med Princ Pract. 2013;22:540–4.
Choseleb E, Patel S, Szomtein S, et al. Reasons and operative outcomes after reversal of gastric bypass and jejunoileal bypass. Obes Surg. 2012;22(10):1611–6.
Geubbels N, Lijftogt N, Fiocco M, van Leersum NJ, Wouters MWJM, de Brauw LM. Meta-analysis of internal herniation after gastric bypass surgery. Br J Surg. Early View (Online Version of Record published before inclusion in an issue)
Higa K, Ho T, Boone K. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003;13:350–4.
Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1040 patients—what have we learned? Obes Surg. 2000;10:509–13.
Hope WW, Sing RF, Chen AY, et al. Failure of mesenteric defect closure after Roux-en-Y gastric bypass. JSLS. 2010;14:213–6.
Himpens J, Verbrugghe A, Cadière G-B, et al. Long-term results of laparoscopic Roux-en-Y gastric bypass: evaluation after 9 years. Obes Surg. 2012;22:1586–93.
Eckhauser A, Torquati A, Youssef Y, et al. Internal hernia: postoperative complication of Roux-en-Y gastric bypass surgery. Am Surg. 2006;72:581–4.
Madan AK, Lo Menzo E, Dhawan N, et al. Internal hernias and nonclosure of mesenteric defects during laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19:549–52.
Aghajani E, Jacobsen HJ, Nergaard BJ, et al. Internal hernia after gastric bypass: a new and simplified technique for laparoscopic primary closure of the mesenteric defects. J Gastrointest Surg. 2012;16:641–5.
Walker AS, Bingham JR, Causey MW, et al. Mesenteric irritation as a means to prevent internal hernia formation after laparoscopic gastric bypass surgery. Am J Surg. 2014;207(5):739–41. discussion 741–2.
Silecchia G, Boru CE, Mouiel J, et al. The use of fibrin sealant to prevent major complications following laparoscopic gastric bypass: results of a multicenter, randomized trial. Surg Endosc. 2008;22(11):2492–7.
Quebbemann BB, Dallal RM. The orientation of the antecolic Roux limb markedly affects the incidence of internal hernias after laparoscopic gastric bypass. Obes Surg. 2005;15(6):766–70. discussion 770.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in this study.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chowbey, P., Baijal, M., Kantharia, N.S. et al. Mesenteric Defect Closure Decreases the Incidence of Internal Hernias Following Laparoscopic Roux-En-Y Gastric Bypass: a Retrospective Cohort Study. OBES SURG 26, 2029–2034 (2016). https://doi.org/10.1007/s11695-016-2049-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2049-8