Abstract
Background
Although medium- to long-term improvement in insulin resistance and T2DM after Roux-en-Y gastric bypass (RYGB) is well documented, few studies have analyzed the acute effects after surgery. Understanding these effects might help explain the physiologic adjustments after surgery and help in managing insulin resistance and controlling the hypoglycemic treatment for bariatric patients.
Methods
We recruited a prospective cohort of 55 consecutive female patients that underwent primary laparoscopic RYGB between January and June/2011. Blood samples were collected preoperatively and at the first, third, and fifth post-operative days after an overnight fast.
Results
There was a significant increase in homeostasis model assessment for insulin resistance (HOMA-IR) on day 1 (2.36 vs 3.12; p = 0.032), followed by a rapid decrease from day 3 onward (3.12 vs 1.70; <0.001). We found a statistically significant difference (p < 0.05) at all time points compared with baseline. HOMA-IR levels at POD5 were 47 % lower than baseline values and were not significantly different from values at 6 months (1.24 vs 0.93; p = 0.09). The blood levels of glucose and insulin closely matched those of HOMA-IR.
Conclusions
RYGB results in a rapid improvement in insulin resistance and a clinically significant decrease in fasting glucose and insulin levels. This improvement is significant at the 3rd post-operative day, and by the 5th day, patients express insulin resistance levels that are similar to those expressed at 6 months after surgery. This work highlights the acute metabolic impact of surgery. Understanding the behavior of insulin and glucose after surgery might improve our knowledge of the pathophysiology of diabetes and lead to novel therapies and tailored surgical approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance is thought to be a fundamental step in the genesis and development of type 2 diabetes mellitus (T2DM) [1]. Obesity has long been thought to produce insulin resistance; however, some authors suggest that it might just be another manifestation of insulin resistance [2]. For overt T2DM to establish, both insulin resistance and β cell dysfunction are required [3].
Bariatric surgery is the only effective and durable treatment for obesity and might result in improvement or resolution of diabetes in up to 80 % of patients [4–6]. Therapeutic interventions on T2DM aim to reduce insulin resistance, increase insulin levels, or a combination of both [7].
After Roux-en-Y gastric bypass (RYGB) surgery, patients have been reported to experience a rapid improvement in insulin resistance and T2DM remission as early as 6 days after surgery [2, 7]. A recent meta-analysis reported that insulin resistance was significantly improved at 2 weeks after RYGB and bilio-pancreatic diversion, but not after sleeve gastrectomy or gastric banding [8].
Although medium- to long-term improvement in insulin resistance and T2DM after RYGB is well documented [9, 10], few studies have analyzed the acute effects after this surgery. Understanding these effects might help to explain some of the physiologic adjustments after surgery and help in managing insulin resistance states and controlling the hypoglycemic treatment for bariatric patients.
Previous studies [7] measuring homeostasis model assessment for insulin resistance (HOMA-IR) in RYGB patients before surgery (after a very-low-calorie diet) and after surgery have reported that, after gastric bypass, the improvement in insulin resistance is greater than that seen after very-low-calorie diets at as early as 6 days after surgery. However, the results are not completely clear on whether RYGB is superior to calorie restriction in terms of improvement in insulin resistance [8].
We proposed to study the changes in the glycemic profile of patients in the days that follow RYGB (up to 5 days) and compare them with the medium-term results at 6 months. Our main objective is not to determine the underlying causes of these changes or compare it with other types of surgery but rather to report and describe its evolution in the first few post-operative days
Patients and Methods
We recruited a prospective cohort of 55 consecutive female patients that underwent primary laparoscopic RYGB between January and June 2011. Blood samples were collected preoperatively and at the first, third, and fifth post-operative days after an overnight fast. According to our protocol, patients started clear fluids on the third post-operative day, and by day 5 they were on full liquid diet. Measures of insulin resistance were calculated with the HOMA2 calculator [11].
All analytical parameters were measured at the São João Hospital Clinical Pathology Department. Serum C-reactive protein (CRP) was assayed using an immuno-turbidimetric assay on an Olympus AU5400® automated clinical chemistry analyzer (Beckman-Coulter®, Izasa, Porto, Portugal). Insulin was measured by way of an electrochemiluminescent immunoassay using a Cobas® e411 automated analyzer (Roche®, Lisbon, Portugal). Blood glucose, total cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol), and triglycerides were measured using conventional methods with an Olympus AU5400® automated clinical chemistry analyzer (Beckman-Coulter®, Izasa, Porto, Portugal). Low-density lipoprotein cholesterol (LDL-cholesterol) was calculated according to Friedewald's equation [12]: [LDL-cholesterol = total cholesterol − HDL-cholesterol − (triglycerides/5)]. Glycated hemoglobin (HbA1c) was determined by an ion-exchange HPLC system with a D-10™ Bio-Rad® analyzer (Bio-Rad®, Porto, Portugal).
Statistical analysis was performed with SPSS statistical package version 18.0. Longitudinal analysis was done with a two-way mixed-model analysis of variance (ANOVA), and pairwise comparisons were followed with Bonferroni correction. Statistical significance was set at p < 0.05.
Results
As for study design, all patients were female. Their mean age was 39 years and the mean body mass index (BMI) was 46.1 (Table 1) with an evolution of obesity of 20 years. The mean fasting glucose level was 93 mg/dL and, overall, patients were hyperinsulinemic (mean 16.7 μUI/L) and insulin resistant (HOMA2-IR = 2.36).
C-reactive protein levels were elevated (mean 13.8 mg/L), and there were no (on average) major abnormalities of lipid profile.
Approximately one in five patients was diabetic and one in four had impairment of glucose metabolism. Slightly more than half of the patients had metabolic syndrome (according to the International Diabetes Federation criteria [13, 14]) at baseline.
At 6 months post-operatively, patients had lost, on average, 31 kg (and 12.2 BMI points), and this weight loss was accompanied by a relevant metabolic improvement. There was a significant decrease on glucose, insulin, HbA1c, CRP, and HOMA-IR levels (Table 2).
The mean scores for HOMA-IR at different time points were significantly different (p < 0.001; Table 3) according to repeated-measures ANOVA with Greenhouse–Geisser correction.
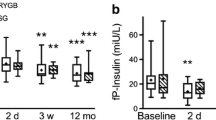
Post hoc tests using Bonferroni correction revealed that, after gastric bypass, there was a significant increase in HOMA-IR on post-operative day (POD) 1 (2.36 vs 3.12; p = 0.032), followed by a rapid decrease from POD 3 onward (3.12 vs 1.70; p < 0.001). There was a statistically significant difference (p < 0.05) at all time points compared with baseline. HOMA-IR levels at POD 5 were not significantly different from values at 6 months (1.24 vs 0.93; p = 0.09). By POD 5, HOMA-IR had a drop of 47 % compared to its baseline value (Figs. 1 and 2).
The HOMA2 index β cell function (Table 4; Fig. 3) suffered a non-significant increase in the first post-operative day, followed by a significant drop in POD 3. On POD 5, there was a trend towards a slight increase, although not statistically significant, followed by a significant drop by 6 months after RYGB.
The blood levels of glucose and insulin (Table 4; Fig. 3) closely matched those of HOMA-IR. We could observe an increase at POD 1 followed by a decrease until POD 5. The values of blood glucose and insulin at 6 months post-operatively were not different from the values at POD 5.
HOMA-IR at baseline was lower in patients with normal glucose metabolism (HOMA-IR = 2.0) than for patients with impairment of the metabolism of glucose (HOMA-IR = 2.9) or overt T2DM (HOMA-IR = 2.2). When stratified by the presence of T2DM or impairment in the metabolism of glucose, the post-operative changes were not different between groups (p = 0.92), and in each group we could observe the same improvement. The HOMA-IR at 5 days was not different between groups (p = 0.81).
Discussion
Our results agree with previous reports [15] that there is a hyperinsulinemic state associated with obesity. This hyperinsulinism is thought to arise from increased insulin production to compensate for insulin resistance. According to some authors [16], besides its effects on glucose and lipid metabolism, this hyperinsulinemic state is also related to intracellular mechanisms that lead to cancer development, hence the need to reverse this pathological condition known as insulin resistance.
The remission rate of T2DM after bariatric surgery is variable [7] and the underlying mechanisms of this improvement are not yet known. Some studies point out that this improvement occurs early on after surgery [2, 9], much before any significant weight loss has occurred.
Some authors have described a rapid improvement in insulin resistance [2, 7, 8, 17, 18] after RYGB or, even without surgery, after prolonged caloric restriction [19]. Several authors have questioned this early improvement in insulin resistance, reporting that there is no significant change in the first days after surgery [20, 21] and that such a change would only occur after a substantial weight loss. As such, the precise behavior of insulin and glucose metabolism after RYGB is not yet known.
Lima et al [22] reported improved insulin resistance at 1 month after RYGB, and according to Rubino et al [23], at 3 weeks after gastric bypass there was a significant decrease in glucose and insulin levels. A recent meta-analysis concluded that HOMA-IR improved by 33 % at 1–2 weeks after gastric bypass and that by 6 months HOMA-IR had improved by 59 % [8]. As early as at 6 days after surgery [2], a decrease in insulin levels and insulin resistance, both in patients with clinical insulin resistance and in patients with normal glucose tolerance tests, has been documented. Furthermore, these improvements were unchanged up to 12 months after surgery despite a continuing weight loss [2]. This early improvement in insulin resistance is clinically significant as up to 30 % of diabetic patients who undergo RYGB surgery might be discharged with no anti-diabetic medication [24].
Our results confirm and extend these findings. We could observe that at post-operative day 3, there is already a significant decrease of glucose and insulin levels and a significant improvement in the insulin resistance index as measured by HOMA-IR. Furthermore, the levels achieved at post-operative day 5 are not statistically different from the levels obtained at 6 months after surgery. Overall, our results at comparable time points seem to be in line with the most recent meta-analysis [8]. In spite of a significant decrease in weight at 6 months after surgery, the results of insulin resistance, fasting glucose, and insulin are not different from those at 5 days when no significant change in weight had occurred.
Prolonged starvation and caloric restriction have been proposed as a tentative hypothesis for the early improvement in insulin resistance [7]. However, the differential results of RYGB and other types of bariatric surgery seem to point that RYGB has (at least) an additive effect to starvation and calorie restriction [7, 24, 25]. In a long-term follow-up study, Pournaras [26] has concluded that in spite of similar weight loss at 2 years, patients with gastric bypass were more likely to be in remission of diabetes than patients with gastric band, which suggests a direct metabolic role of surgery, not related to calorie restriction and weight loss. Similarly, Woelnerhanssen et al. reported that the improvement in insulin resistance after gastric bypass “did not parallel” the weight loss [15], and when compared with a very-low-calorie diet, RYGB has been shown to lead to greater improvement in insulin resistance, especially in diabetic patients [27].
However, other studies [28–31] concluded that the improvement in insulin resistance was directly related to post-operative weight loss and caloric restriction. The report [2] that in the sub-acute setting (3 months) there was a slight increase in insulin resistance supports that calorie restriction might play a role in the early improvements widely reported.
In spite of being difficult to understand this conundrum without a sham-surgery control group, our results point against that because: (1) there is an improvement in glucose, insulin, and insulin resistance between POD 3 and 5, when patients who were starving (up to day 3) started on full liquid diet, and (2) the results at POD 5 are not different from the measurements at 6 months, when the patients have long been on a regular diet.
Despite previous reports that insulin resistance increases acutely after abdominal surgery [32], such findings have never been reported after bariatric surgery [8]. The post-operative increase in insulin resistance after major abdominal surgery has been related to surgical stress [32] and is thought to be more important in extra-hepatic tissues. This finding suggests that calorie restriction per se (which “usually” happens after a major abdominal surgery) should not be, by itself, a driving mechanism of the improvement seen in insulin resistance after RYGB. Our results confirm the original findings by Thorell [32] which indicate that, starting in the post-operative day 1, there is in fact an increase in insulin resistance. However, the post-operative insulin resistance should persist for at least 5 days. According to our results, the physiologic effects of RYGB rapidly reverse the putative post-operative insulin resistance, which might explain why it has never been reported before.
During RYGB, a small gastric pouch (~30 mL) is constructed in the proximal stomach and the remaining stomach and duodenum are excluded from the alimentary tract. Digestive transit is then re-routed to a jejunal loop at approximately 70 cm of the angle of Treitz. This “alimentary” limb is then connected with the proximal jejunum (biliary limb) at a distance of approximately 150 cm. Several studies have proposed that the anatomical changes after RYGB might account for the improvement in glucose metabolism.
Two major theories have arisen: according to the “foregut theory” [33], it is the exclusion of the duodenum that is responsible for this improvement, while according to the “hindgut” theory [34], it is the rapid arrival of undigested food to the ileum that drives the metabolic improvement. The results in human subjects have been conflicting [4], but whatever the prevailing theory proves to be, it appears that gastric bypass surgery “fundamentally alters the physiology of the foregut” [2].
Several studies concluded that RYGB leads to an increase in GLP-1 expression and that this will regulate and improve β cell function [15, 18, 26, 35–39] and β cell proliferation [24, 40]. Interestingly, GLP-1 levels were not correlated with calorie restriction or weight loss.
An animal study with a hyperinsulinemic euglycemic clamp was used to compare tissue-specific changes in insulin sensitivity, and it was concluded that after RYGB, there is an improvement in the hepatic insulin sensitivity that is not related to weight loss [41]. This improvement in hepatic insulin sensitivity has also been reported in humans shortly (1 month) after surgery [2, 18], and Bikman et al [42] concluded that insulin sensitivity was greater in patients at 1 year after RYGB than in weight-matched controls and that glucose transport in muscle was also improved.
It has been reported [2, 22, 43] that the improvement in insulin resistance after gastric bypass is not related to a modification of the production of insulin in response to a glucose challenge but rather due to a decrease in the fasting production of insulin. Indeed Reed et al [17] concluded that RYGB corrected the fasting hyperinsulinemia associated with obesity, without significant changes in glucose tolerance. Even more, weight-stable patients after RYGB had lower fasting insulin levels than weight-matched controls [17].
Our results rely on the HOMA2 calculation for insulin resistance and β cell function [11]. This model is intended to be applied in steady-state parameters and not to evaluate changes after stimulation. As such, we can conclude that our results represent the homeostatic period achieved by overnight fasting. We could indeed observe a significant decrease in fasting insulin production from post-operative day 3 onward. Both the decrease in fasting insulin levels and the improvement in insulin sensitivity might “unstress” the pancreatic β cell and allow the pancreas to produce appropriate amounts of insulin in response to physiological stimulation [17]. Our short-term fluctuations in β cell function suggest that the pancreas does indeed respond to the needs elicited by sensitivity to insulin in order to maintain adequate glucose levels. If in the first post-operative day there is a physiologic need to “boost” insulin production due to post-operative insulin resistance, after day 3, with increasing sensitivity to insulin, β cell function decreases with decreasing needs for insulin production.
In apparent contrast with these findings, some studies have reported that metabolic improvement after RYGB occurs through an increase in the production of GLP-1 and insulin, which might explain why some patients come to develop nesidioblastosis [24, 44]. To this, some authors reply that hyperinsulinism is a consequence of persistent hyperfunctioning β cells from the pre-operative insulin resistance state. Our results do not support this increased production theory as we consistently found lower levels of insulin after surgery and an increase in sensitivity to insulin, but only a dynamic study of insulin response after a test meal can further explore this theory. A controlled animal study with obese Zucker rats [45] concluded that both the insulin peripheral sensitivity and post-prandial production were improved after gastric bypass.
Both of these results can be combined in a “unified theory”: RYGB improves and corrects insulin resistance and the fasting hyperinsulinemic state, and the unstressed pancreatic β cells can now produce increased amounts of insulin in response to an oral challenge. As such, the physiological changes after RYGB might control glucose metabolism in both fronts: it corrects insulin resistance and it allows the pancreas to increase insulin production on demand.
Improvement in hepatic insulin sensitivity has been reported to be more pronounced in patients with diabetes [2, 46], and the early improvement in insulin resistance might be due to an increase in β cell function, especially in previously hyperglycemic [47] and diabetic patients [48]. Interestingly, patients with diabetes did not suffer the slight increase in insulin resistance at 3 months that was observed for patients with a normal glucose tolerance test [2].
In our results, we could not detect any difference in the metabolic behavior between patients with and without diabetes. Although patients with impairment of glucose metabolism had higher pre-operative levels of insulin resistance and the measurements at 5 days and 6 months are not different, improvement in insulin resistance and β cell function was not significantly different. Once again, only dynamic studies with evaluation of the response profile to oral feeding might untangle these results.
Conclusion
RYGB results in a rapid improvement in insulin resistance and a clinically significant decrease in the fasting glucose and insulin levels. This improvement is significant at POD 3, and by POD 5 patients express insulin resistance levels that are similar to those expressed at 6 months after surgery. The insulin resistance state associated with major abdominal surgery seems to occur on the first post-operative day, but it is rapidly reversed by the physiologic effects of gastric bypass or calorie restriction. Whether surgery itself or an improved lifestyle (dietary patterns) is responsible for this change, patients in hypoglycemic therapy should be closely monitored and their medications may be discontinued early after surgery.
There is still much to understand on the physiologic adaptations after gastric bypass and further studies are required. Several mechanisms still require explanation, and further studies, measuring GLP-1, glucagon, GIP, ghrelin, and other enteric peptides, might render novel insights in this area. Furthermore, this study only addressed changes in fasting states, and the study of changes after meals might also help explain the physiologic effects of gastric bypass.
Understanding the behavior of insulin and glucose after surgery might improve our understanding of the pathophysiology of diabetes and lead to novel therapies and tailored surgical approaches. Our findings altogether reinforce the theory of “metabolic surgery” and can be useful in predicting early glycemic control.
References
Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95(5):875–92.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–81.
Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96(9):E1372–9.
Laferrère B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine. 2011;40(2):162–7.
Meijer RI, van Wagensveld BA, Siegert CE, et al. Bariatric surgery as a novel treatment for type 2 diabetes mellitus: a systematic review. Arch Surg. 2011;146(6):744–50.
Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Foo J, Krebs J, Hayes MT, et al. Studies in insulin resistance following very low calorie diet and/or gastric bypass surgery. Obes Surg. 2011;21(12):1914–20.
Rao RS, Yanagisawa R, Kini S. Insulin resistance and bariatric surgery. Obes Rev. 2012;13(4):316–28.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50. discussion 350–2.
Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. discussion 84–5.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95.
Friedewald W, Levy R. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499.
Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–80.
Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. 2009. p. 1640–5.
Woelnerhanssen B, Peterli R, Steinert RE, et al. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy—a prospective randomized trial. Surg Obes Relat Dis. 2011;7(5):561–8.
Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7(7):1003–15.
Reed MA, Pories WJ, Chapman W, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96(8):2525–31.
Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes Relat Metab Disord. 2010;34(3):462–71.
Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–42.
Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54(8):2093–102.
Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14(1):15–23.
Lima MMO, Pareja JC, Alegre SM, et al. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(8):3871–5.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236–42.
Ashrafian H, Athanasiou T, Li JV, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev. 2011;12(5):e257–72.
Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009;35(6 Pt 2):518–23.
Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252(6):966–71.
Plum L, Ahmed L, Febres G, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring). 2011;19(11):2149–57.
Ballantyne GH, Wasielewski A, Saunders JK. The surgical treatment of type II diabetes mellitus: changes in HOMA insulin resistance in the first year following laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic adjustable gastric banding (LAGB). Obes Surg. 2009;19(9):1297–303.
Lee W-J, Lee Y-C, Ser K-H, et al. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18(9):1119–25.
Ballantyne GH, Farkas D, Laker S, et al. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16(9):1189–97.
Gumbs AA, Modlin IM, Ballantyne GH. Changes in insulin resistance following bariatric surgery: role of caloric restriction and weight loss. Obes Surg. 2005;15(4):462–73.
Thorell A, Efendic S, Gutniak M, et al. Insulin resistance after abdominal surgery. Br J Surg. 1994;81(1):59–63.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741–9.
Morínigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16(12):1594–601.
Liu Y, Zhou Y, Wang Y, et al. Roux-en-Y gastric bypass-induced improvement of glucose tolerance and insulin resistance in type 2 diabetic rats are mediated by glucagon-like peptide-1. Obes Surg. 2011;21(9):1424–31.
Perugini RA, Malkani S. Remission of type 2 diabetes mellitus following bariatric surgery: review of mechanisms and presentation of the concept of ‘reversibility’. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):119–28.
Laferrère B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes Relat Metab Disord. 2011;35 Suppl 3:S22–5.
Yin DP, Gao Q, Ma LL, et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Ann Surg. 2011;254(1):73–82.
Laferrère B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 2009;35(6 Pt 2):513–7.
Scheen AJ, De Flines J, De Roover A, et al. Bariatric surgery in patients with type 2 diabetes: benefits, risks, indications and perspectives. Diabetes Metab. 2009;35(6 Pt 2):537–43.
Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–8.
Bikman BT, Zheng D, Pories WJ, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93(12):4656–63.
de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg. 2009;19(3):313–20.
Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–54.
Meirelles K, Ahmed T, Culnan DM, et al. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249(2):277–85.
Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35(1):137–42.
Lin E, Liang Z, Frediani J, et al. Improvement in ß-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab. 2010;299(5):E706–12.
Lin E, Davis SS, Srinivasan J, et al. Dual mechanism for type-2 diabetes resolution after Roux-en-Y gastric bypass. Am Surg. 2009;75(6):498–502. discussion 502–3.
Acknowledgments
The authors have no conflict of interest to report. All original data were collected and analyzed by the authors. Funding for this study was granted through FCT (Harvard Medical School Portugal Program—HMSP-ICJ/SAU-ICT/0007/2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faria, G., Preto, J., da Costa, E.L. et al. Acute Improvement in Insulin Resistance After Laparoscopic Roux-en-Y Gastric Bypass: Is 3 Days Enough to Correct Insulin Metabolism?. OBES SURG 23, 103–110 (2013). https://doi.org/10.1007/s11695-012-0803-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0803-0