Abstract
Background
The aim of this study was to investigate the effects of Roux-en-Y gastric bypass (RYGB) on glucose tolerance and insulin resistance in type 2 diabetic rats and the possible mechanisms involved in this process.
Methods
Thirty Goto-Kakizaki (GK) rats were randomly divided into three groups: RYGB operation, sham operation, and food restriction groups. Ten Wistar rats were used as non-diabetic control. The body weight and food consumption of rats were recorded 1 week before or every week after surgery. The fasting blood sugar and oral glucose tolerance test were performed using blood glucose meter. The levels of plasma insulin or glucagon-like peptide-1 (GLP-1) were evaluated by enzyme-linked immunosorbent assay. The insulin resistance was quantified using homeostasis model assessment method. The expression of GLP-1 receptor, Bcl-2, Bax, and caspase-3 was determined by Western blotting.
Results
Our results revealed that RYGB efficiently improved both glucose tolerance and insulin resistance in GK diabetic rats by upregulating GLP-1/GLP-1R expression. In addition, GLP-1R agonist exendin-4 dose-dependently increased insulin secretion in RIN-m5F cells and regulated the proliferation and apoptosis of these cells.
Conclusions
RYGB provides a valuable therapeutic option for patients with type 2 diabetes. GLP-1 may contribute to the regulation of pancreatic β-cell function through its receptor following RYGB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) results from the interactions of diverse environmental factors and genetic variants [1]. It exhibits an increased prevalence and is closely correlated with long-term microvascular and macrovascular complications, which are the major causes of increased morbidity and mortality in T2DM. Traditional pharmacological therapies, such as the initial oral monotherapy and insulin therapy at the advanced stages, are commonly used for treatment of patients with T2DM [2]. However, these therapies cannot prevent the incidence of T2DM-induced complications nor improve the prognosis of T2DM patients. Therefore, it is crucial to find an effective treatment for T2DM.
Accumulating evidence have shown that Roux-en-Y gastric bypass (RYGB) surgery provides long-term control of body weight and diabetes and has been considered to be an effective and safe therapy for T2DM patients [3–5]. Although the percentage of resolution of T2DM following RYGB has been reported to be approximately 83% [3], it is not known how RYGB surgery improves glucose homeostasis in T2DM. A number of studies have demonstrated that gut hormones may play a role in the regulation of body weight and glucose metabolism as the immediate response to this surgery [5, 6].
GLP-1, a 30 amino acid peptide secreted mainly by intestinal L cells in response to nutrient ingestion [7], is a potent insulin secretagogue that has multiple synergistic effects on glucose-stimulated insulin secretion [8]. However, the exact mechanism by which GLP-1 mediates the glucose tolerance and insulin resistance (IR) is not clear. In the present study, using T2DM model of Goto-Kakizaki (GK) rats, we investigated the effects of RYGB surgery on glucose tolerance and insulin resistance in GK diabetic rats and clarified the functional roles of GLP-1 in this process. We also studied the relationship between RYGB and GLP-1R. Furthermore, the roles of GLP-1R agonist, exendin-4 in the regulation of insulin secretion, and beta cell proliferation were also examined. The findings from our study will identify the mechanisms of the improvement of T2DM following RYGB surgery and thus provide a theoretical basis for clinical treatment of T2DM.
Materials and Methods
Reagents
Enzyme-linked immunosorbent assay (ELISA) kits for rat insulin and GLP-1 were purchased from Abcam Inc. (Cambridge, MA, USA) and Phoenix Pharmaceuticals (Burlingame, CA, USA), respectively. Exendin-4 was obtained from Sigma (St. Louis, MO, USA). Polyvinylidene difluoride (PVDF) membrane was purchased from BIO-RAD (Hercules, CA, USA). Rabbit anti-GLP-1R from Abcam Inc, rabbit anti-Ki-67, Bcl-2, Bax, caspase-3, and β-actin antibodies from Wuhan Boster Biological Technology, LTD were used in this study.
Cell Cultures
Rat insulinoma cell line, RIN-m5F cells were purchased from American Type Culture Collection. Cells were routinely cultured in RPMI 1640 culture medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 mg/L) in a humidified atmosphere of 5% CO2 incubator at 37°C. Subculture was carried out when cells reached 80% confluent. The normal glucose concentration in culture medium was 25.2 mmol/L. For high glucose stimulation, 33.3 mmol/L glucose was applied into culture medium.
Animals
Eight-week-old male GK rats (specific pathogen-free; n = 30) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Age-matched male Wistar rats (n = 10) were provided by laboratory animal center of affiliated Shengjing Hospital of China Medical University. Animals were maintained under standard conditions (12:12-h light/dark cycle) with free access to food and water before operation. All animals were treated as approved by the China Medical University Animal Research Committee.
Experimental Design
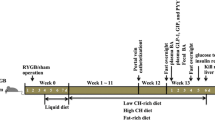
Rats were randomly allocated into four groups, GK RYGB, GK sham, GK food restriction (FR), and Wistar non-diabetic control, with ten rats in each group. In GK RYGB group, anesthesia was induced by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g body weight). Following disinfection with 2% Anerdian, the peritoneum was entered through a midline incision. The conjunction of esophagus and stomach was transected, and the stump of stomach was sewn closed with 6–0 sutures. The jejunum was cut at 10 cm distal to the Ligament of Treitz, and anastomosis was performed between the distal part of jejunum and esophagus. An enterotomy was made at 10 cm distal to the esophagojejunostomy. The abdomen was closed thereafter. Rats in the GK sham operation group received similar pre- or post-operative care as GK RYGB rats. However, in GK sham operation rats, transections of the gastrointestinal tract were performed at the sites where gastrotomy and enterotomy were performed for the RYGB, and reanastomosis was performed at the original cutting sites. Therefore, the physiological flow of food through the gastrointestinal tract remained intact. For rats in the FR group, accessible chow was restricted to only 1/3 of the normal daily intake. In addition, ten male Wistar rats were used as non-diabetic control. The body weight and food consumption of rats were recorded 1 week prior to or every week after surgery. Food intake rate was calculated by the following equation: food intake rate = daily food consumption (g)/rat body weight (kg).
Measurement of Fasting Blood Sugar and Oral Glucose Tolerance Test
Fasting blood sugar (FBS) levels were measured 1 week prior to or every week after surgery using blood glucose meter (Bayer, Germany) after 12–14 h fasting. Two grams per kilogram body weight glucose was given to rats by gavage. Blood samples were collected from the tail vein at 0, 30, 60, 90, and 120 min after the glucose gavage, and the glucose concentrations were immediately examined by a blood glucose meter. Area under the curve (AUC) was calculated using the trapezoidal rule.
ELISA
The levels of plasma insulin or GLP-1 were measured using ELISA kits according to the manufacturer’s instructions. After 12 h fasting, 0.5-mL blood sample was collected from the tail vein at 0 or 30 min after glucose gavage (2 g/kg body weight). Sera were isolated by centrifugation and stored at −80°C until used. Anti-rat insulin antibody was added to the 96-well plate (100 μL for each well) and incubated at 37°C for 60 min. After three times washing, 100 μL of concentrated enzyme solution was added to each well, and the plate was incubated at 37°C for 30 min. After washing, 100 μL of substrate solution was applied and the plate was incubated at 37°C for 25 min in dark. Finally, sealer was removed and the stop solution was loaded (100 μL for each well). The absorbance at 450 nm was recorded by the plate reader (ELX808, BioTek, USA) immediately after thorough mixing. The insulin concentration in the supernatant of RIN-m5F culture medium was examined in a similar manner.
Insulin Resistance Analysis
Insulin resistance was quantified by homeostasis model assessment (HOMA) method with the following formula: HOMA-IR = (G 0 × I 0)/22.5, where G 0 is the FBS level (millimoles per liter) and I 0 (micro-units per milliliter) is the fast plasma insulin level.
Western Blotting
To evaluate the GLP-1R protein expression, membrane proteins were extracted from RIN-m5F cells. Whole cell protein was extracted for detection of Ki-67, Bcl-2, Bax, and caspase-3 levels. Protein extracts were separated on 10% sodium dodecyl sulfate–polyacrylamide gel, transferred to PVDF membrane, blocked with 10% skim milk in Tris-buffered saline containing Tween 20 (TBS-T), and then probed with rabbit anti-GLP-1R, Ki-67, Bcl-2, Bax, or caspase-3 antibodies (1:500 dilution) at 4°C overnight. Secondary horseradish peroxidase-conjugated anti-rabbit IgG was used at a dilution of 1:2,000 for 2 h at room temperature. After three times washes with TBS-T, immunoreactive bands were visualized with BCIP/NBT alkaline phosphatase substrate system. β-Actin (1:1,000 dilution) was used as control. The intensity of band was further quantified using the BandScan software (FUJIFILM, Japan).
Statistical Analysis
The data were analyzed using SPSS 11.5 software and were plotted as mean ± SD. Statistically significant differences were carried out by one-way analysis of variance. P < 0.05 was recognized as significant.
Results
Effects of RYGB on Rat Body Weight and Food Intake
A total of 30 GK rats were randomly assigned into three groups, GK RYGB, GK sham, and GK FR, with ten rats in each group. Ten male Wistar rats were used as non-diabetic normal control. After operation, two rats from the GK RYGB group died due to anastomotic leakage or bleeding. Rats in other groups all survived throughout the experiment. No differences were observed in rat body weight or food consumption rate among these four groups 1 week prior to surgery, while decreased body weight was detected in rats from GK RYGB, GK sham, and GK FR groups following operation (Fig. 1a). In GK sham group, the average body weight was reduced after operation but slightly increased 1 week post-operation. Four weeks after surgery, the lowest body weight was found in GK FR rats. Food consumption was significantly decreased in rats from GK RYGB, GK sham, and GK FR groups 1 week post-operation, but gradually increased food intake was observed in these three groups (Fig. 1b). By week 4 post-operation, rats in GK FR group exhibited the lowest food intake rate (P < 0.05), and no difference was found in food consumption among GK RYGB, GK sham, and Wistar control groups.
RYGB Improved Glucose Tolerance and Insulin Resistance
We next investigated the effect of RYGB on glucose tolerance and insulin resistance in diabetic rats. As shown in Fig. 2a, elevated FBS level was found in GK diabetic rats compared to Wistar rats 1 week pre-operation (P < 0.05). The FBS levels presented a stable trend in rats from GK sham and GK FR groups post-operation, while a dramatic reduction was detected in rats received RYGB surgery. Moreover, the FBS value of GK RYGB rats recovered to normal level after the second week post-operation (P > 0.05 compared to Wistar group). To further evaluate the influence of RYGB on glucose tolerance, oral glucose tolerance test (OGTT) was performed. As expected, increased pre-operative glucose tolerance AUC was seen in GK rats (P < 0.05 compared to Wistar rats), whereas no difference was found among these GK rats (P > 0.05; Table 1). Three weeks after surgery, the OGTT AUC level of GK RYGB rats was remarkably reduced by 40.6% compared with the pre-operative value (P < 0.05), suggesting that RYGB efficiently improved glucose tolerance in diabetic rats. A similar but less pronounced effect was found in the GK FR group. In addition, diabetic rats showed an elevated baseline plasma insulin concentration and reduced insulin release capacity to respond to glucose stimuli compared to non-diabetic rats (Fig. 2b). Significant reduction of basal plasma insulin concentration was detected in GK RYGB rats post-operation (P < 0.05 compared with that of pre-operation). Thirty minutes after glucose administration, the plasma insulin level was obviously enhanced, revealing that RYGB promoted the response of glucose-stimulated insulin release in diabetic rats. To confirm the hypothesis that RYGB may improve insulin resistance in GK rats, HOMA-IR value was examined. Note that RYGB dramatically attenuated insulin resistance post-operation (Fig. 2c). These results suggested that RYGB efficiently improved both glucose tolerance and insulin resistance in diabetic rats.
RYGB improved glucose tolerance and insulin resistance of diabetic rats. a The FBS value of rats in each group was assessed 1 week prior to and every week after surgery using a blood glucose meter. b The levels of plasma insulin were evaluated by ELISA pre- and 3 weeks post-operation. c Insulin resistance was quantified by HOMA method pre- or 3 weeks post-operation as described in “Materials and Methods.” *P < 0.05 compared with GK RYGB group; #P < 0.05 compared with Wistar group
RYGB Increased Plasma GLP-1 Concentration and GLP-1R Level in the Pancreatic Tissue of GK Rats
To explore the possible mechanisms underlying RYGB-induced improvement of glucose tolerance and insulin resistance in diabetic rats, we investigated the plasma level of GLP-1. Decreased plasma GLP-1 concentrations were found in diabetic rats 1 week prior to surgery (P < 0.05 compared with that of Wistar rats), while the level of GLP-1 gradually increased following RYGB operation (Fig. 3a). Interestingly, no statistically difference in GLP-1 concentration was found between GK RYGB rats and Wistar rats 3 weeks after RYGB surgery. Importantly, by the fourth week post-operation, the plasma GLP-1 value in GK RYGB rats was significantly higher than that of control rats (P < 0.05). Furthermore, increased GLP-1R expression was observed in rat pancreatic tissues after RYGB surgery (P < 0.05, compared to that of the control Wistar rats; Fig. 3b). These findings demonstrated that RYGB surgery could increase the levels of GLP-1 and GLP-1R in the diabetic rats, implying that RYGB may improve glucose tolerance and insulin resistance by upregulating GLP-1/GLP-1R expressions. To further investigate the roles of GLP-1/GLP-1R in diabetes, we investigated the expression of GLP-1R in RIN-m5F cells. As shown in Fig. 4a, cells treated with 33.3 mmol/L high glucose demonstrated significantly decreased GLP-1 level in RIN-m5F cells (P < 0.05 compared with control).
RYGB increased plasma GLP-1 and GLP-1R levels in the pancreatic tissue of GK rats. a The plasma GLP-1 level was analyzed 1 week prior to and every week after surgery by ELISA. *P < 0.05 compared with Wistar group. b Four weeks after surgery, the GLP-1R expression in the rat pancreatic tissues was examined by Western blotting. Relative GLP-1R band intensity was quantified and normalized to β-actin. *P < 0.05 compared with GK RYGB group; #P < 0.05 compared with Wistar group
High glucose reduced the GLP-1R level in RIN-m5F cells. a RIN-m5F cells were treated with normal culture medium (containing 25.2 mmol/L glucose) or high glucose medium (containing 33.3 mmol/L glucose) for 24 h. Membrane proteins were extracted and the GLP-1R expression was evaluated by Western blotting. Relative GLP-1R band intensity was quantified and normalized to β-actin. *P < 0.05 compared with control (normal culture medium treatment). b Cells were incubated with different doses of exendin-4 (0–100 nmol/L). One hour after incubation, the supernatant of culture medium was collected and the released insulin concentrations were determined by ELISA analysis. *P < 0.05 compared with 0 nmol/L exendin-4 treatment. c Cells were treated with 50 nmol/L exendin-4 for 24 h. Protein expression of Ki-67, Bcl-2, Bax, and caspase-3 was determined by Western blotting. d Relative band intensity was quantified and normalized to β-actin. *P < 0.05 compared with control
Effect of Exendin-4 on Insulin Excretion
To investigate the mechanisms involved in promoting the effect GLP-1 on insulin secretion, we employed exendin-4, a GLP-1R agonist, which has been reported to be a more potent stimulator of insulin secretion than GLP-1 due to its long half-life [9]. We found that exendin-4 dose-dependently increased the insulin concentration in RIN-m5F culture medium (Fig. 4b). To explore the potential mechanism of exendin-4-promoted insulin secretion in RIN-m5F cells, expressions of the proliferation-associated nuclear antigen Ki-67 [10], apoptosis-related proteins, Bcl-2, Bax, and caspase-3 [11] were examined. As shown in Fig. 4c, d, 50 nmol/L of exendin-4 substantially upregulated Ki-67 expression in RIN-m5F cells 24 h after stimulation (P < 0.05 compared with control). Administration of the same dose of exendin-4 enhanced the level of Bcl-2 but reduced the level of Bax protein 24 h after stimulation. The Bcl-2/Bax ratio (3.2 ± 0.4) was remarkably elevated by exendin-4 treatment compared with controls (0.8 ± 0.2; P < 0.05). Meanwhile, downregulation of caspase-3 protein level was found in exendin-4 treatment group (P < 0.05 compared with control). These findings suggested that the GLP-1R agonist, exendin-4, promoted insulin secretion activity of RIN-m5F cells by affecting the proliferation and apoptosis of these cells.
Discussion
RYGB operation has been demonstrated to be an effective and safe way to achieve sustained improvement in T2DM patients [3–5]. One common explanation is that RYGB surgery invokes a pleiotropic endocrine response, with altered gastrointestinal hormone secretions [5, 6]. The gut gluco-incretin hormone GLP-1, which stimulates insulin secretion in response to enteral nutrients, is a candidate mediator of the anti-diabetic effects of RYGB [8]. In the present study, we confirmed that RYGB surgery largely improved the glucose tolerance and insulin resistance in type 2 diabetic rats by upregulating the expression of GLP-1 and GLP-1R.
RYGB Efficiently Improved Glucose Tolerance and Insulin Resistance in Diabetic Rats
While decreased body weight and food consumption were found in GK rats immediately following RYGB surgery (Fig. 1), by the fourth week after operation, both body weight and food intake rate gradually increased to normal levels. Although the food consumption was enhanced, the FBS value of GK RYGB rats recovered to normal level after the second week post-operation (P > 0.05 compared with Wistar group; Fig. 2a), demonstrating that the reduced FBS level was not a result from decreased food intake. A recent report also indicated that duodenal–jejunal exclusion improved glucose tolerance in GK rats without affecting body weight or food intake [12]. Importantly, the food restriction only showed limited effect on the improvement of glucose tolerance, whereas RYGB GK rats remarkably reduced the OGTT AUC level by 40.6% compared with the pre-operative value (P < 0.05; Table 1), suggesting that RYGB-induced improvement of glucose tolerance was probably not associated with food intake in diabetic rats. Previous studies have suggested that RYGB significantly promoted the response of glucose-stimulated insulin release post-operatively [13, 14]. Here, our results showed that RYGB surgery improved insulin resistance in GK rats. Collectively, we did not find direct correlations between the post-operative stress-induced initial lost of body weight, reduction of food intake, and the improvement of glucose tolerance/insulin resistance followed by RYGB. The possible mechanisms for the improvement of glucose tolerance and insulin resistance is likely resulted from the altered anatomical structures in gastrointestinal tract. Besides, decreased food and glucose intake as well as the drop of body fat may also contribute to the functional recovery of pancreatic β-cells.
The Involvement of GLP-1 in RYGB-Induced Amelioration of T2DM
Two hypotheses have been proposed to explain the effects of RYGB on T2DM—the hindgut hypothesis and the foregut hypothesis. The former states that diabetes control results from expedited delivery of chyme to the distal intestine, enhancing a physiologic signal that improves glucose metabolism [15, 16]. The latter theory contends that exclusion of the duodenum and proximal jejunum from the transit of nutrients prevents the secretion of a putative signal that promotes insulin resistance and T2DM [17, 18]. Importantly, the enhanced secretion of gut-derived incretin hormone, GLP-1, is widely accepted to be responsible for the improvement of glucose tolerance [6, 19]. Our present study demonstrated that diabetic rats exhibited decreased plasma GLP-1 concentrations prior to surgery (P < 0.05 compared to Wistar rats), whereas RYGB gradually upregulated the post-operative GLP-1 to normal level by week 3 post-operation (Fig. 3). It should be noted that on the fourth week post-operation that the plasma GLP-1 concentration in GK RYGB rats was higher than that of normal rats (P < 0.05). It is possible that since RYGB surgery enhanced the nutrient delivery to the distal ileum, it therefore stimulated GLP-1 secretion by intestinal L cells. However, we cannot rule out the possibility that RYGB surgery reduced the production of certain hormones, which might negatively regulated GLP-1 secretions. Interestingly, no obvious alternations of GLP-1 level were observed in FR group, suggesting that food restriction and loss of body weight did not contribute to the changes in GLP-1 level in diabetic rats. Besides, other hormones with possible roles in glucose tolerance and insulin resistance were not evaluated in this study; therefore, GLP-1 could be only one of the mediators in the improvement of glucose tolerance and insulin resistance in this setting.
Potential Mechanisms of Exendin-4-Mediated Upregulation of Insulin Secretion
GLP-1R is widely expressed in α and β cells of islets. Given the fact that the plasma half-life of the intact GLP-1 peptide is short in vivo [20], the GLP-1R agonist, exendin-4, is considered to be a more potent stimulator of insulin secretion [9]. To explore the potential mechanism of the improvement of T2DM after RYGB, we used a rat insulinoma cell line, RIN-m5F cells, and analyzed the effects of exendin-4 on the insulin secretion, proliferation, and apoptosis of pancreatic β-cells in vitro. Consistent with in vivo studies which showed the reduced level of GLP-1R in diabetic rats, high glucose-induced downregulation of GLP-1R protein expression in RIN-m5F cells (Fig. 4a), implying that decreased GLP-1R level may inhibit the bio-activity of GLP-1 and thus contribute to the progression of T2DM. Furthermore, exendin-4-promoted insulin secretion from RIN-m5F cells was accompanied with enhanced expression of the proliferation-associated nuclear antigen Ki-67 [10], suggesting that the promoted activity of GLP-1R can stimulate cell proliferation (Fig. 4c, d). In addition, elevated Bcl-2/Bax ratio and reduced caspase-3 [11] activity were observed upon exendin-4 treatment, suggesting that GLP-1R agonist prevented apoptotic cell death under high glucose conditions. These results demonstrated the critical role of GLP-1R in high glucose environment and implied that GLP-1R may be an alternative target for gene therapy of T2DM.
In conclusion, our data illustrated that RYGB efficiently improved glucose tolerance and insulin resistance in GK diabetic rats via upregulation of GLP-1 expression. RYGB operation may provide a valuable therapeutic option for patients with T2DM.
References
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46.
DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131(4):281–303.
Schauer PR et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. discussion 84–5.
Pories WJ et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50. discussion 350–2.
Rubino F et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236–42.
Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89(6):2608–15.
Mojsov S et al. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261(25):11880–9.
Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001;56:377–99.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Gerdes J et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–5.
Marzo I et al. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med. 1998;187(8):1261–71.
Kindel TL et al. Duodenal–jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13(10):1762–72.
Korner J et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90(1):359–65.
Korner J et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring). 2006;14(9):1553–61.
Patriti A et al. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004;14(6):840–8.
Mason EE. The mechanisms of surgical treatment of type 2 diabetes. Obes Surg. 2005;15(4):459–61.
Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25(4):527–31.
Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236(5):554–9.
Patriti A et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg. 2005;15(9):1258–64.
Deacon CF et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44(9):1126–31.
Conflict of Interest
None of the authors has any conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Zhou, Y., Wang, Y. et al. Roux-en-Y Gastric Bypass-Induced Improvement of Glucose Tolerance and Insulin Resistance in Type 2 Diabetic Rats Are Mediated by Glucagon-Like Peptide-1. OBES SURG 21, 1424–1431 (2011). https://doi.org/10.1007/s11695-011-0388-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-011-0388-z