Abstract
Aim of this study is to compare the efficacy of BioEnterics Intragastric Balloon (BIB®) followed by diet with BIB followed by another BIB. A prospective study was designed: a homogeneous group of 100 obese patients (age range 25–35, BMI range 40.0–44.9, M/F ratio 1/4) was allocated into two groups according to procedure: BIB (6 months) followed by diet therapy (7 months; group A = 50 pts), BIB positioning followed by another BIB after 1 month (group B = 50 pts). Baseline demographics were similar in both groups (Group A 10M/40F; mean age 31.4 ± 2.6; range 25–35; mean weight 106.3 ± 12.5 Kg; range 88–150; mean BMI 42.6±2.7 Kg/m2; range 40.2–43.8; Group B 10M/40F; mean age 32.1 ± 2.1; range 25–35; mean weight 107.1 ± 11.9 Kg; range 90–150; mean BMI 42.9 ± 2.3; range 40.2–43.9). In both groups, weight loss parameters (Kg, BMI, and % EBL) were considered. Statistics were by Fisher’s exact test (p < 0.05 was considered significant). At the time of 1st BIB removal, weight loss parameters in both groups were not significantly different: Group A: mean weight was 83.7±19.1 (range 52–151); mean BMI 34.2 ± 3.9 (range 32.4–43.8); and mean %EBL 43.5 ± 21.1 (range 0–68). Group B: mean weight was 84.9 ± 18.3 (range 50–148); mean BMI 34.8 ± 3.3 (range 32.4–43.8); and mean % EBL 45.2 ± 22.5% (range 0–68). At the study end, weight loss parameters were significantly lower in patients who underwent consecutive BIB (p < 0.05): mean BMI was 30.9 ± 7.2 Kg/m2 (range 24–40), and 35.9 ± 9.7 Kg/m2 (range 34–42); mean % EBL was 51.9 ± 24.6% (range 0–100) and 25.1 ± 26.2% (range 0–100) in group B and A, respectively. As compared with diet, a second intragastric balloon can be positioned without difficulties, achieving good results with continuous weight loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The BioEnterics® Intragastric Balloon (BIB®) is a well-established device for temporary treatment in morbidly obese patients [1–4]. The BIB® is potentially attractive to healthcare practitioners whose patients have experienced poor results with dietary programs, drug treatment, and behavioral therapy [5]. Moreover, many aspects of this device are unknown, and only a few studies were prospectively conducted to evaluate its efficacy [1–6]. BIB was mainly positioned before bariatric surgery in a planned strategy to reduce co-morbidities, lowering the surgical and anesthesiological risks [7–9]. Unfortunately, this strategy can be uneventful in many cases. In fact, it has been reported that half of patients scheduled for surgery refused the following step [1, 2]. Moreover, in many bariatric centers, the waiting list is very long. Due to this delay, the effect of the BIB treatment is lost before the definitive treatment. So, the idea to place a second consecutive intragastric balloon was developed. To evaluate this therapeutic option, a prospective study was designed with the aim of comparing the efficacy of BioEnterics Intragastric Balloon (BIB®) followed by diet to BIB followed by another BIB.
Methods
Patient Selection
From January 2007 to December 2007, a homogeneous group of 100 obese patients (age range 25–35, BMI range 40.0–44.9, M/F ratio 1/4) accepted to participate at the present study and were randomly allocated into two groups according to procedure: BIB (6 months) followed by diet therapy (7 months; group A = 50 pts), BIB positioning followed by another BIB after 1 month (group B = 50 pts).
Baseline demographics were similar in both groups (Group A 10M/40F; mean age 31.4 ± 2.6; range 25–35; mean weight 106.3 ± 12.5 Kg; range 88–150; mean BMI 42.6 ± 2.7 Kg/m2; range 40.2–43.8; Group B 10M/40F; mean age 32.1 ± 2.1; range 25–35; mean weight 107.1 ± 11.9 Kg; range 90–150; mean BMI 42.9 ± 2.3; range 40.2–43.9).
BIB Positioning
BIB (Allergan, Irvine, CA, USA) was used in all cases. The Helicobacter Pylori test was performed in all patients. After sedation (propofol 2 mg/Kg i.v.), the patient was placed in a lateral decubitus position, and esophagus, stomach, and duodenum were endoscopically examined. The instrument was retrieved, and the BIB was inserted in the stomach; inflation was performed under direct vision by using saline (500 ml) and methylene blue (10 ml) solution. After 6 months, the BIB was removed by endoscopy after complete deflation and with a dedicated instrument. Patients of both groups received the same medical treatment during the study. On the first postoperative day, intravenous saline (30–35 ml/Kg/die) with omeprazole (20 mg/die), ondansetron (8 mg/die), and butylscopolamine bromide (20 mg × 3/die) were given to all patients. On the second postoperative day, if they were able to tolerate fluids, patients were discharged with drug therapy: omeprazole (20 mg/die), anti-emetics (if needed), and a 1,000 Kcal diet (glucide 146 g, lipids 68 g; proteins 1 g/Kg ideal weight according to the Lorentz’ formula).
Study Outline
This study was approved by local ethical committee and patients enrolled signed a specific informed consent. Patients were randomly allocated to their treatment by sorting a sealed envelope, with a computer-generated randomization list. Graphic representation of this study is reported in Fig. 1. This was conducted according to criteria of the so-called intention-to-treat analysis. Time elapsed from 1st balloon removal and 2nd balloon placement is 30 days. This period allows the stomach to restore the pre-BIB placement conditions. Patients were weighed weekly. In both groups, weight loss parameters (Kg, BMI, %EBL) were considered. Results are expressed as mean ± standard deviation and range for numerical variables and absolute numbers. Statistical analysis was done by means of Student’s t test for numerical variables and χ 2 test or Fisher’s exact test for categorical variables. P < 0.05 was considered significant.
Results
BIB was well tolerated in patients of both groups with slight duration of post-placement symptoms (Table 1).
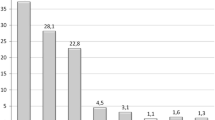
At time of 1st BIB removal, weight loss parameter in both groups were not significantly different: Group A mean weight was 83.7 ± 19.1 Kg (range 52–151); mean BMI 34.2 ± 3.9 Kg/m2 (range 32.4-43.8); and mean %EBL 43.5 ± 21.1% (range 0–68; Table 2). Group B: mean weight was 84.9 ± 18.3 Kg (range 50–148); mean BMI 34.8 ± 3.3 Kg/m2 (range 32.4–43.8); and mean %EBL 45.2 ± 22.5% (range 0–68). During the months of therapy suspension after the 1st BIB, patients of both groups regain a mean of 0.9 ± 0.9 of BMI. At the study end, weight loss parameters were significantly lower in patients who underwent consecutive BIB group B patients (p < 0.05): mean BMI was 30.9 ± 7.2 Kg/m2 (range 24–40), and 35.9 ± 9.7 Kg/m2 (range 34–42); mean %EBL was 51.9 ± 24.6% (range 0–100) and 25.1 ± 26.2% (range 0–100) in groups B and A, respectively (Table 2, Fig. 2). After 2 years of follow-up, the rate of patients present was significantly higher in Group B (44/50; 88% vs 33/50; 66.6%; p < 0.001). Most of the patients absent underwent a bariatric surgical procedure: 14/50 (28%) and 5/50 (10%) in group A and B, respectively (p < 0.001; Table 3). Mean BMI was significantly lower (p < 0.05) in Group B after 1 year (33.1 vs 37.1 Kg/m2) and after 2 years (36.8 vs 41.1 Kg/m2) of follow-up (Fig. 2). Several complications were diagnosed in patients enrolled in this study. A detail of pathologies and their outcome after the 1st and the 2nd step of this study are reported in Tables 4 and 5.
Discussion
The BIB is a device used for temporary treatment of obesity [1–4]. Intragastric balloon positioning is potentially attractive to healthcare practitioners who have experienced poor results with dietary programs, drugs, and behavioral therapy [5]. It has been mainly recommended as a weight reduction adjuvant before bariatric surgery and before all kinds of planned surgery in the morbidly obese patients to reduce life-threatening co-morbidities and lesser surgical risk [7–10].
The effectiveness of the intragastric balloon has at least two components linked to balloon itself and the concomitant conventional treatment. A recent meta-analysis estimates balloon efficacy with the loss of 6.7 Kg at time of removal [1]. These results are greater than the efficacy evaluated for sibutramine (4.4 Kg) and orlistat (3.9 Kg) [11]. However, long-term follow-up is noticeable scarce. In a recent study, we reported that in short–medium-term follow-up BIB is significantly better than diet in support weight loss [5]. Melissas, Hervé, and Sallet reported different experiences that demonstrate than in a subset of patients compliant to diet therapy after BIB removal weight loss was maintained [4, 7, 9].
On the other end, it has been reported that half of patients scheduled for surgery after BIB removal declined any bariatric operation [1, 2]. Thus, in this population, it is mandatory to offer a treatment to avoid the regain of their initial weight, nullifying the therapeutic attempt [7–9].
The idea to insert a second BIB a few weeks from first balloon removal was developed both to prolong the efficacy of the BIB and ameliorate the weight loss. In our daily experience, most patients ask to not remove the balloon because of good results in terms of weight loss, and the waiting list for bariatric surgery is 6–12 months. Patients were clearly informed that the positioning of a second intragastric balloon was not a definitive treatment of their obesity but only a bridge for a surgical bariatric treatment. In the present study, the second balloon was placed without difficulties and adjunctive complications. Only one patient decided to stop the trial because psychological intolerance after more than 1 year treatment. As compared with diet, a second intragastric balloon can be positioned without difficulties, achieving significant good results with continuous weight loss as compared to in patients treated with BIB followed by diet.
The second BIB was tolerated without significant complications. We suggest this protocol in those patients who refused the surgical bariatric treatment or in those who referred in centers with a long waiting list or in those patients with BMI < 35 without clear surgical indications to prevent weight gain (Fig. 3).
References
Imaz I, Martinez-Cervell C, Garcia-Alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes surg. 2008;18:841–6.
Spyropoulos C, Katsakoulis E, Mead N, et al. Intragastric balloon for high risk superobese patients: a prospective analysis of efficacy. SOARD. 2007;3:78–83.
Genco A, Bruni T, Doldi SB, et al. BioEnterics Intragastric Balloon: the Italian experience with 2, 515 patients. Obes Surg. 2005;15:1161–4.
Sallet JA, Marchesini JB, Paiva DS, et al. Brazilian multicenter study of the intragastric balloon. Obes Surg. 2004;14:991–8.
Genco A, Balducci S, Bacci V, et al. Intragastric balloon or diet alone? A retrospective evaluation. Obes Surg. 2008;18:989–92.
Genco A, Cipriano M, Bacci V, et al. Bioenterics Intragastric Balloon (BIB): a short term, double-blind, randomized, controller, crossover study on weight reduction in morbidly obese patients. Int J Obes. 2006;30:129–33.
Melissas J, Muozas J, Filis D, et al. The intragastric balloon. Smoothing the path to bariatric surgery. Obes Surg. 2006;16:897–902.
Angrisani L, Lorenzo M, Borrelli V, et al. Is bariatric surgery necessary after intragastric balloon treatment? Obes Surg. 2006;16:1135–7.
Hervé J, Wahlen CH, Schaeken A, et al. What becomes of patients one year after the intragastric balloon has been removed? Obes Surg. 2005;15:864–70.
Genco A, Cipriano M, Materia A, et al. Laparoscopic sleeve gastrectomy versus intragastric balloon: a case-control study. Surg Endosc. 2009;23:1849–53.
Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–46.
Conflict of interest
Authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genco, A., Cipriano, M., Bacci, V. et al. Intragastric Balloon Followed by Diet vs Intragastric Balloon Followed by Another Balloon: A Prospective Study on 100 Patients. OBES SURG 20, 1496–1500 (2010). https://doi.org/10.1007/s11695-010-0231-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0231-y