Abstract
Microbial exopolysaccharides (EPS) are extracellular polymeric substances synthesized by bacteria, fungi or algae which are now one of the important food ingredients. In this study, glucan EPS was isolated from lactic acid bacteria (LAB) of fermented food origin and was incorporated in yoghurt to enhance its techno-functional attributes and rheological properties. Glucan EPS concentration at 1% showed significant improvement in syneresis, wheying off, water-holding capacity, and sensorial properties of yoghurt. The water holding capacity (WHC) increased from 47% to about 63% while syneresis decreased from 52% to nearly 30%. Rheological features such as viscosity, storage modulus, loss modulus, and firmness were significantly higher for 1% EPS containing yoghurt (1% ECY) than that of the control. The average particle size of 1% ECY was increased from 2.35 µm to 3.46 µm. The microstructure of EPS containing yoghurt was studied by scanning electron microscopy and confocal laser scanning microscopy. EPS supports the growth of probiotic microflora of yoghurt, which was revealed by analyzing the microbial count of yoghurt. The antioxidant activity as 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid (mg Trolox/100 g sample) and reducing power assay (mg ascorbic acid/100 g sample) for 1% ECY and control yoghurt were found to be 27.9 (mg Trolox/100 g sample), 6.44 (mg Trolox/100 g sample) and 0.25 (mg ascorbic acid/100 g sample), 0.19 (mg ascorbic acid/100 g sample), respectively. Significant enhancement of these bio-functional attributes of EPS containing yoghurt makes the glucan EPS a suitable hydrocolloid to develop various functional foods.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased consumer awareness about the relationship between healthy food habits and well-being, the importance of functional foods has tremendously grown. Yoghurt is one of the popular fermented dairy-based product, commonly produced by the addition of lactic culture namely Streptococcus salivarius ssp. thermophiles and Lactobacillus delbrueckii ssp. bulgaricus. They convert lactose to lactic acid and synthesize flavour components including volatile and non-volatile acids as well as carbonyl compounds that are responsible for the characteristic flavour of yoghurt [1]. In the past few decades, yoghurt has gained importance from a functional food perspective due to its rich pool of various functional and bioactive components. Variety of health attributes associated with yoghurt consumption includes prevention of osteoporosis, weight management, improved lactose tolerance, strengthening of immune system, cancer prevention etc. [2]. Along with these biological roles, other key parameters which are important in yoghurt are structural, rheological and textural properties which ultimately decides consumer’s sensorial acceptance. Hence, to obtain these attributes, several studies were conducted in recent years stating the use of hydrocolloids, kind of polysaccharides, such as gelatin, carrageenan, various gums and modified starches have been successfully used. Parallelly, extensive work has been conducted to enhance these techno-functional attributes by using bacterial exopolysaccharides (EPS). Many lactic acid bacteria (LAB) can produce EPS, carrying a wide range of applications such as stabilizer, emulsifier, viscosity enhancer, gelling agent and water-binding agents in many food products like jam, jellies, yoghurt, bakery products, beverages and salad dressings which contributes to texture, consistency, mouthfeel, and reduced syneresis of the products [3]. Especially, in dairy-based fermented products such as yoghurt and cheese, EPS incorporation was done successfully to obtain proper consistency and texture of the product [4]. Numerous studies have also indicated that EPS can enhance the textural, physical, microstructural and sensorial properties of yoghurt [4,5,6,7]. Furthermore, few EPS reported in previous studies showed excellent antioxidant properties [8]. EPS has been known for imparting various physicochemical and functional properties such as emulsification, anti-syneresis, flocculation, water solubility, prebiotic properties etc. [9]. The quantities reported for EPS production by various LAB strains that have got Generally Regarded as Safe (GRAS) status with various functional attributes varies from 25 mg L− 1 to 600 mg L− 1. These amounts are lower as compare to EPS produced by microbes that have not got GRAS status (10–25 g L− 1) [10].
In this study, glucan EPS produced from Enterococcus hirae OL616073, it is able to produce 20 g L− 1 thereby opening the door for its sound use in favour of industrial scale-up. Further, this EPS has shown to possess various functional properties including foaming capacity, emulsifying and good water holding capacity [11]. Moreover, in detail antioxidant potential and in vitro probiotic stimulation abilities are also studied for this glucan EPS (unpublished results). Therefore, by virtue of these properties and higher production rates, this study aims at evaluating the effect of glucan EPS on various techno-functional, microstructural, textural, rheological and bio-functional properties of yoghurt.
Materials and methods
Ingredients
Milk was procured from the local dairy co-operative outlet of Pondicherry, India. Skim milk powder (SMP having 35% of protein) used was from well-known national brands. EPS (glucan) used was produced from the potential probiotic strain Enterococcus hirae OL616073 formerly isolated from traditional Indian fermented food (Idli) [11]. Standardized yoghurt cultures (Streptococcus salivarius ssp. thermophiles & Lactobacillus delbrueckii ssp. bulgaricus) were procured from National Collection of Dairy Cultures, NDRI, Karnal, India in the form of freeze-dried culture (NCDC 144 mixed culture), activated and stored in deMan Rogosa Sharpe (MRS) media. All other chemicals and microbial media used were procured from Sigma Aldrich Chemicals Pvt. Ltd. (Bangalore, India) and HiMedia Laboratories Pvt. Ltd. (Mumbai, India) were either of analytical or HPLC grade.
EPS production
The EPS production was carried out according to the method explained by Kavitake et al. [11] from probiotic strain Enterococcus hirae OL616073. The culture was inoculated in sterile MRS broth (supplemented with 2% sucrose) and incubated for 48 h in a static condition at 30 °C. To remove the cell biomass, the fermented broth was centrifuged at 12,000 x g for 15 min and the supernatant was collected. Then, 2% (w/v) trichloro acetic acid (TCA) solution was added to the supernatant and the precipitated protein impurities were removed by centrifugation (10,000 x g for 30 min at 4 °C). Then three volumes of the ice-cold ethanol were added to the supernatant and kept overnight for precipitation at 4 °C. The precipitate was collected by centrifugation (10,000 x g for 15 min) and dissolved in deionized water. It was washed with ethanol 2–3 times to remove the media impurities from the EPS. Then the crude EPS suspension was dialyzed against deionized water using 12–14 kDa molecular weight cut-off (MWCO) dialysis membrane at 4 °C for two days. The dialyzed contents were then freeze-dried, weighed, and stored. The sugar content in the extracted (partially purified) EPS was quantified by the phenol–sulfuric acid method.
Yoghurt production
Yoghurt was produced by following the method of Wacher-Rodarte et al. [12] with few modifications. The milk procured was standardized to 15% total solids (w/v) and heated at 90 °C for 10 min. Then the samples were cooled to 42 °C in a water bath containing ice and subsequently inoculated with the yoghurt cultures (2%), poured into 100 mL PET bottles. Later incubated at 42 °C for about 7–8 h and stored at refrigeration temperature (4 °C) for 24 h before testing. All the analysis were done after 24 h but before 48 h of storage.
The effect of glucan EPS was studied by adding and dissolving (a process of dissolution involves 30 min hydration and 10 min vortexing) it into milk prior to incubation. Different concentrations of EPS (0.10, 0.25, 0.50, 0.75 and 1.00% (w/v), were used and the milk without EPS was taken as control.
Selection of EPS concentration level based on techno-functional and sensory properties
Wheying off
Wheying off was measured by using a siphon-off method described by Hassan et al. [13] with few modifications. Briefly, the set of refrigerated yoghurt samples were taken out and immediately kept at 45° C to allow the whey present on the surface to collect on the side of the cup within 10 s. Thereby, whey separated on the surface for spontaneous serum separation was collected by pipetting it out.
Water holding capacity
The water-holding capacity (WHC) of yoghurt was estimated by the protocol given by Mudgil et al. [14] with few modifications. Briefly, 10 g of yoghurt sample (X) was subjected to centrifugation at 950 g for 10 min at 4° C. The whey expelled (Y) was removed and the pellet was weighed. It was estimated as:
Syneresis
Syneresis (%) was estimated by the protocol given by Amatayakul et al. [15] with some modifications. Ten gram of the yoghurt sample was centrifuged at 950 g for 15 min. The syneresis was calculated as a percentage.
Sensory evaluation
Sensory evaluation performed in this study was carried out by healthy, non-smokers and lactose-tolerant subjects with consent to participate in the sensory evaluation of the yoghurt samples. Ten semi-trained sensory panelists from the Department of Food Science and Technology, Pondicherry University (5 women and 5 men, ages 25–55 years old) were chosen based on voluntariness with the understanding that the samples under evaluation were safe. The participants in this research were regular users of this kind of product. They rinsed their palates with water in between for each sample. A nine-point Hedonic scale, which indicates the degree of participants’ general liking or disliking of quality characteristics such as appearance, taste, texture, aroma, and overall acceptability, was used to ask participants to rate the product. According to the evaluator, each attribute’s score ranged from 1 to 9 (1: extremely dislike; 9: extremely like) [16]. The average intensity score for each attribute was determined and tallied when the evaluation process was finished.
Physicochemical properties of yoghurt
pH and acidity
The pH of the yoghurt sample was measured using a digital pH meter (Eutech Instruments, Cyberscan 2100, Singapore). The titratable acidity of the yoghurt was analyzed by the method given by AOAC [17]. It was determined by titrating with NaOH (0.1 N), using phenolphthalein as an indicator and expressed as % lactic acid
Color parameters
L*, a*, b*, chroma, hue, whiteness index, yellowness index, Browning index and ΔE (Degree of color change).
Hunter Lab ColorFlex (Reston, VA, USA) was used to measure the color of yoghurt samples [18]. Results were expressed as L*, a*, b*, chroma (C*), hue (H*), whiteness index (WI) yellowness index, Browning index and ΔE.
Particle size analysis
The particle size analysis of yoghurt was done by using Malvern Mastersizer, (Malvern Instruments Ltd., Worcestershire, UK). The sample was prepared by diluting it 20 times with ethylene glycol to avoid particle sedimentation. A hundred micrograms of sample was suspended in 2 mL of ethylene glycol and ultrasonicated for 20 min in a bath sonicator. Average particle size was estimated and results were expressed in µm. The refractive index was calculated using Abbe’s refractometer yoghurt (1.365) and ethylene glycol (1.429).
Fourier transform infrared microscopy
Fourier transform infrared microscopy (FTIR) was used to detect the structural and functional groups of yoghurt. Approximately 5 mg of yoghurt powder sample was mixed with dry potassium bromide (KBr) thereby compressing the mixture into pellet form. The spectrum was recorded between the wavelength ranges of 400–4000 cm− 1 at a resolution of 4 cm− 1 using FT-IR Thermo Nicolet: 6700, USA.
Microstructural analysis of yoghurt
Scanning electron microscopy (SEM)
A very thin layer of the sample was smeared on a coverslip and air-dried, thereby subjected to metallization (sputtering) for about 45 min with a thin layer of gold nano-particles in a sputter coater. Typical microstructures at different magnifications (1.00 K and 2.00 K) were taken using a Hitachi S-570 SEM, operating at 15 kV (Hitachi S-3400 N), Japan.
Confocal laser microscopy (CLSM)
Sample preparation was done according to the protocol given by Kristo et al. [19] with slight modifications. Yoghurt samples were incubated for 3 h by staining with rhodamine B dye at 4 °C before observing in the microscope. The samples were observed under CLSM (M/S Nikon Corporation, Model Nikon (A1R), Tokyo, Japan) equipped with multiple laser lines including Diode (405 nm, 440/445 nm, 488 nm, 561/594 nm, 638/640 nm), AR laser (457 nm, 488 nm, 514 nm), He-Ne laser (543 nm) with the wavelength range of 400–750 nm, fixed with 4 Photo Multiplier Tubes detector and 6 Filter cubes. The emission spectral range for sample observation kept was 565–600 nm.
Rheological properties of yoghurt
Viscosity and viscoelastic properties
Compact rheometer (RHEOPLUS MCR 52, Anton Paar, GmbH, Austria) equipped with a Peltier plate temperature controller over an extended period of time was used. The apparent viscosity was measured by using a parallel plate (PP-50) geometry, a probe with a diameter of 50 mm, and the gap between the plates as 1 mm at different shear rates (0.1 to 100 s− 1). An amplitude sweep test was performed to determine the linear viscoelastic range with a constant angular frequency of 10 rad/s and varying strain between 0.1–100 rad/s. Subsequently, a frequency sweep test was performed at maximum strain to measure the storage modulus (G’), loss modulus (G’’), and complex viscosity (η*).
Texture analysis
Texture analysis of yoghurt sample was done as described by Behare et al. [20]. The texture analyzer (TA-XT2i, Stable Micro Systems, UK) fitted with 25 kg load cell was calibrated by using 5 kg standard weight prior to use. A 25 mm diameter perplex cylindrical probe (P25) was used to measure yoghurt firmness set in 50 mL sterilized sample container at a temperature 25 ± 1 °C.
Antioxidant assays
Preparation of sample aliquots for antioxidant assays
0.5 g of yoghurt sample was weighed and 1 mL of 100% of methanol was added to it thereby centrifugation was done at 1600 g for 15 min. Supernatant was used as sample aliquot.
ABTS
ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) free radical scavenging activity of yoghurt was analyzed by the method described by Re et al. [21] with some modifications. ABTS radical cation (ABTS+) stock solution having a concentration of 7 mM was prepared in potassium persulfate solution (2.45 mM) and kept in the dark at room temperature for 12–16 h before use to produce free radicals. For further study, the ABTS+ solution was diluted with methanol to an absorbance of 0.76 ± 0.02 at 734 nm to obtain a working solution. Methanol was taken as a reagent blank. To 3.0 mL working solution of ABTS+, 30 µL of sample aliquot was added; the absorbance (at 734 nm) was taken after 6 min of initial mixing. The results were expressed in mg trolox equivalent/100 g sample.
Reducing power assay
Reducing power assay was analyzed by the method given by Benslama and Harrar [22]. Standard curve was plotted using ascorbic acid and results were expressed as mg/100 g ascorbic acid equivalent.
Microbial analysis of yoghurt
Spread plate method was used to determine the colony-forming units as log CFU/g by serial dilution and plating of yoghurt samples (control and 1% ECY) on MRS agar plates and incubating at 40 °C for 48 h.
Statistical analysis
The data obtained during optimization were analyzed statistically using Duncan’s Multiple Range Test and paired-t test in SPSS tool (IBM SPSS Statistics Version 20) both at p < 0.05 level of significance. All results are presented as the mean ± SD.
Result and discussion
Selection of EPS concentration level based on techno-functional properties of yoghurt
Wheying off
Wheying off is a kind of textural defect in yoghurt occurs due to the weakening of the gel network. This is mainly caused by shrinkage of the yoghurt gel when it loses its ability to bind all of the serum phase [23]. Therefore, spontaneous serum separation on the surface of a gel network without applying external pressure leads to wheying off; while whey syneresis is quantified by disrupting the gel network mechanically [15]. Wheying off on the surface was analyzed by pipetting out the whey present on the surface for selection of EPS concentration in yoghurt samples. In case of control, the wheying off was excessive (~ 1.7 mL for 100 mL yoghurt) while with increasing concentration of EPS wheying off went on decreasing and ultimately in the yoghurt sample containing 1% EPS whey separation on the surface was almost negligible (less than 0.1 mL for 100 mL yoghurt) as shown in Fig. 1(a). One set of yoghurt was kept in test tubes to observe the visual serum separation as indicated in Fig. 1 (b). It was mainly due to the presence of higher level of EPS in the serum phase which increased the viscosity of yoghurt by aiding in bridges formation with the casein matrix, it was observed that EPS and casein interactions can modify the gel structure. Further, EPS increased the density of protein network due to the presence of α-1-6 and α-1-3 linked D-glucose units of glucan EPS causing an increase in the adsorption of water in gel structure as mentioned in our previous work on physicochemical characterization of glucan EPS [11]. EPS entanglement with the casein matrix was also confirmed with scanning electron micrographs of yoghurt by Teggatz, and Morris [24]. Previously, Han et al. [6] indicated the less whey separation in EPS rich yoghurt compared to control. Therefore, it is suggested that EPS can be a good alternative to hydrocolloids for cultured dairy products where texture is of prime importance.
(a): Wheying off in yoghurt samples at different EPS Levels (0.00 to 1.00%). (b): One set of yoghurt at different EPS Levels (0.00 to 1.00%) was kept in glass tubes for visualization (Arrow indicating whey separation). (c): Syneresis and water holding capacity (WHC) of yoghurt samples at different EPS levels (Alphabets a to d and A to C indicate significant differences at various concentrations of EPS in syneresis and WHC, respectively)
Water holding capacity
Water holding capacity or water retention capacity is the total amount of water that can be absorbed in the three-dimensional structure of the protein. It is an important structural characteristic of yoghurt. Figure 1(c) indicates that with increasing concentration non-significant difference (p < 0.05) was found between control (WHC: ~47%) and 0.1% (WHC: 48%) of EPS. However, WHC increased with increasing EPS concentration. As a result, significant increase was found (p < 0.05) when the EPS concentration increased from 0.1 to 0.75% (WHC: ~60%) and thereby non-significant difference was observed at 1% level of EPS (WHC: ~63%). The increase in WHC was mainly due to the interaction between proteins micelles and EPS [25]. Especially, EPS when present at higher level, it causes the formation of protein aggregates, and the water retention ability increases with the formation of larger protein aggregates. Several reports have suggested that EPS enrichment enhances the water holding capacity in yoghurt [26, 27]. Furthermore, in fermented milk-based pudding also, İspirli et al. [28] has reported reduced serum separation by enriching it with 1% of glucan type EPS with enhanced bio-functional attributes. Recently, Du et al. [29] revealed nearly 10% increment in WHC when 0.6% level of glucan EPS was added ex situ compared to control sample. Significant increase in WHC was observed by Pan et al. [30] also after adding 0.5% of dextran EPS obtained from Leuconostoc pseudomesenteroides. Amani et al. [27] has suggested that an EPS and protein interaction plays a very significant role to bind the serum phase and thereby enhancing the WHC. However, non-significant increase in WHC was found when concentration of EPS increased from 0.75 to 1%. Hence, it can be concluded that glucan EPS at 1% can influence the WHC of yoghurt gel positively.
Syneresis
Syneresis is simply shrinkage of a gel network which leads to oozing out of water. The effect of different concentrations of EPS on the syneresis of yoghurt was studied. Significant (p < 0.05) reduction of syneresis was found between control and yoghurt containing different EPS concentrations, Fig. 1(c). The syneresis was significantly decreased to an extent of ~ 31% (case of 1% EPS level) from ~ 52% (case of control). At higher concentrations of EPS, syneresis was less due to the water imbibing ability of the EPS and the presence of EPS in the protein matrix of yoghurt subsequently modifies the yoghurt gel during the coagulation process. Previously, similar results have been reported by several authors [4, 5, 7, 26]. These studies have shown that the yoghurt made with EPS or EPS producing strains showed significantly (p < 0.01) less syneresis as compared to that of control. In previous reports, EPS indicated reduced degree of syneresis in starch paste at the level of 0.50% using galactan EPS [9]. Further, syneresis decreases as the pH increases, however, no significant difference in the pH values was found. Hence, 1% of this EPS can be successfully used as anti-syneresis agent to reduce the syneresis and enhance the quality of yoghurt without affecting the pH significantly.
Sensory evaluation
Sensory evaluation of yoghurt samples incorporated with EPS on various important attributes such as color, odour, taste, mouthfeel, texture and overall acceptability conducted is indicated in Fig. 2. In case of EPS enriched yoghurts, texture and mouth feel scores were increased as compared to control. This can be correlated with texture enhancing effects of EPS on yoghurt while overall acceptability, odour and taste got marginally affected. The texture and mouthfeel scores were higher for 1% EPS containing yoghurt while taste, odour and overall acceptability scores were almost comparable with that of control. The effect of EPS on sensory properties of yoghurt studies have indicated that addition of EPS does not affect the sensory qualities much however exhibited better textural properties and mouthfeel. The mouthfeel of ECY was better due to enhanced microstructural characteristics like stiff and dense protein network with large size pores [8].
Based on these techno-functional parameters and sensory analysis, 1% ECY concentration was selected and used for further studies in yoghurt.
Physicochemical properties of yoghurt
pH and acidity
Statistically, non-significant difference was found between control and 1% ECY, however, 1% ECY had slightly lower pH values than control (Table 1). Ramchandran and Shah [31] and Hassan et al. 2015 [13] have reported similar results stating non-significant (p > 0.05) difference in pH of the hydrocolloid (EPS, cress seed mucilage and guar gum) containing and non-containing yoghurt.
ECY showed significantly higher titratable acidity (p < 0.05) than control. Contrarily, Güler-Akin et al. [5] reported that the titratable acidity of EPS containing yoghurt was lower than non EPS containing yoghurt. However, in this study, slightly higher acidity values for EPS-enriched yoghurt were observed which might be due to the EPS utilizatio n and acid production by microorganisms present in yogurt.
Color parameters
L*, a*, b*, chroma (C*), Hue angle (H*), whiteness index, yellowness index, browning index and ΔE (degree of color change). The color measurements are presented in terms of L*, a*, b*, chroma, hue angle, whiteness index, yellowness index, and browning index values, along with a degree of change in color of yoghurt sample after EPS enrichment i.e. ΔE. In the case of 1% ECY sample, whiteness index, an important quality parameter related to the processing, and storage of yoghurt as well as L* value related to luminosity, were decreased significantly (p < 0.05) (Table 1). On the other hand, a* and b* values and yellowness index were higher for 1% ECY samples which can be correlated with slight reddish and yellowish color of EPS reported in previous report by Kavitake et al. [11]. The shifting of a* values from − 1.60 to + 1.42 indicates the shifting slight greenness to slight redness [32], while a color shift in case of b* values indicates the increase in slight yellowness of yoghurt sample which again can be attributed to the color provided by EPS. Further, higher intensity of chroma value is associated with higher degree of colorfulness with respect to grey color of same lightness, which again was higher for EPS enriched sample. Hue angle, H*, is classified on the basis of four quadrants to define the color. In case of control H* lied in the second quadrant (90º to 180 º) indicating greenish hue (characteristic of whey due to the presence of riboflavin) while for EPS enriched sample it was between 0º to 90º i.e. first quadrant depicting red-yellow region (due to EPS). Additionally, browning index was also estimated which may indicate the prior processing of milk and degree of browning in the same. BI was found to be more for 1% ECY sample compared to control, however, in both the cases the values of BI were not that high. The degree of color change (ΔE) was found to be 9.58 due to the addition of EPS in yoghurt (Table 1).
Particle size analysis
Yoghurt is a gel formed by casein particle aggregation which leads to gelation. The nature of final yoghurt gel is decided based on the theory given by Mitchell [33]. This says that gel is made up of chains of polymer segments (particles in case of yoghurt), the more aggregation and cross-linking, the higher the particle size. In these experiments, higher values of particle size i.e. for 1% ECY (3460 d.nm ~ 3.46 μm) as compared to that of control (2354 d.nm ~ 2.35 μm) indicates the formation of bonds and series of polymer-like structure which was formed due to the cross-linking between EPS and milk components that comprises various components (fat, protein, carbohydrate sugars, bacteria etc.). However, in this experiment, the particle size values were very much less as compared to the literature available (previously reported ~ 10–20 μm) which was due to the ultrasonication treatment given to yoghurt which caused collisions and disruption of gel particles.
FTIR analysis
FTIR spectra for both the control and 1% ECY sample are presented in Fig. 3. The almost same pattern of functional groups was recorded in both the yoghurt samples with the main difference related to polysaccharides composition which could demonstrate the presence and nature of EPS observed mainly in the regions near 1043 and 1414 cm− 1 due to strong stretching vibrations in this region Wang et al. [34]. The presence of a peak near 1043 cm− 1 confirmed the presence of monosaccharide characteristics having a pyranose ring which might be glucan EPS or another similar pyranose ring containing EPS and a peak near 1414 cm− 1 which is a characteristic peak of carboxyl group indicating the acidic nature of EPS [35,36,37], suggesting the desirable changes in treated samples is due to the presence of EPS. However, the presence of other bands (additional peaks indicated within a circle) in the treated sample near the region 422 cm− 1, 477 cm− 1, and 619 cm− 1 could be correlated with the complexity of food constituents [38], which might have produced some by-products by reacting with EPS or some other interfering components, other than native milk lactose, proteins, and fat. As, milk lactose, proteins, and fat indicate the peak near 1039 cm− 1, 1546 cm− 1, and 2873, 1747 cm− 1 due to hydroxyl (OH), amide group (CONH), and carbon-hydrogen (C-H), carbonyl (C-O) groups, respectively [39].
Microstructural analysis of yoghurt
The mechanism of interactions at the microstructural level was analyzed by using SEM and CLSM. The CLSM analysis also was done along with SEM for further visualization of a fully hydrated sample as SEM alone is not suitable for observing highly hydrated samples. Also, the visualization of a completely hydrated sample can also avoid the artifacts related to conventional SEM as reported by Hassan et al. [40]. However, in our case, both SEM, as well as CLSM micrographs, revealed almost similar morphology and rearrangements. As shown in Fig. 4a, b, the control samples the proteins are more randomly arranged while in the 1% ECY sample they became more structured as EPS distributed the protein matrix more uniformly. Yoghurt sample enriched with EPS had larger spaces and pockets between the protein matrix, probably these larger pores made the protein matrix tightly packed (thick and dense) leading to more ordered structure in case of 1% ECY sample. On the other hand, in case of control yoghurt sample protein strands and small pores were observed which may not be able to hold enough amount of serum. This is in agreement with early reports, Folkenberg et al. [41], and Puvanenthiran et al. [42] indicated that EPS scatters in the pores effectively by surrounding the protein matrix and increases the density of protein matrix by aggregation. Kristo et al. [43] also recorded exactly similar confocal micrographs in the presence of EPS having very large voids in the microstructure thereby forming dense protein strands. The microstructural visualization in our case also showed that in the 1% ECY sample the density of protein matrix increased due to increased porousness, which thereby led to an increase in the compactness of proteins. This compactness of proteins might also be the reason for a change in physical characteristics like decreased syneresis and increased WHC [44]. Since the denser network has formed due to higher aggregation consequently immobilized a significant amount of free water. Recently, Wu et al. [45] revealed a very interesting comparison between the porous and non-porous structures of yoghurt. Porous structure helps strengthen of gel network as it increases its capacity to hold excess of water while non-porous structure had lower WHC. Secondly, in the case of dairy-based products like yoghurt and related products, whey proteins are also present which can act with EPS synergistically to enhance water binding ability by increasing the density of protein matrix and imbibing more water in the gel system [46].
(a): SEM images of control and 1% EPS containing yoghurt (1% ECY) at different magnifications (1000 and 2000 X). (b): CLSM images of control and 1% ECY showing (more scattered protein particles are present in the control sample while in case of 1% ECY more cross-linked polymer-like dense protein strands are formed)
Rheological properties of yoghurt
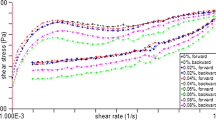
Apparent viscosity
The apparent viscosity (η) was recorded at a shear rate (\(\dot{\gamma }\)) of 50 s− 1, which is comparable with those shear rates occurring in the mouth [47]. The viscosity of 1% ECY was significantly higher as compared to control presented in Table 2 (for comparison purpose, indicated at shear rate 50 s− 1) and Fig. 5(a). The possible reason could be the binding of serum present in yoghurt by the EPS that led to an increase in serum phase viscosity and followed by increased product viscosity. Moreover, in our case higher EPS concentrations viz. 1% level occupied more space hence casein micelles and other milk constituents probably were compelled to interact with each other leading to the formation of more cross-linked compact strands which thereby increased the stiffness and viscosity of yoghurt gel, this hypothesis could be supported by the SEM and CLSM micrographs. Stiffer gels could be derived from the cross-linking of the EPS molecules with whey proteins and casein particles within the gel network which resulted in increased viscosity. Costa et al. [48] speculated that the presence of EPS in the serum phase or on the surface of protein particles can be the driving force responsible for yoghurt properties. They also reported that the presence of EPS may influence the protein aggregates formation, larger the protein aggregates more the ability to retain water which thereby increases the solvation characteristics of the protein matrix [48]. Hence, glucan EPS can influence the formation of a protein network and ultimately lead to the formation of larger pores with more rearrangements, in regard to possible formation of large and thicker protein aggregates. This influences viscosity, mouthfeel, and creaminess of the product positively. Therefore, higher viscosity of 1% ECY can be correlated with smooth and creamy mouthfeel which in turn also led to an increase in texture and mouthfeel for sensory scores.
Viscoelastic properties
Viscoelastic parameters including G’, G” and η* of both control and 1% ECY samples are represented in Table 2 (expressed at 10 rad/s for comparison) and Fig. 5(b). G’ indicates the elastic or solid-like behaviour and it measures the deformation energy stored in the sample during the shear process [49] while G” represents the viscous behaviour of the product. As indicated in Table 2, the G’ values were significantly higher for 1% ECY than that of the control indicating high elastic behaviour or more firm, compact, and solid-like properties of EPS treated sample. The results of the present study are not in agreement with Doleyres et al. [50] who reported lower G’ values for EPS-treated yoghurt. However, the higher values of G’ reported by Prasanna et al. [7] again indicate the interactions between the EPS and the proteins of the gel matrix indicating higher viscosity. Predominantly, two explanations can be anticipated for such interactions, firstly, in their native state milk proteins possess a net negative charge, however below the isoelectric point (for casein ~ 4.6) and (for whey proteins ~ 5.2) they acquire a net positive charge, thus the pH of yoghurt is obvious below than the isoelectric point of milk proteins. Hence, positively charged milk proteins and slightly anionic EPS led to the formation of a continuous gel network, which in turn resulted in high viscoelastic moduli and viscosity [24]. The second justification stands in the prior heat treatment given to milk which induces denaturation in whey proteins, denatured whey proteins interact with these colloidal particles which can form large aggregates via electrostatic interactions, and acts as a bridging link in the protein network leading to higher G’ and G” values.
Also, for polymeric systems, at higher shear rates the apparent or steady-state viscosity is considered as less reliable so complex viscosity or dynamic viscosities are calculated. The complex viscosity of the 1% ECY sample was significantly higher than the control sample. Hence, the addition of EPS has a positive impact on the rearrangement of gel formation by interacting with milk proteins which resulted in higher product viscosity.
Firmness
EPS addition to yoghurt significantly affected the texture of yoghurt, the firmness values are presented in Table 2. The formation of a dense network between EPS and casein micelles might be the possible reason that led to an increase in the strength of coagulum and subsequently firmness [44]. Contrarily, some authors revealed that EPS-enriched yoghurt had lower firmness values than the control [46, 47]. However, on the opposite side, similar to our results it has been reported that firmness values were higher for EPS enriched samples as compared to control [7, 51]. Therefore, the dynamics of changes occurring between gel and proteins is a highly complex phenomenon, it is still not clear how EPS interacts with milk protein and modifies texture. The possible mechanism advocated for increased firmness is the presence of net negative charge and the high molecular weight of EPS which induces strong casein-casein interactions [52]. To some extent changes occurring in colloidal calcium phosphate, whey proteins and other milk components are also responsible for protein bridge formation [53]. This may be the negative charge and high molecular weight with a varying chain length of glucan EPS could be the possible mechanism responsible for increased firmness. Therefore, improving the texture as firmness using glucan EPS could be important for enhancing the texture of yogurt and related products.
Antioxidant assays
The presence of EPS in yoghurt sample increased the antioxidant activities performed by ABTS radical scavenging and reducing power assay as compared to the control yoghurt (Table 2). EPS has also been previously reported to show antioxidant activity in a dose dependent manner [8]. Both the ABTS and reducing power assay are based upon single electron transfer mechanism and allows the determination of hydrophilic antioxidants efficiently. But the values for both the parameters found to be different. This could be because two antioxidant conditions were measured under different reaction conditions, particularly in terms of pH value (pH for reducing power assay being 3.6, while that for ABTS being 7.4). The presence of carboxyl group of this EPS also has caused the electrons withdrawal and ultimately increasing the antioxidant activity. To some extent milk proteins also contributes to significant amount of antioxidant activity and this perhaps could be the reason that plain yoghurt sample also has some of the radical scavenging potential. Perhaps, the changes in molecular characteristics and interaction of EPS with other milk components during fermentation had also led to higher antioxidant activity. Moreover, it may be concluded that during fermentation process interaction of EPS with milk proteins can also influence the charge, molecular characteristics and hydrophobicity of protein leading to higher radical scavenging activity in case of EPS treated sample.
Microbial analysis of yoghurt
Yoghurt bacteria are well known for maintaining the healthy gut function. The bacterial population present in control was ~ 8.0 log CFU/g, whereas in the 1% ECY it was found to be ~ 8.3 log CFU/g. This indicates that presence of EPS has supported the growth of yoghurt starters. The slight increase in acidity for 1% ECY can also be correlated with the increase in microbial count. Moreover, recent reports on prebiotic assay done for galactan EPS has indicated appreciable amounts of short-chain fatty acids production which makes it a good candidate as prebiotic food source [54]. Good prebiotic potential of glucan type EPS added (at 1%) in probiotic milk pudding was observed by İspirli et al. [28], along with improved technologically important aspects (syneresis, texture etc.) of the product.
Conclusion
This work shows that adding 1% of ex-situ EPS to yoghurt can improve its technological and biological qualities to a great extent without affecting its overall or sensory qualities. The water-binding ability of yoghurt with glucan EPS was found to be greatly enhanced, indicating the potential of this natural hydrocolloid for usage in both dairy and non-dairy products. Yoghurt with glucan EPS had good firmness, viscosity, and mouthfeel because to its high water retention capacity, which also improved its microstructural qualities. Analysis of the yogurt’s microbes and antioxidants showed that it could enhance its biological performance. Because of its technical and health-promoting qualities, glucan EPS therefore has potential for use in a variety of food applications to develop the functional foods.
References
S.S. Senadeera, P.H.P. Prasanna, N.W.I.A. Jayawardana, D.C.S. Gunasekara, P. Senadeera, A. Chandrasekara, Heliyon. (2018) https://doi.org/10.1016/j.heliyon.2018.e00955
P.H. Pradeep Prasanna, D. Charalampopoulos, Int. J. Dairy. Technol. 132–141 (2019). https://doi.org/10.1111/1471-0307.12568
A. Patel, J.B. Prajapati, Adv. Dairy. Res. (2013). https://doi.org/10.4172/2329-888X.1000107
T. Yang, K. Wu, F. Wang, X. Liang, Q. Liu, G. Li, Q. Li, Int. Dairy. J. (2014). https://doi.org/10.1016/j.idairyj.2013.08.007
M.B. Güler-Akin, M. Serdar Akin, A. Korkmaz, Int. J. Dairy. Technol. (2009). https://doi.org/10.1111/j.1471-0307.2009.00499.x
X. Han, Z. Yang, X. Jing, P. Yu, Y. Zhang, H. Yi, L Zhang Biomed. Res. Int. (2016). https://doi.org/10.1155/2016/7945675
P.H.P. Prasanna, A.S. Grandison, D. Charalampopoulos, Food Res. Int. (2013). https://doi.org/10.1016/j.foodres.2012.11.016
B.R. Ghalem, Am. J. Chem. Biochem. Eng. (2017) http://www.sciencepublishinggroup.com/j/ajcbe
P.B. Devi, D. Kavitake, P.H. Shetty, Int. J. Biol. Macromol. (2016). https://doi.org/10.1016/j.ijbiomac.2016.09.054
P. Ruas-Madiedo, J. Hugenholtz, P. Zoon, Int. Dairy. j. 12(2–3), 163–171 (2002). https://doi.org/10.1016/S0958-6946(01)00160-1
D. Kavitake, S. Tiwari, P.B. Devi, I.A. Shah, G.B. Reddy, P.H. Shetty, Int. J. Biol. Macromol. (2024). https://doi.org/10.1016/j.ijbiomac.2023.129105
C. Wacher-Rodarte, M.V. Galvan, A. Farres, F. Gallardo, V.M. Marshall, M. Garcia-Garibay, J. Dairy. Res. (1993). https://doi.org/10.1017/S0022029900027564
L.K. Hassan, H.F. Haggag, M.H. ElKalyoubi, M.A. El-Aziz, M. El-Sayed, A.F. Sayed, Ann. Agric. Sci. (2015). https://doi.org/10.1016/j.aoas.2014.11.021
D. Mudgil, S. Barak, B S Khatkar Food Biosci. (2016). https://doi.org/10.1016/j.fbio.2016.02.003
T. Amatayakul, F. Sherkat, N.P. Shah, Int. J. Dairy. Technol. (2006a). https://doi.org/10.1111/j.1471-0307.2006.00264.x
I.O. Korkmaz, C. Bilici, S. Korkmaz, Int. J. Gastro Food Sci. 23, 100291 (2021). https://doi.org/10.1016/j.ijgfs.2020.100291
AOAC, Official Methods of Analysis of AOAC International, 16th edP (Cuniff, vol. II (Maryland, USA, 1999)
M. Vargas, M. Cháfer, A. Albors, A. Chiralt, C. González-Martínez, Int. Dairy. J. (2008). https://doi.org/10.1016/j.idairyj.2008.06.007
E. Kristo, Z. Miao, M. Corredig, Int. Dairy. J. (2011). https://doi.org/10.1016/j.idairyj.2011.02.002
P.V. Behare, R. Singh, R. Nagpal, K.H. Rao, J. Food Sci. Technol. (2013). https://doi.org/10.1007/s13197-013-0999-6
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans. Free Radic Biol. Med. (1999)
A. Benslama, A. Harrar, Int. J. Herb. Med. 4(6), 158–161 (2016)
J.A. Lucey, Int. J. Dairy. Technol. (2004). https://doi.org/10.1111/j.1471-0307.2004.00142.x
J.A. Teggatz, H.A. Morris, Food Struc. 9(2), 9 (1999)
P. Buldo, C. Benfeldt, D.M. Folkenberg, H.B. Jensen, J.M. Amigo, S. Sieuwerts, R. Ipsen, LWT-Food Sci. Technol. (2016). https://doi.org/10.1016/j.lwt.2016.04.050
H. Abbasi, M.E. Mousavi, M.R. Ehsani, Z.E. D-Jomea, M. Vaziri, J. Rahimi, S. Aziznia, Int. J. Dairy. Technol. (2009). https://doi.org/10.1111/j.1471-0307.2009.00513.x
E. Amani, M.H. Eskandari, S. Shekarforoush, Food Sci. Nutr. (2017). https://doi.org/10.1002/fsn3.427
H. İspirli, F. Demirbaş, E. Dertli, J. Food Sci. Technol. (2018). https://doi.org/10.1007/s13197-018-3181-3
H. Du, X. Wang, H. Yang, F. Zhu, J. Cheng, X. Peng, X. Liu, Food Control. 109900 (2023). https://doi.org/10.1016/j.foodcont.2023.109900
L. Pan, Q. Wang, L. Qu, L. Liang, Y. Han, X. Wang, Z. Zhou, J. Dairy. Sci. (2022). https://doi.org/10.3168/jds.2021-20997
L. Ramchandran, N.P. Shah, LWT-Food Sci. Technol. (2010). https://doi.org/10.1016/j.lwt.2010.01.012
F. Yazici, A. Akgun, J. Food Eng. (2004). https://doi.org/10.1016/S0260-8774(03)00237-1
J.R. Mitchell, J. Text, Studies, (1980) https://doi.org/10.1111/j.1745-4603.1980.tb01312x
Y. Wang, Z. Ahmed, W. Feng, C. Li, S. Song, Int. J. Biol. Macromol. (2008). https://doi.org/10.1016/j.ijbiomac.2008.06.011
Z. Chen, J. Shi, X. Yang, B. Nan, Y. Liu, Z. Wang, Int. Dairy. J. (2015). https://doi.org/10.1016/j.idairyj.2014.10.004
W. Li, X. Xia, W. Tang, J. Ji, X. Rui, X. Chen, M. Dong, J. Agri Food Chem. (2015). https://doi.org/10.1021/acs.jafc.5b01086
D. Kavitake, P.B. Devi, S.P. Singh, P.H. Shetty, Int. J. Biol. Macromol. (2016). https://doi.org/10.1016/j.ijbiomac.2016.01.099
A. Kennas, H. Amellal-Chibane, F. Kessal, F. Halladj, J. Saudi Soc. Agric. Sci. (2020). https://doi.org/10.1016/j.jssas.2018.07.001
F.A. Iñón, S. Garrigues, M. de la Guardia, Anal. Chim. Acta. (2004). https://doi.org/10.1016/j.aca.2004.03.014
A.N. Hassan, J.F. Frank, K.B. Qvist, J. Dairy. Sci. (2002). https://doi.org/10.3168/jds.S0022-0302(02)74243-4
D.M. Folkenberg, P. Dejmek, A. Skriver, R. Ipsen, J. Texture Stud. (2005). https://doi.org/10.1111/j.1745-4603.2005.00010.x
A. Puvanenthiran, R.P.W. Williams, M.A. Augustin, Int. Dairy. J. (2002). https://doi.org/10.1016/S0958-6946(02)00033-X
E. Kristo, Z. Miao, M. Corredig, The role of exopolysaccharide produced by Lactococcus lactis subsp. cremoris in structure formation and recovery of acid milk gels. Int. Dairy J. (2011). https://doi.org/10.1016/j.idairyj.2011.02.002
V.R. Harwalkar, M. Kalab, F. Microstruc, (1986) https://digitalcommons.usu.edu/foodmicrostructure/vol5/iss2/13
J. Wu, J. Cheng, B. Adhikari, F. Xue, J. Future Foods. (2023). https://doi.org/10.1016/j.fufo.2023.100242
T. Amatayakul, A.L. Halmos, F. Sherkat, N.P. Shah, Int. Dairy. J. (2006b). https://doi.org/10.1016/j.idairyj.2005.01.004
A. Skriver, J. Holstborg, K.B. Qvist, Relation between sensory texture analysis and rheological properties of stirred yogurt. J. Dairy. Res. (1999). https://doi.org/10.1017/S0022029999003763
N.E. Costa, D.J. O’Callaghan, M.J. Mateo, V. Chaurin, M. Castillo, J.A. Hannon, T.P. Beresford, Int. Dairy. J. (2012). https://doi.org/10.1016/j.idairyj.2011.08.004
A.G.D. Cruz, R.N. Cavalcanti, L.M.R. Guerreiro, A.D.S. Sant’Ana, L.C. Nogueira, C.A.F.D. Oliveira, H.M.A. Bolini, J. Food Eng. (2013). https://doi.org/10.1016/j.jfoodeng.2012.08.018
Y. Doleyres, L. Schaub, C. Lacroix, J. Dairy. Sci. (2005). https://doi.org/10.3168/jds.S0022-0302(05)73100-3
D. Jaros, H. Rohm, A. Haque, C. Bonaparte, W. Kneifel, Vol. 57, No. 6, 325–328, (2002)
S. Mende, M. Peter, K. Bartels, H. Rohm, D. Jaros, Food Hydrocoll. (2013). https://doi.org/10.1016/j.foodhyd.2012.12.011
M. Alexander, D.G. Dalgleish, Colloids Surf. B. (2004) https://doi.org/10.1016/j.colsurfb.2004.08.012
P.B. Devi, D. Kavitake, J. Jayamanohar, P.H. Shetty, Food Res. Int. 143, 110333 (2021). https://doi.org/10.1016/j.foodres.2021.110333
Acknowledgements
Authors Swati Tiwari, Digambar Kavitake and Palanisamy Bruntha Devi are grateful to ICMR-Senior Research Fellowship (Ref: No.3/1/2/287/2021-Nut.), SERB - National Post-Doctoral Fellowship (SERB-NPDF) (PDF/2021/000551) and DST-WISE KIRAN project grant (DST/WOS-A/LS -259/2019 (G) for financial assistance. We acknowledge Ministry of Food Processing Industries (MoFPI), India (Project ID: Q-11/5/2020-R&D). We also acknowledge Pondicherry University for providing infrastructural facilities. The technical assistance for CLSM given by IMTECH, Chandigarh, FTIR facility by IIT-Madras and rheological measurement facilities by NDRI, Karnal, India are deeply acknowledged.
Author information
Authors and Affiliations
Contributions
Swati Tiwari: Experiment, writing, original draft, review and editing. Digambar Kavitake: Conceptualization, experimentation, writing, review and editing. Palanisamy Bruntha Devi: Writing, review and editing. Bhavesh Baria: Experiment, editing. Kritika Agarwal: Experiment, editing. Ramasamy Ravi: Writing, review and editing. Ashish Kumar Singh: Writing, review and editing. Prathapkumar Halady Shetty: Conceptualization, design of the study, review, editing and supervision.
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiwari, S., Kavitake, D., Devi, P.B. et al. Functional enhancement of yoghurt through incorporation of glucan exopolysaccharide from Enterococcus hirae OL616073 of food origin. Food Measure 18, 5462–5476 (2024). https://doi.org/10.1007/s11694-024-02580-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02580-0