Abstract
Tannins are the molecules that are responsible for the astringency perceptions of wines. However, the mechanisms by which tannins influence the astringency perceptions of wines remain unclear. In the current study, the grape varieties had greater effects on the tannin profiles than did the aging periods. The percentage of galloylated flavanols (%G) was positively correlated with drying astringency and negatively correlated with velvety astringency. The mean degree of polymerization (mDP) of tannins had a positive correlation with drying astringency. In comparison to monomeric flavan-3-ols, the flavan-3-ol subunits of tannins are the most important determinants of astringency perceptions. Furthermore, findings justified the inconsistencies on the correlation between the tannins profiles and the astringency reported of previous studies. The current findings enhance the understanding of the relationship between the tannin profiles and the astringency perceptions of wines.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The condensed tannins, which are also known as the proanthocyanidins, are polyphenolic compounds that are widely found in the fruits, barks, leaves, and seeds of plants. They are the secondary metabolites that protect plants from the biotic and the abiotic stresses, thus, play an important role in the plant adaptations to the environments [1,2,3]. During winemaking processes, the maceration process extracts the condensed tannins from the grapes into the wine [4]. The flavan-3-ols, which encompass ( +)-catechin (C); ( −)-epicatechin (EC); ( +)-gallocatechin (GC); ( −)-epigallocatechin (EGC); ( −)-epicatechin gallate (ECG); and ( −)-epigallocatechin gallate (EGCG), are the important monomers of the condensed tannins.
The tannin concentrations and composition in wines are initially influenced by the grape varieties [5] and subsequently modified during wine aging [6]. The tannin concentration and composition are the most important factors to winemakers: they determine the colors and the astringencies of wines. Bindon et al. [7] found that the grape tannin composition significantly influences the formation of polymeric pigments in model wine. Moreover, these authors reported that the astringency of red wine is affected by several factors such as the tannin structure and concentration. According to Quijada-Morín et al. [8], the subunit compositions of wine tannins have a greater effect on the astringency than do the total concentration and the mean degree of polymerization (mDP) of the tannins. However, Kyraleou et al. [9] suggested that tannin concentration has a higher correlation with astringency than does the tannin structural compositions. The percentage of galloylated flavanols (%G) is another factor that regulates astringency perception; nevertheless, the contribution of the %G towards astringency is argued. Several studies reported the positive correlation between the %G and the astringency [10, 11]; however, several studies indicated the absence of correlation [9, 12] or even the negative one between %G and astringency [13]. On the basis of previous studies' findings, the wine astringency is influenced by the concentration, the mDP, and the subunit compositions of the wine tannins. However, the relationships between the concentrations and the compositions of grape proanthocyanidins and the sensory properties of grapes or wines that were reported in previous studies are limited to particular grape varieties [14] or particular regions [10] from which the grapes were obtained. Moreover, some of the previous studies derived the relationships between the concentrations and the compositions of grape proanthocyanidins and the sensory properties of grapes or wines from the constructed models [11, 12]; the experimental results derived from wines are required to validate the results obtained by the constructed models. In addition, a previous study focusing on grape variety effect on tannin profiles and astringency was reported [15]. At present, relatively little is still known about the variety effect on astringency perceptions of wines.
The current study aims to provide comprehensive and systematic evaluations on the effects of grape varieties and the aging periods on the tannin profiles and the astringencies of wines. The two main objectives were: (1) to evaluate the tannin profiles of the wines that were produced from three grape varieties and were subjected to three aging periods and (2) to investigate the effects of the tannin profiles on the astringency perceptions of the wines. The findings of this study improve current understanding on the relationships between the tannin profiles and the astringencies of wines.
Materials and methods
Chemicals and reagents
Acetonitrile and methanol were obtained from Tedia (USA). Phloroglucinol, trifluoroacetic acid (99%, spectroscopy grade), acetic acid, l ( +)-ascorbic acid, EC, C, and ECG were purchased from Sigma-Aldrich (USA).
Wine samples
Red wines of Merlot (ML), Cabernet Gernischt (CG), and Pinot Noir (PN) grape varieties were selected in this work. The wines were produced in three continuous vintages (2015, 2016, and 2017) in Wuwei City, Gansu Province, China (Table 1). All the wines were produced at commercial scale in Mogao International Winery (36°11 N, 103°69 E). Similar production processes were used for each vintage and variety. About 150 kg grapes were destemmed, crushed, and treated with 60 mg/L SO2 addition, the must was transferred into 200 L stainless steel tanks, added with 20 mg/L pectinase (BXL, Gestown, China), and then macerated at room temperature for 8 h. After that, grape must was inoculated with 250 mg/L activated commercial yeast (BV818, Angel, China), and then fermented with the skins and seeds. When alcoholic fermentation was completed (about 2 g/L reducing sugar), the wine was treated with 60 mg/L SO2 and then macerated at 25 °C for 1 week. After that, separated red wine from pomace using a plate-and-frame filter press, and stored at 12 °C for 3 months. The wine was clarified using 1.0 g/L bentonite (NC, Erbsloeh) before bottling, and then were transported to Northwest A&F University kept in the original bottles (750 mL, Amber, Cork) and stored in a wine cellar under 15 ± 2 °C and 65 ± 5% RH.
Each red wine of each grape variety consisted of 3 treatments (1-, 2- and 3-year-aged, respectively). Each treatment consisted of 6 bottles with a total of 54 bottles in this study. Three bottles of each treatment were used for chemical measurements, and the other three bottles were used to sensory analysis. One bottle of wine was considered as witness. After the aging for 1, 2, or 3 years, wines were used for chemical measurements and sensory analysis at room temperature (20 ± 2 ℃). For different chemical measurements, 3 bottles of each treatment were opened, and were immediately divided into 50 mL centrifuge tubes and stored in a refrigerator at − 80 ℃.
Spectrophotometric analyses
Color parameters of wines was analyzed according to the procedures described by Chira et al. [14]. Briefly, wine samples were placed in 2-mm-path-length glass cell to be scanned in transmission mode (380–700 nm) under 1 mm optical way using a Shimadzu UV-2450 spectrophotometer (Shimadzu Corporation, Japan). Spectra data were converted to the various CIE coordinates using 10° Standard Observer and Standard Illuminant D65. The distilled water was used as reference. The total phenolic content and the total anthocyanin content were quantified according to the protocols described by Perez-Magarino and Jose [16]. The total phenolic content was measured by Folin–Ciocalteu reagent, and the total anthocyanin content was quantified according to the color variation as a function of pH. The total phenolic content was expressed as gallic acid equivalent, and the total anthocyanin content was expressed as mg/L of malvidin-3-glucoside. The spectrophotometric analyses were performed in triplicate.
Ultra-performance liquid chromatography (UPLC) analyses of tannins
Instruments and mobile phases
The UPLC analyses were conducted by an ACQUITY UPLC I-Class system (Waters Corporation, USA). The column was an ACQUITY UPLC BEH HILIC C18 (2.1 mm × 50 mm), and the phases consisted of phase A (2% (v/v) aqueous phosphoric acid solution) and phase B (20% (v/v) of phase A in acetonitrile); the flow rate was 0.20 mL/min. The mobile phase gradients were adjusted to 10% B at 0–6 min, 10–15% B at 6–9 min, 15–20% B at 9 − 12 min, 20–30% B at 12–13 min, and 30–10% B at 13–15 min. The eluted peaks were detected at 280 nm by a diode array detector (PDA eλ).
Determination of monomeric flavan-3-ol concentrations
In order to determine the concentrations of monomeric flavan-3-ols, 10 mL of each wine sample were added into a 50 mL centrifuge tube and then, 10 mL of ethyl acetate were also added into the wine. The mixture was subjected to vortex mixing for 2 min at 3500 r/min, and the organic phase was carefully transferred into a 50 mL round-bottom flask by a needle; the latter process was performed in triplicate. The organic phases were combined, and the ethyl acetate was evaporated by a rotary evaporator under vacuum at 35 ℃. The residue was dissolved in 2 mL of methanol and filtered through a 0.22 µm PFTE membrane. The methanolic samples were stored at − 80 ℃ until further analyses.
Determinations of the subunit compositions and the mDP of condensed tannins
The condensed tannins were extracted according to the report of Yacco et al. [17]. Briefly, 50 mL of each wine sample was injected into a chromatography column that was packed with Toyopearl chromatography resin (HW-40C, 30 mm, 250 mm). The column was pre-equilibrated with 0.05% (v/v) trifluoroacetic acid (TFA) aqueous solution. The injected wine was firstly eluted with 300 mL of 0.05% (v/v) TFA aqueous solution to remove sugars and organic acids and then was eluted with 300 mL of the 1:1 mixture of methanol and 0.05% (v/v) TFA aqueous solution to remove anthocyanins and low molecular weight phenolics. Finally, the injected wine was eluted with 150 mL of the 2:1 mixture of acetone and 0.05% (v/v) TFA aqueous solution to elute the condensed tannin fraction. The tannin fraction was concentrated under reduced pressure at 38 ℃ to remove the acetone and was lyophilized to obtain dry tannin extract. After that, the dry weight of tannin was measured, and then was stored at − 80 ℃ until further analyses.
The condensed tannins that were extracted from the wines were characterized by the acid-catalyzed cleavage of the tannins in the presence of excess phloroglucinol; the characterizations followed the procedures described by Kennedy and Jones [18] with minor modifications. Briefly, each of the dry tannin extracts of the wines was dissolved in methanol and was added with phloroglucinol reagent (a solution of 0.1 N hydrochloric acid in methanol containing 50 g/L phloroglucinol and 10 g/L ascorbic acid) at 1:1 (v/v) ratio. The mixture was maintained at 50 °C for 20 min. The reaction was terminated by adding 40 mM cold aqueous sodium acetate solution into the mixture at 5:1 (v/v) ratio. The solution was injected into the UPLC system, and the eluted peaks were detected by a diode array detector (PDA eλ) at 280 nm. A chromatogram that illustrates the peaks of monomeric flavan-3-ols is shown in Supplementary Fig. 1. In order to calculate the percentages of terminal and extension subunits, the calibration curves of the flavan-3-ols-the C, EC, ECG, EGC, GC and EGCG were prepared; the reference compounds (i.e., the standard compounds) of the flavan-3-ols were used to construct the standard curves (Supplementary Table 1). The mDP of the condensed tannins was calculated on the basis of the ratio between the sum of all constitutive units (in mole) and the sum of the terminal units (in mole). The determination of the composition and the mDP of condensed tannins were performed in triplicate.
Sensory analyses
The sensory analyses of ML, CG, and PN wines were accomplished by a sensory panel following the processes of the sensory analyses conducted by Ristic et al. [19]. Briefly, eleven professionally-trained panelists (nine females and two males) from the College of Enology, Northwest A & F University, were appointed for the sensory analyses. To ensure that all panelists exercised similar criteria in evaluating the sensory properties of the wines, we equipped the panelists with a sensory evaluation training; the solutions of 3 g/L commercial tannins, 15 mg/L quinine sulfate, 15 g/L sucrose, and 3 g/L tartaric acid were selected to train the panelists for distinguishing astringent, bitter, sweet, and sour tastes, respectively. The sensory analyses of the ML, the CG, and the PN wines were performed in a standard wine sensory laboratory. The laboratory was equipped with a separate compartment at ambient temperature (20 ± 2 °C) that was free of noise or other interferences. Each panelist evaluated 30 mL of each wine sample. The panelist covered his or her tongue with the wine and gently stirred the wine by the tongue. After 3–5 s, the panelist took a breath, held the breath for 3 s, and expectorated the wine. Upon completing the sensory analysis of one wine sample, the panelists were allowed to rinse their mouths twice with deionized water, to chew soda crackers to recover their taste buds, and to wait for at least 30 s before evaluating the subsequent sample. Each panelist completed the sensory evaluation of each wine sample within 3 min. When the panelists evaluated the astringencies of the wine samples, the astringencies were qualitatively expressed by descriptors such as puckery, coarse or emery, grainy, dryness, silky, and velvety (Table 2). Subsequently, the intensities of the drying astringency descriptors (puckery, coarse, grainy, and dryness) and the intensities of the velvety astringency descriptors (silky and velvety) were evaluated by 5-point scales (1: no perception, 2: slight perception, 3: moderate perception, 4: strong perception, 5: very strong perception). Then the two astringency descriptors were selected, and the intensity of drying astringency or the intensity of velvety astringency, Modified frequency (MF %), a mixture of intensity and frequency of detection, was calculated to grade the intensity of astringency with the formula:
F (%) denoted the frequency of the astringency descriptor, and I (%) denoted the average intensity of each astringency descriptor. The average intensity of each astringency descriptor was calculated on the basis of the intensities of the descriptor reported by all panelists.
Statistical analysis
Statistical analyses were conducted by SPSS Statistics 20.0 (IBM, the USA). The significant differences in the results were evaluated by by ANOVA and Tukey’s HSD test; the significant differences in the results were indicated by p < 0.05. Figures were prepared by Origin Pro 9.1 (OriginLab Corporation, the USA). The principal component analysis (PCA) with cross validation was performed by Unscrambler X10.3 (CAMO Software, Norway).
Results and discussion
The effects of the grape varieties and the aging periods on the phenolic compositions and the color parameters of the wines.
Phenolic composition and color parameters of wines are shown in Table 3. The total phenolic contents of ML and CG wines after 2-year aging (ML2 and CG2 wines; 1643.15 and 1606.22 mg/L, respectively) were 209.5 and 303.6 mg/L, 388.6 and 278.3 mg/L higher than in the two other aging periods, while the total phenolic contents of PN wines were no significant differences between values of total phenolic contents nevertheless the aging period. Among ML, CG, and PN wines, the ML, the CG, and the PN wines that were 2-year-aged had the highest total tannin contents (38.1 mg/L) and it was 1.5 and 13.5 mg/L higher than in the two other aging periods. Thus, the grape varieties greatly affect the phenolic compositions of the wines, which is in agreement with the previous reports of Heras-Roger et al. [20] and Leeuw et al. [21]. Although the phenolic compositions could be influenced by the the climate, and the harvest year, it was hypothesized that the grape varieties had stronger influences on the phenolic compositions of the wines than did the aforementioned factors.
Overall, the longer is the aging period, the lower are the total anthocyanin contents and the higher are the color intensity of the wines; the anthocyanins are decomposed during the wine aging [22]. The reduction in total anthocyanin contents were observed to be followed by the formation of polymeric pigments that increased hues and color intensity of the wines during aging [6]. In addition, the anthocyanins might directly or indirectly condense with flavanols pinotins, portisins, flavanyl-pyranoanthocyanins, or the other polymeric pigments and formed stable color pigments [23,24,25]. Although a series of more stable anthocyanins were formed during wine aging and these pigments probably were less likely to accumulate [26].
Effect of grape varieties and aging periods of wines on the latter's concentrations in monomeric flavan-3-ols.
Effect of grape varieties and aging periods of wines on the latter's concentrations in monomeric flavan-3-ols are illustrated in Fig. 1C, EC and EGC are the major monomeric flavan-3-ols of ML, CG, and PN wines. All wines had low concentrations of GC, ECG, and EGCG. The concentrations of the monomeric flavan-3-ols in PN wines decreased significantly over time. In fact, the concentration of EGCG in the PN wine after 3-year aging (PN3 wine) is below the detection limit of the UPLC system; similar finding was reported by Gambuti et al. [27] and Ćurko et al. [6].
As opposed to the changes in the concentrations of the monomeric flavan-3-ols in PN wines, the concentrations of the monomeric flavan-3-ols in ML and CG wines are the highest after 2-year aging (ML2 and CG2 wines; 278.5 mg/L and 200.2 mg/L, respectively) but the lowest after 3-year aging (ML3 and CG3 wines; 195.3 and 142.1 mg/L, respectively). During wine aging, condensed tannins undergo spontaneous cleavage and formation of interflavan bonds [28], polymerize with the other condensed tannins, and promote the condensations between anthocyanins and flavanols. The lower monomeric flavan-3-ol concentrations of ML3 and CG3 wines than those of ML2 and CG2 wines may be attributed to the plausible participations of the monomeric flavan-3-ols in ML3 and CG3 wines in polymerization and condensation reactions. On the other hand, the higher monomeric flavan-3-ol concentrations of ML2 and CG2 wines than the monomeric flavan-3-ol concentrations of ML1 and CG1 wines and the monomeric flavan-3-ol concentrations of ML3 and CG3 wines can be ascribed to the higher interflavan-bond cleavage rates of the condensed tannins or the higher initial concentrations of the monomeric flavan-3-ols in the ML2 and the CG2 wines.
Effect of grape varieties and wine aging periods on the latter's tannin profile
During wine aging, condensed tannins undergo structural rearrangements, namely the interflavan bond cleavage and formation, and precipitation reactions; the structural changes of the tannins are reflected by the changes in the mDP of the tannins. The early stages of wine aging are characterized by the intensive polymerizations, thus, the increases in the mDP of the tannins [6, 29]. However, the mDPs of condensed tannins generally decrease after long-term aging [14]. As shown in Table 4, the mDPs of the wines after 1-year aging (ML1, CG1, and PN1 wines; 2.81, 2.94 and 2.02, respectively) are significantly higher than those of the wines after 3-year aging (ML3, CG3, and PN3 wines; 2.58, 3.05 and 1.76, respectively) but significantly lower than those of the wines after 2-year aging (ML2, CG2, and PN2 wines; 2.78, 3.06 and 2.26, respectively).
Tannins are mainly composed of C, EC, ECG, EGC, GC and EGCG terminal and/or extension subunits. As shown in Table 4, the C is the most abundant terminal subunit of the condensed tannins extracted from the wine samples, while the EC is the most abundant extension subunit of the tannins. This finding is supported by Basalekou et al. [30], who confirmed that EC is the dominant extension unit in the red wine of Mandilari variety. Percentages of EC extension subunits of tannins extracted from young wines (2017 vintages) are 1.5–7.5% higher than those of tannins extracted from aged wines (2015 vintages); the EC extension subunits of the tannins extracted from aged wines were gradually oxidized during aging. According to Mcrae et al. [25], significantly higher eECs of the wines after 1-year aging than those of wines after 3-year aging can be assigned to the gradual oxidation of tannins during aging. During the aging period, the C was always the most abundant terminal subunit and ML, CG and PN were 19.6–22.4%, 18.8–19.5% and 29.2–37.1%, respectively; the EC is the most abundant extension unit and ML, CG and PN were 47.2–50.5%, 50.6–52.3% and 35.1–45.9%, respectively. In addition, the %PC of ML, CG and PN were 83.6–85.6%, 80.7–84.5% and 90.6–93.6%, respectively; %PD of ML, CG and PN were 13.5–13.6%, 15.1–19.1 and 6.4–9.0%, respectively. Although the chemical compositions of wine may affect the changes in tannin structure during the aging of the wine, the effect diminishes after long-term aging [6]. Nonetheless, there is no consistent conclusion on the percentage changes of ECG subunits during wine aging [10, 31]. Mcrae et al. [25] proposed that the lack of consistent conclusion is more heavily influenced by the differences in the structures of the extracted tannins than by the aging periods; thus, the current findings may hypothesize that the grape varieties had stronger influences on the structures of the condensed tannins extracted from ML, CG, and PN wines than did the aging periods. Furthermore, reductions in %G values of the wines during aging were noticed; the %G values of the wines after 3-year aging are 0.30–0.75% lower than of the wines after 2-year aging and 0.18–0.46% lower than of the wines after one-year aging (Table 4). Low %G values of wines after 3week-year aging may be due to the consumptions of the galloylated flavanols (e.g., the EGC) in the wines during the reactions with oxygen [32]. The current hypothesis on the reactions between the galloylated flavanols and oxygen is supported by Mcrae et al. [25] who demonstrated that the tannins in aged wines exhibit similar characteristics to oxidized tannins. Overall, the current findings suggest that grape varieties have greater influences on the tannin profiles of the wine samples than do the aging periods, in spite of the significant changes in the tannin profiles during the aging of the wines.
To further investigate the effects of the grape varieties and the aging periods on the tannins profiles and TA of the wines, PCA was performed (Fig. 2). The score plot (Fig. 2A) reveals wines clustering: PN wines are clustered on the left-hand side of the plot, while GC and ML wines are clustered on the right-hand side of the plot. On the other hand, 3-years-aged wines were located above 2-years-aged and 1-year-aged ones, respectively. These findings indicated that the grape varieties were the primary factors that explained the differences in the physicochemical parameters of the wines; the aging periods of the wines were the secondary factors that explained the differences.
The physicochemical parameters of the wines were analyzed by PCA (Fig. 2B) where the first and second components (PC1 and PC2) explained together 73% of the total variances. mDP, %PD, eEGC, GC, EGCG and eEC were positively correlated with PC1; while, tC, ECG and %PC were negatively correlated with the first principal component. TP and C are negatively correlated with the PC2. Overall, Fig. 2A and B indicated that grape varieties significantly affected most wines' physicochemical parameters of the wines. On the other hand, the aging periods significantly affected the TP, the C, and the tEC.
Effect of grape varieties and wines aging periods on the latter's astringency perceptions
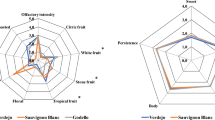
The astringencies of the wines were evaluated by descriptors, such as puckery, coarse or emery, grainy, dryness, silky, and velvety (Fig. 3). As observed, the grape varieties and the aging periods greatly influenced the astringency perceptions of the wines. In general, the wines that were aged for 3 years exhibited palpable velvetiness. The reactions between tannins and anthocyanins are the major phenolic reaction that occur during wine aging; the reactions continuously reduce the astringency of the wine [33]. The wines that were aged for 1 year impose strong puckery and dryness perceptions, which can be explained by the high concentrations of tannins in the young wines. During wine aging, puckery perception gradually softens [34].
Correlations between the astringency perceptions and the concentrations of monomeric flavan-3-ols and between the astringency perceptions and tannin profile.
Pearson’s correlation was employed to analyze the relationships between the astringency perceptions and tannins profiles of the wines (Table 5). As observed, drying astringency showed significant correlations with TT.
A strong positive correlation between drying astringency and total tannin content (TT) was observed (R = 0.77). This is in accordance with previous reports of several studies [9, 35]. However, other researchers found no statistical significant correlation between the astringency and total tannin concentration [8]. Kyraleou et al. [9] proposed that the differences in the determination methods of tannin concentration lead to the differences in the reported findings. The current findings demonstrated that there is neither a significant correlation between drying astringency and total anthocyanin content (TA) nor a significant correlation between velvety astringency and TA; which is in accordance with the findings of Landon et al. [36].
The galloylated flavanols (%G) are the other critical factors that determine astringency. As shown in Table 5, there is no significant correlation between the %G and drying astringency nor between the %G and velvety astringency. Nevertheless, there is no agreement in literature on the correlation between the %G and the astringency. The studies conducted by Chira et al. [10] and Ćurko et al. [11] reported a positive correlation between the %G and the astringency. However, the study of Chira et al. [13] showed a negative correlation between %G values and the astringencies of grape seed extracts. Classifying astringency into drying and velvety astringencies may be crucial to investigate the correlation between the %G and the astringency; hence, the lack of the classification justifies the inconsistent reports on the correlation between the %G and the astringency. The effect of the %PC on the astringency was illustrated by Sáenz-Navajas et al. [37]: (1) a strong negative linear relationship for %PC < 68%, (2) a moderately strong positive linear relationship for 68% < %PC < 76%, and (3) no relationship for %PC > 76%. Table 5 indicates that neither is the %PC significantly correlated with drying astringency nor is the %PC significantly correlated with velvety astringency. The absence of significant correlation could be justified on the basis of the high %PC values of the wines (> 80%, Table 4) and the absence of the relationship between the %PC and the astringency for %PC > 76% [37]. Similar to the inconsistent reports on the correlation between the %G and the astringency, the reports on the correlation between the PD% and the astringency are conflicting [37, 38]. The contributions of the conformational differences among tannins may explain the conflicting reports; however, measuring tannin conformations in wine is challenging [37].
In general, large condensed tannins bind strongly to saliva proteins and enhance the astringency perceptions [39]. In this work, the mDP of tannins showed no significant correlation with drying astringency (Table 5). At present, the contribution of the mDP towards astringency is argued. Several studies found that there was a significant positive correlation between mDP and astringency [40, 41]. Interestingly, the studies by Quijada-Morín et al. [8] and Wollmann and Hofmann [12] reported that there is no correlation between the mDP of tannins and the astringency perception, while the studies by Kyraleou et al. [9] and Sáenz-Navajas et al. [37] suggested the partial correlation between the mDP of tannins and the astringency perception. Kyraleou et al. [9] reported that the astringency is significantly correlated with the mDP of the oligomeric fraction of tannins, but not with mDP of the polymeric fraction of tannins. Likewise, Sáenz-Navajas et al. [37] presented that for low mDP values (below 1.4), the astringency increases with the increase in mDP; however, for high mDP values (above 1.4), the astringency decreases with the increase in mDP. The effect of the mDP of tannins on the astringency is related to the tannin hydrophobicity [34]. Large condensed tannins are likely to carry hydrophobic functional groups that increase the astringency [42]. Nonetheless, due to the conformational rearrangements and the polymerizations of the tannins during wine aging, the increase in astringency is not proportional to the increase in the number of hydrophobic functional groups [34].
Both velvety astringency and drying astringency were negatively correlated with the concentration of EGC (Table 5). This finding agrees with the previously-reported similar finding [9, 30]. Interestingly, none of the C concentration, the EC concentration, the GC concentration, the ECG concentration, and the EGCG concentration is significantly correlated with drying astringency and/or velvety astringency. Similar observation was reported by Kallithraka et al. [43] and Quijada-Morín et al. [8]. According to Ramos-Pineda et al. [44], the wine flavanols may synergistically interact with saliva proteins, which could explain why astringency is more influenced by the monomeric flavan-3-ols composition than by the total concentration. The importance of the flavan-3-ol subunits of tannins for the astringency perception is emphasized by Quijada-Morín et al. [8].
Conclusion
In the current study, the grape varieties and aging periods have a significant effects on the tannin profiles of ML, CG, and PN wines; the tannin profiles significantly influenced the astringency perceptions (the drying astringencies and the velvety astringencies) of the wines. In comparison with monomeric flavan-3-ols, the flavan-3-ol subunits of tannins are the more important determinants of astringency perceptions. Interestingly, the flavan-3-ol subunits had stronger influences on drying astringency than they did on velvety astringency. In addition, there was no significant correlation between the %PC and the astringency perceptions, which can be attributed to the high %PC values of the wines. Furthermore, the mDP of tannins exhibited a significant positive correlation with drying astringency. The findings of this study contributed to the current understanding of the relationships between the tannin profiles and the astringency perceptions of wines. In addition, it also provided winemakers with insights into the evaluations and modifications of astringency perceptions based on tannin profiles of wines. Further studies are needed to gain insight into the influence of ageing conditions such as oxygen, temperature or light on tannin profiles and the astringency perceptions of wines.
Abbreviations
- C:
-
( +)-Catechin
- EC:
-
(–)-Epicatechin
- GC:
-
( +)-Gallocatechin
- ECG:
-
(–)-Epicatechin gallate
- EGC:
-
(–)-Epigallocatechin
- EGCG:
-
(–)-Epigallocatechin gallate
- tC:
-
( +)-Catechin terminal subunit
- tEC:
-
(–)-Epicatechin terminal subunit
- tEGC:
-
(–)-Epigallocatechin terminal subunit
- eC:
-
( +)-Catechin extension subunit
- eEC:
-
( −)-Epicatechin extension subunit
- eEGC:
-
( −)-Epigallocatechin extension subunit
- eECG:
-
( −)-Epicatechin gallate extension subunit
- %PC:
-
The percentage of procyanidins
- %PD:
-
The percentage of prodelphinidins
- %G:
-
The percentage of galloylated flavanols
- mDP:
-
Mean degree of polymerization
References
E. Petrussa, E. Braidot, M. Zancani, C. Peresson, A. Bertolini, S. Patui, A. Vianello, Plant flavonoids–biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 14(7), 14950–14973 (2013). https://doi.org/10.3390/ijms140714950
S. Quideau, D. Deffieux, C. Douat-Casassus, L. Pouységu, Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 50(3), 586–621 (2011). https://doi.org/10.1002/anie.201000044
E.Q. Xia, G.F. Deng, Y.J. Guo, H.B. Li, Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 11(2), 622–646 (2010). https://doi.org/10.3390/ijms11020622
M. Guaita, A. Bosso, Polyphenolic characterization of grape skins and seeds of four Italian red cultivars at harvest and after fermentative maceration. Foods 8(9), 395 (2019). https://doi.org/10.3390/foods8090395
A.M. Jordo, J.M. Ricardo-Da-Silva, Evolution of proanthocyanidins during grape maturation, winemaking, and aging process of red wines, in Red Wine Technology, ed. by A. Morata (Elsevier, Amsterdam, 2019), pp. 177–193
N. Ćurko, K.K. Ganić, M. Tomašević, L. Gracin, M. Jourdes, P.L. Teissedre, Effect of enological treatments on phenolic and sensory characteristics of red wine during aging: micro-oxygenation, sulfur dioxide, iron with copper and gelatin fining. Food Chem. 339, 127848 (2021). https://doi.org/10.1016/j.foodchem.2020.127848
K. Bindon, S. Kassara, Y. Hayasaka, A. Schulkin, P. Smith, Properties of wine polymeric pigments formed from anthocyanin and tannins differing in size distribution and subunit composition. J. Agric. Food Chem. 62(47), 11582–11593 (2014). https://doi.org/10.1021/jf503922h
N. Quijada-Morín, J. Regueiro, J. Simal-Gándara, E. Tomás, J.C. Rivas-Gonzalo, M.T. Escribano-Bailón, Relationship between the sensory-determined astringency and the flavanolic composition of red wines. J. Agric. Food Chem. 60(50), 12355–12361 (2012). https://doi.org/10.1021/jf3044346
M. Kyraleou, Y. Kotseridis, S. Koundouras, K. Chira, P.L. Teissedre, S. Kallithraka, Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L. cv. syrah grapes under semiarid conditions. Food Chem. 203, 292–300 (2016). https://doi.org/10.1016/j.foodchem.2016.02.052
K. Chira, N. Pacella, M. Jourdes, P.L. Teissedre, Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 126(4), 1971–1977 (2011). https://doi.org/10.1016/j.foodchem.2010.12.056
N. Ćurko, K.K. Ganić, L. Gracin, M. Ðapić, M. Jourdes, P.L. Teissedre, Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 145, 15–22 (2014). https://doi.org/10.1016/j.foodchem.2013.07.131
N. Wollmann, T. Hofmann, Compositional and sensory characterization of red wine polymers. J. Agric. Food Chem. 61(9), 2045–2061 (2013). https://doi.org/10.1021/jf3052576
K. Chira, L. Zeng, A.L. Floch, L. Péchamat, M. Jourdes, P.L. Teissedre, Compositional and sensory characterization of grape proanthocyanidins and oak wood ellagitannin. Tetrahedron 71(20), 2999–3006 (2015). https://doi.org/10.1016/j.tet.2015.02.018
K. Chira, M. Jourdes, P.L. Teissedre, Cabernet sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. Eur Food Res Technol 234, 253–261 (2012). https://doi.org/10.1007/s00217-011-1627-1
K. Chira, G. Schmauch, C. Saucier, S. Fabre, P.L. Teissedre, Grape variety effect on proanthocyanidin composition and sensory perception of skin and seed tannin extracts from Bordeaux wine grapes (Cabernet Sauvignon and Merlot) for two consecutive vintages (2006 and 2007). J. Agric. Food Chem. 57(2), 545–53 (2009). https://doi.org/10.1021/jf802301g
S. Perez-Magarino, G.S. Jose, Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 96(2), 197–208 (2006). https://doi.org/10.1016/j.foodchem.2005.02.021
R.S. Yacco, A.A. Watrelot, J.A. Kennedy, Red wine tannin structure-activity relationships during fermentation and maceration. J. Agric. Food Chem. 64(4), 860–869 (2016). https://doi.org/10.1021/acs.jafc.5b05058
J.A. Kennedy, G.P. Jones, Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 49(4), 1740–1746 (2001). https://doi.org/10.1021/jf001030o
R. Ristic, K. Bindon, L.I. Francis, M.J. Herderich, P.G. Iland, Flavonoids and C13-norisoprenoids in Vitis vinifera L. cv. Shiraz: relationships between grape and wine composition, wine colour and wine sensory properties. Aust. J. Grape Wine R 16(3), 369–388 (2010). https://doi.org/10.1111/j.1755-0238.2010.00099.x
J. Heras-Roger, C. Díaz-Romero, J. Darias-Martín, A comprehensive study of red wine properties according to variety. Food Chem. 196, 1224–1231 (2016). https://doi.org/10.1016/j.foodchem.2015.10.085
R.V. Leeuw, C. Kevers, J. Pincemail, J.O. Defraigne, J. Dommes, Antioxidant capacity and phenolic composition of red wines from various grape varieties: specificity of Pinot Noir. J. Food Compos. Anal. 36(1–2), 40–50 (2014). https://doi.org/10.1016/j.jfca.2014.07.001
X.K. Zhang, F. He, B. Zhang, M.J. Reeves, Y. Liu, X. Zhao, C.Q. Duan, The effect of prefermentative addition of gallic acid and ellagic acid on the red wine color, copigmentation and phenolic profiles during wine aging. Food Res. Int. 106, 568–579 (2017). https://doi.org/10.1016/j.foodres.2017.12.054
F. He, N.N. Liang, L. Mu, Q.H. Pan, J. Wang, M.J. Reeves, C.Q. Duan, Anthocyanins and their variation in red wines. II. Anthocyanin derived pigments and their color evolution. Molecules 17(2), 1483–1519 (2012). https://doi.org/10.3390/molecules17021483
L.F. Casassa, J.F. Harbertson, Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 5, 83–109 (2014). https://doi.org/10.1146/annurev-food-030713-092438
J.M. McRae, R.G. Dambergs, S. Kassara, M. Parker, D.W. Jeffery, M.J. Herderich, P.A. Smith, Phenolic compositions of 50 and 30 year sequences of Australian red wines: the impact of wine age. J. Agric. Food Chem. 60(40), 10093–10102 (2012). https://doi.org/10.1021/jf301571q
X.K. Zhang, Y.B. Lan, Y. Huang, X. Zhao, C.Q. Duan, Targeted metabolomics of anthocyanin derivatives during prolonged wine aging: evolution, color contribution and aging prediction. Food Chem. 339, 127795 (2021). https://doi.org/10.1016/j.foodchem.2020.127795
A. Gambuti, A. Rinaldi, M. Ugliano, L. Moio, Evolution of phenolic compounds and astringency during aging of red wine: effect of oxygen exposure before and after bottling. J. Agric. Food Chem. 61(8), 1618–1627 (2013). https://doi.org/10.1021/jf302822b
H. Fulcrand, M. Duenas, E. Salas, V. Cheynier, Phenolic reactions during winemaking and aging. Am J Enol Vitic 57(3), 289–297 (2006). https://doi.org/10.1016/j.scienta.2005.07.007
M. Cano-López, F. Pardo-Mínguez, G. Schmauch, C. Saucier, P.L. Teissedre, J.M. López-Roca, E. Gómez-Plaza, Effect of micro-oxygenation on color and anthocyanin-related compounds of wines with different phenolic contents. J. Agric. Food Chem. 56(14), 5932–5941 (2008). https://doi.org/10.1021/jf8006147
M. Basalekou, M. Kyraleou, C. Pappas, P. Tarantilis, Y. Kotseridis, S. Kallithraka, Proanthocyanidin content as an astringency estimation tool and maturation index in red and white winemaking technology. Food Chem. 299, 125135 (2019). https://doi.org/10.1016/j.foodchem.2019.125135
M. Kyraleou, S. Kallithraka, E. Gkanidi, S. Koundouras, K.N. Kilcawley, Discrimination of five greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds. J. Food Compos. Anal. 92, 103547 (2020). https://doi.org/10.1016/j.jfca.2020.103547
V. Ferreira, V. Carrascon, M. Bueno, M. Ugliano, P. Fernandez-Zurbano, Oxygen consumption by red wines. Part I: consumption rates, relationship with chemical composition, and role of SO2. J. Agric. Food Chem. 63(51), 10928–10937 (2015). https://doi.org/10.1021/acs.jafc.5b02988
B. Sun, A.C. Neves, T.A. Fernandes, A.L. Fernandes, N. Mateus, V. De Freitas, C. Leandro, M.I. Spranger, Evolution of phenolic composition of red wine during vinification and storage and its contribution to wine sensory properties and antioxidant activity. J. Agric. Food Chem. 59(12), 6550–6557 (2011). https://doi.org/10.1021/jf201383e
W. Ma, A. Guo, Y. Zhang, H. Wang, Y. Liu, H. Li, A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Tech. 40(1), 6–19 (2014). https://doi.org/10.1016/j.tifs.2014.08.001
A. Rinaldi, A. Gambuti, L. Moio, Application of the SPI (saliva precipitation index) to the evaluation of red wine astringency. Food Chem. 135(4), 2498–2504 (2012). https://doi.org/10.1016/j.foodchem.2012.07.031
J.L. Landon, K. Weller, J.F. Harbertson, C.F. Ross, Chemical and sensory evaluation of astringency in Washington state red wines. Am. J. Enol. Vitic. 59(2), 153–158 (2008). https://doi.org/10.1016/0014-2999(94)90534-7
M.P. Sáenz-Navajas, S. Ferrero-Del-Teso, M. Romero, D. Pascual, P. Fernández-Zurbano, Modelling wine astringency from its chemical composition using machine learning algorithms. OENO One 53(3), 499–509 (2019). https://doi.org/10.20870/oeno-one.2019.53.3.2380
R. Ferrer-Gallego, N. Quijada-Morín, N.F. Brás, P. Gomes, V. de Freitas, J.C. Rivas-Gonzalo, M.T. Escribano-Bailón, Characterization of sensory properties of flavanols—a molecular dynamic approach. Chem. Senses. 40(6), 381–390 (2015). https://doi.org/10.1093/chemse/bjv018
W. Ma, P. Waffo-Teguo, M. Jourdes, H. Li, P.L. Teissedre, Chemical affinity between tannin size and salivary protein binding abilities: implications for wine astringency. PLoS One 11(8), e0161095 (2016). https://doi.org/10.1371/journal.pone.0161095
J.M. McRae, A. Schulkin, S. Kassara, H.E. Holt, P.A. Smith, Sensory properties of wine tannin fractions: implications for in-mouth sensory properties. J. Agric. Food Chem. 61(3), 719–727 (2013). https://doi.org/10.1021/jf304239n
A. Rinaldi, M. Jourdes, P.L. Teissedre, L. Moio, A preliminary characterization of Aglianico (Vitis vinifera L. cv.) grape proanthocyanidins and evaluation of their reactivity towards salivary proteins. Food Chem. 164, 142–149 (2014). https://doi.org/10.1016/j.foodchem.2014.05.050
G.R. Scollary, G. Pásti, M. Kállay, J. Blackman, A.C. Clark, Astringency response of red wines: potential role of molecular assembly. Trends Food Sci. Tech. 27(1), 25–36 (2012). https://doi.org/10.1016/j.tifs.2012.05.002
S. Kallithraka, D. Kim, A. Tsakiris, I. Paraskevopoulos, G. Soleas, Sensory assessment and chemical measurement of astringency of greek wines: correlations with analytical polyphenolic composition. Food Chem. 126(4), 1953–1958 (2011). https://doi.org/10.1016/j.foodchem.2010.12.045
A.M. Ramos-Pineda, I. García-Estévez, N.F. Brás, D.V.E.M. Martín, M. Dueñas, M.T. Escribano-Bailón, Molecular approach to the synergistic effect on astringency elicited by mixtures of flavanols. J. Agric. Food Chem. 65(31), 6425–6433 (2017). https://doi.org/10.1021/acs.jafc.7b01600
Acknowledgements
We are thankful to Mogao International Winery for providing the wines of this work.
Funding
This research was supported by The National Key Research and Development Project (item no. 2019YFD1002500-04) and The Key Industry Innovation Chain (Group), Agriculture of Shaanxi Province, China (No. 2020ZDLNY05-05).
Author information
Authors and Affiliations
Contributions
CY conceptualized this work. ZW, YR, JY, CY, and ZW developed the methods. JY performed the experiments and validated the data. ZW prepared this manuscript. YR reviewed and edited this manuscript. All authors have read this manuscript and agreed this manuscript to be published after peer review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11694_2022_1355_MOESM2_ESM.docx
Supplementary file2 (DOCX 15 KB) Retention times, regression equations and determination coefficients for six flavan-3-ols monomers
Rights and permissions
About this article
Cite this article
Wang, Z., Yang, J., Ren, Y. et al. The effects of the grape varieties and the wine aging periods on the tannin profiles and the astringency perceptions of wines. Food Measure 16, 2726–2737 (2022). https://doi.org/10.1007/s11694-022-01355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01355-9