Abstract
Gallic acid and phloroglucinol are the main phenolic compounds of the pistachio green hull (Pistachia vera) extract. The anti-radical and -peroxide activities of gallic acid and phloroglucinol were compared through DPPH radicals scavenging, bleaching of β-carotene (BCB), and Rancimat assays. The gallic acid molecules (log P = − 0.46) with an electron-donating carboxylate anion had significantly higher radical scavenging activities than phloroglucinol molecules (log P = 1.38) in DPPH (IC50 = 30.53 vs. 45.72 μM), BCB (IC50 = 43.66 vs. 66.15 μM), and Rancimat (OSI = 4.89 vs. 2.26 h) assays. The combinational kinetic model was successfully used for the determination of kinetic parameters, such as induction period (IP), the maximum concentration of lipid hydroperoxides (PVmax), and critical reverse micelle concentration (CMC) in soybean oil triacylglycerols (TAGs) peroxidized at 60 °C. The kinetics parameters, antioxidant effectiveness (F), and activity (AA) revealed gallic acid had the highest inhibitory effect during TAGs peroxidation due to the improved interfacial performance. Gallic acid and phloroglucinol were able to protect TAGs against peroxidation (IP = 388.34–816.21 vs. 25.53–122.4 h) in terms of the extent of their participation in the main reaction of chain termination and pro-oxidative side reactions of chain initiation, and anti-oxidative side reactions of chain propagation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The oxidation of lipid, the reaction of unsaturated fatty acids by oxygen in lipid systems, is the most common chemical reaction that leads to severe losses in the sensory attributes, quality of nutrition, shelf-life, and safety of food systems [1]. The fatty acids' position on the molecule of glycerol and the degree of unsaturation is the important intrinsic factors affecting the lipid oxidation rate. Lipid oxidation occurs quickly for polyunsaturated fatty acids and relatively slowly for saturated fatty acids; for instance, the oxidation rate of linolenic acid (C18:3) was considered to be 2500 times more than stearic (C18:0) acid [2, 3]. In the glycerol backbone, the position sn-2 compared to sn-3 and sn-1 can protect unsaturated fatty acids more from oxidizing agents. In position sn-2 of glycerol, the availability of the fatty acids is lower than the sn-3 and sn-1 for being oxidized by reactive radicals [4]. The major challenge in the oil industry is the inhibition of lipid oxidation, which can effectively be tackled by adding antioxidant compounds. Due to the carcinogenic effects of synthetic antioxidants (BHA, BHT, and TBHQ), the addition of natural phenolic antioxidants can be considered the most effective method to improve the oxidative stability of lipid systems [5].
Commercial vegetable oils normally contain small amounts of water and various types of surface-active agents like phospholipids, free fatty acids, sterols, mono- and/or diacylglycerols that during the refining process are not entirely removed. Bulk oils also including many other surface-active agents, e.g., hydroperoxides (LOOH), ketones, aldehydes, and alcohols that are derived from lipid oxidation [6]. The amount of water increases with the mono- and, or bimolecular decomposition reactions of LOOH under lipid oxidation [7]. According to the association colloids hypothesis, the LOOH produced during peroxidation tends to entrap traces of water and to form micelles beyond their critical reverse micelle concentration (CMC). During lipid peroxidation, the micelles grow in size and number as the concentration of LOOH and other surface-active agents increases [8]. CMC marks the transition from the initiation stage, where micelles are stable, to the propagation stage with extensive micellar collisions [9]. Addition of antioxidant molecules to bulk oil prolonged the induction period (IP) by stabilizes reverse micelles and scavenging lipid radicals at the interfaces. Molecules of antioxidant positioning their nonpolar tails and polar head groups at the oil phase and the reverse micelles interface, so stabilize reverse micelles by reducing the interfacial tension. Therefore, antioxidant performance in lipid systems is attributed to its innate potency as a chelating agent or radical scavenger, interaction with other reactants, and locating into the water–oil interface (oxidation site) [10].
Gallic acid and phloroglucinol are the main phenolic compounds of the aqueous extract of pistachio (Pistachia vera) green hull (PGH). Previous studies indicated that the PGH extract had significantly higher anti-microbial, -mutagenicity, -radicals, and -peroxide activities than synthetic antioxidants in biological, lipid, and emulsion systems. These studies have shown that high levels of gallic acid and phloroglucinol are the main reasons for the excellent antioxidant activity of PGH extract [11,12,13]. However, in the literature, there are no data that compare the antioxidant potency of gallic acid and phloroglucinol to show which phenolics is leading to the unique antioxidant action of the PGH extract. Studies have shown that antioxidants' performance is drastically dependent on the oxidative environment used to estimate their activity, such as the alcoholic environment of DPPH⋅ assay, dispersed emulsion systems, and bulk oils of different unsaturation [14].
Therefore, the present study aimed to estimate the antiradical activities of phloroglucinol and gallic acid using various antioxidative evaluation assays, including DPPH, β-carotene bleaching, and, Rancimat methods and investigate the mechanism of action of these phenolic compounds in stripped soybean oil during peroxidation at 60 °C.
Materials and methods
Chemicals
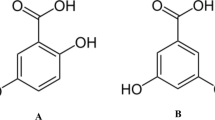
The refined soybean-seed oil was obtained by a local oil refining factory (Aliagolestan Co., Gorgan, Iran). Gallic acid, phloroglucinol (Fig. 1) of analytical grade, was supplied from Sigma-Aldrich (St. Louis, MO). All the other chemicals and solvents applied in this research were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO).
HPLC analysis
The extraction of PGH phenolic compounds was carried according to the optimal method described by Rajaei et al. [15]. An Azura high-performance liquid chromatography (HPLC) system (Knauer, Berlin, Germany) equipped with a UV–Vis photodiode array detector (DAD 2. 1L, Knauer) was employed for the identification of phenolic compounds according to the method of Lalegani et al. [12]. The polyphenols in a 10 μL of sample solution were separated with a column 5 µm ODS3 reversed-phase Prodigy (250 × 4.6 mm; Phenomenex, USA) at room temperature and detected by UV–Vis spectra at 190 to 700 nm. The mobile phase used for gradient condition consisted of solvent A (water/acetic acid (97/3, v/v)) and solvent B (methanol) with a flow rate of 1 mL/min.
DPPH free radical assay
DPPH radical-scavenging potency was examined according to the method developed by Delfanian et al. [16]. Briefly, 100–200 µL of antioxidant solutions (10–50 µM) was added to 3 mL of methanolic DPPH⋅ solution (0.1 mM). After 30 min incubation at 25 °C, the absorbance of solution (Asample) was read at 517 nm against a blank (Acontrol). The ability of phenolic antioxidants in radical scavenging (%RSA) was investigated from Eq. (1):
The IC50, [AH] required to scavenge 50% of DPPH⋅, was measured by interpolating the linear regression analysis.
β-Carotene bleaching method
Briefly, 10 mL of chloroform was mixed with 0.2 mg of the β-carotene. One milliliter of the β-carotene solution was added to 200 mg of Tween 40 and 20 mg linoleic acid. Chloroform was evaporated under a stream of nitrogen gas. Then, 50 mL of oxygenated distilled water was added to the mixture by vigorous shaking. About 100–200 µL of antioxidant compounds were mixed with 4 mL of the emulsion. The absorbance of the emulsion was recorded immediately at t = 0 min at 470 nm. The vials were placed in a water bath at 45 °C for 35 min, and finally, the absorbance of the emulsion was recorded [17]. The percentage inhibition (%I) was calculated from Eq. (2):
where AC(t) and AS(t) are the absorbance of the control and sample at t = 30 min, respectively, and AC(0) is the absorbance of the control at t = 0 min.
Oxidative stability (Rancimat) test
Gallic acid and phloroglucinol at 1.2 mM were added to 3 g of stripped soybean oil. Rancimat (Metrohm 743, Herisau, Switzerland) test was performed at 120 °C with a 15 L/h airflow rate to measure oxidative stability index (OSI) [18].
Partition coefficient (log P)
To determine the partition coefficient of the antioxidants, the solution of each antioxidative compound in 1-octanol (3 mM) was stored for 1 h at 60 °C. Then, the maximum absorbance of solutions was determined immediately by the UV spectrum (A0). Five milliliters of acetate buffer (0.1 M, pH 5.5) was added to 5 ml of the above solutions and vortexed for 1 min. After 30 min, the maximum absorbance of the 1-octanol layer was read (Ax) [19]. Partition coefficient was calculated from Eq. (3):
Soybean oil purification
Purification of soybean oil was carried according to the chromatographic method described by Yoshida et al. [20] with a chromatographic glass column (36 × 3.4 cm I.D.) packed with aluminum oxide 60 (activated for 3 h at 200 °C) with a ratio of 1 (oil) to 1 (absorbent). As a final step, the amount of phenolic compounds [21], tocopherols [22], and hydroperoxides (see below) were determined to ensure the purification process efficiency. The purified soybean oil triacylglycerols were contained inconsiderable values of PV (< 1 meq/kg), phenols and tocopherols (< 1 mg/kg).
Peroxide value (PV)
The spectrophotometric method developed by Shantha and Decker [23] was employed to measure LOOH accumulation of soybean oil TAGs to determine the PV. In brief, the oil samples (0.001–0.4 g) were dissolved in chloroform–methanol (9.8 ml, 7:3 v/v), and vortexed for 5 s. Then, 50 µl of the clear solution of iron (II) chloride, and 50 µl ammonium thiocyanate solution (30% w/v) was added on a vortex mixer for 5 s. The solution was saved for 5 min at 25 °C, and then the solution absorbance was read at 500 nm.
Fatty acid composition
The fatty acids profile of the soybean oil was determined by converting fatty acids into their methyl esters (FAMEs). In brief, 0.3 g of oil was dissolved in 7 ml of hexane and then methylated with 2 ml of methanolic potassium hydroxide solution (7 N) at 50 °C for 10 min. The gas chromatograph HP-5890 (Hewlett-Packard, CA, USA) equipped with a flame ionization detector (FID) was employed to determine FAMEs. The capillary column used was CP-FIL 88 (Suppl Co., Inc., Bellefonte, PA, USA). The flow rate of carrier gas (nitrogen) was 0.75 ml/min. Both the injector and the detector temperature were maintained at 250 °C, and that of the oven at 198 °C. The fatty acid composition was calculated in relative area percentages [24].
The calculated oxidizability (Cox) value of the oils was determined by the unsaturated C18 fatty acids percentage:
Preparation of oxidizing systems
The soybean oil TAGs (28.5 g) treatment with 1.2, 2.4, 4.8, and 9.6 mmol of gallic acid and phloroglucinol stored in a Petri dish with a diameter of 19.5 cm in a 1-mm layer (kinetic regime) and then oxidized in accelerated oil oxidation at 60 °C [20].
Kinetic parameters
The combinational kinetic model described by Farhoosh [25] was used to determine the PV-based kinetic parameters. The kinetic curve of LOOH accumulation was drawn by plotting PV changes over time (Fig. 2). IP (Eq. (5)) and CMC (Eq. (6)) of LOOH was calculated from the x-and y-coordinates of the intersection point of two straight lines fitted on the initiation and propagation stages of the kinetic curves, respectively. The second straight line precisely arose from the sigmoidal kinetic model (Eq. (7)) fitted on the whole range of PV changes over time.
where ki (meq kg−1 h−1), k1 (h−1) and k2 (kg meq−1 h−1) are the parameters of the equations; Cc (kg meq−1) is an integration constant and PV0 (meq kg−1) is PV at t = 0.
Antioxidative power of phloroglucinol and gallic acid in peroxidation of soybean oil TAGs was examined by effectiveness factor (F), oxidation rate ratio (ORR), antioxidant activity (AA), and mean rate of antiradical consumption (\({\bar{W}}\) AH).
Stabilization factor or antioxidant efficiency, which showing the potency of an antioxidant (AH) in prolonging IP, was calculated by Eq. (9).
where IP0 and IPAH are the IP in the absence and presence of phenols, respectively.
The parameter ORR, an inverse measure of antioxidant strength, was determined by Eq. (10).
where Ki and Ki0 are the pseudo-zero order rate constants in the presence and absence of phenolic antioxidants, respectively.
Antioxidant activity (AA) was obtained with Eq. (11).
The parameter \({\bar{W}}\) AH, the average rate of AH consumption, was calculated by Eq. (12) [26].
Mechanism of action
In bulk oils, the antioxidant action mechanism of a phenolic antioxidant is related to the extent of participation of antioxidant molecules (AH) and radicals (A⋅) in a series of oxidation reactions. Factor F represents the possibility of blocking peroxyl radicals (LOO⋅) through the main reaction of chain termination23. If the relationship between factor F and concentration of antioxidant [AH]0 is linear, the AH molecule participates in the reaction 23 whereas, its nonlinear relationship reveals the participation of the AH, besides the main reaction 23, in the chain initiation reaction 17 and, or 18. It will be possible to identify the occurrence of side reactions 17 and 18 by the following regression equation:
where f is the stoichiometric coefficient of inhibition denoting how many radicals perish in an AH, Wi is the mean rate of initiation during IP, n is the kinetic reaction order, and Keff is the rate constant of the AH consumption in side reaction(s) of chain initiation. Considering to regression equation, the linear relationship at n = 0 reveals that the AH does not take part in the side reactions, whereas, linear relationship at n = 1 and n = 2 represents the AH participate in one and both of the reaction(s) 17 and 18, respectively.
Following dependency can be employed for evaluation of the possibility of A⋅ participation in the side reactions of chain propagation 20, 21, and 22:
The linear relationship at n = − 1 shows antioxidant radical does not take part in the chain propagation reactions whereas, the linear relationship at n = − 0.5 reveals that the A⋅ predominantly participates in reaction 21. Nonlinear relationship at n = − 1 and − 0.5 denotes that the A⋅ takes part in more than one reaction of chain propagation. Moreover, no dependency (n = 0) indicates the antioxidant molecules are so active that peroxyl radicals react faster with antioxidant molecules than with LH (oil reactant) [27].
Statistical analysis
Each analysis was carried out in triplicate, and results were analyzed by one-way analysis of variance (ANOVA). Duncan's multiple range tests were employed to determine the significant differences of means at P < 0.05. Regression analyses and ANOVA were performed using SlideWrite 7.01, Excel, and SPSS Statistics 19 software.
Results and discussion
HPLC analysis of the extracted polyphenols
HPLC analysis was used to identify major phenolic compounds of aqueous extract of PGH (Fig. 3). Three major phenolics were found in PGH extract, including phloroglucinol (peak 1), gallic acid (peak 2), and galloyl-shikimic acid (peak 3), with retention times 4.92, 6.12, and 7.39 min, respectively. The content of gallic acid and phloroglucinol was 22.30 and 5.36 mg/g extract, respectively. The galloyl-shikimic acid is one of the isomers of gallic acid, which is not considered in this study. These results were in accordance with Garavand et al. [28] and Sadeghinejad et al. [13], who reported gallic acid and phloroglucinol were to major phenolic components of aqueous and alcoholic extract of PGH. These studies also showed that the high level of antioxidative potency of PGH extract is due to the presence of a large amount of gallic acid and phloroglucinol. Therefore, pure gallic acid and phloroglucinol were used to compare their antioxidative potency and mechanism of action in inhibiting bulk phase oil peroxidation from an interfacial phenomena standpoint.
Soybean oil characterization
Table 1 is shown the chemical properties of the purified and unpurified soybean oils. As can be seen, the process of purification did not affect the composition of fatty acids of the soybean oil. It was in accordance with those reported for soybean oil in literature. The purified soybean oil contained a negligible amount of phenolic compounds, tocopherols, and hydroperoxides, showing the minor components' efficient removal that may interfere with the antioxidant agents.
Antioxidative performance
In this section, the antioxidant capacities of gallic acid and phloroglucinol were evaluated using different chemical methods: β-carotene bleaching, DPPH⋅ scavenging, and Rancimat assays.
The ability of antioxidative compounds for scavenging DPPH free radicals is presented in Table 2. The antiradical activities of gallic acid and phloroglucinol increased as the antioxidant concentrations increased from 10 to 50 μM. The percent values of RSA for gallic acid at all concentrations were significantly higher than phloroglucinol. Considering IC50, the gallic acid concentration required to scavenge 50% of DPPH⋅ was considerably lower than phloroglucinol (30.53 μM, 45.72 μM). This result was in accordance with Lalegani et al. [12] that reported the anti-DPPH⋅ activity of gallic acid was significantly more potent compared to phloroglucinol. Some reports in the literature showing among a group of antioxidative agents, gallic acid had the most potent anti-DPPH⋅ [29, 30]. The antiradical activity of antioxidative compounds depends on the number of electron donor hydroxy and carboxyl substitutions that increasing the phenoxy radical's stability. Gallic acid with a carboxyl group and three hydroxyl groups was the most reactive antioxidant than phloroglucinol with three hydroxyl groups (Fig. 1). Carboxyl is considered an electron-withdrawing functional group, which is expected to raise the O–H bond dissociation enthalpy (BDE) of the phenolic ring. Such a discrepancy has been attributed to the proton dissociation from the –COOH group of gallic acid in polar media, generating carboxylate anion (-COO). The O–H BDE value in gallic acid decreases owing to the electron-donating impact of the -COO, which favors H-atom transfer and electron-donating-based radical scavenging. The OH-BDE values of gallic acid and phloroglucinol are 72.2 and 75.3 kcal mol−1, respectively [31]. Intramolecular hydrogen bonds (IHB) are another possible explanation for the difference in anti-DPPH⋅ potency of phloroglucinol and gallic acid. The IHB between polar solvents and these functional groups can play an essential role in the DPPH⋅ scavenging activity. Intramolecular hydrogen bonds have a considerable contribution to lowering the O–H BDE value of phenolic antioxidants. Furthermore, the solubility and polarity of a phenolic antioxidant significantly improve its availability and molecular mobility and enhances the anti-radical ability to scavenge free DPPH· [10]. Table 2 shows the partition coefficient of gallic acid was significantly lower than that of phloroglucinol (− 0.46 vs. 1.38), arising from the carboxyl/carboxylate group of high potency to create more hydrophilic interactions between the antioxidant and the polar molecules of protic methanol as a reaction solvent.
In the BCB method, gallic acid and phloroglucinol prevented the extent of β-carotene bleaching by neutralizing the linoleate-free radical and other free radicals formed in the emulsion. As shown in Table 2, a concentration-dependent antioxidant potential was observed for both of the antioxidant components studied. A comparison of antioxidant capacity in the emulsion system showed that gallic acid was the most reactive antioxidant than phloroglucinol. So, the IC50 value of gallic acid was lower than the phloroglucinol. This means that a significantly lower concentration of gallic acid (43.66 µM) was required to scavenge 50% of linoleate-free radicals than phloroglucinol (66.15 µM). In general, gallic acid with more robust interfacial performance due to its carboxyl group indicated a higher ability to lie in the actual site of oxidation (oil–water interface). The obtained results were contrary to Alavi Rafiee et al. [14] that reported that the pyrogallol behaved more effectively in the emulsion than the gallic acid. This difference between the antioxidant potency of pyrogallol and phloroglucinol has been attributed to the hydroxyl group's position in the phenolic ring.
In the Rancimat test, the oxidative stability index (OSI) of soybean oil was significantly promoted with the phenolics added. The OSI value of purified soybean oil was only 0.32 h (Table 2). A higher induction period was obtained for soybean oil containing gallic acid compared to phloroglucinol means that gallic acid was the most effective antioxidant to prolong the time of lipid oxidation. Concerning the carboxyl group's electron-withdrawing character, which is not markedly dissociated in the hydrophobic media, gallic acid was expected to behave as a weaker antioxidant than phloroglucinol in the lipid system. However, the less hydrophobic molecules of gallic acid than phloroglucinol (log P = − 0.46 vs. 1.38) would have been able to decrease interfacial tension more efficiently and create more stable reverse micelles assembled by less aggregatable hydroperoxides.
Mechanism of antioxidant action
The kinetic parameters representing the inhibited bulk oil oxidation in the presence of the antioxidant components are shown in Table 3. The combinational kinetic model was used to determine kinetic parameters, including IP, CMC, and PVmax, for soybean oil treatments peroxidized at 60 °C. The CMC or PVIP marks the transition from the initiation stage, where micelles are stable, to the propagation stage with extensive micellar collisions. At this stage, extensive micelle collisions increase the bimolecular reactions of hydroperoxides, and oxidation enters the propagation phase,
The IP of soybean oils was significantly prolonged by adding different gallic acid concentrations and phloroglucinol (Table 3).
In the bulk oils, the mechanism of the inhibitory impact of a phenolic agent related to the extent of participation of AH molecules and A⋅ in the following oxidation reactions: (1) Reactions of chain initiation:
(2) Reactions of chain propagation:
(3) Reactions of chain termination:
Considering the kinetic parameters F, ORR, and AA, which is the ratio of F to ORR, the greater extents of strength and effectiveness were observed for gallic acid. Mechanically, the phenolic antioxidants presented nonlinear dependencies of the F on the [AH] during the soybean oil triacylglycerols (TAGs) peroxidation (Fig. 4), indicating the AH molecules participate not only in the main chain termination reaction (reaction 22) but also take part in side reaction(s) of chain initiation 17 and, or 18. Moreover, the rate of TAGs oxidation inhibited by phenolic antioxidants was dependent on their concentration, meaning that the AH compounds were very active that the peroxyl radical (LOO⋅) reacted with the AH molecules faster than the LH (oil reactant). Concentration dependency of the mean rate of AH consumption, \({{\bar{W}}}_{AH}\), (Eq. 13) in oil samples at n = 1 and n = 2 (Fig. 5) was linear, demonstrating the AH molecules take part in both side reactions 17 and 18. The TAGs containing gallic acid had higher Keff values (2.7907 M−1 s−1), which shows their AH molecules more participate in the side reaction 16. While, the Keff values for TAGs containing phloroglucinol (0.0001 M−1 s−1) were lower, which indicates their AH molecules participated lower in both side reactions [26].
The linear dependency was found between the Ki versus the [AH]n (Eq. 14) at n = − 1 for lipid systems contained a different concentrations of gallic acid (Fig. 6). This signifies that their A⋅ did not participate in the side reactions of chain propagation (20, 21, and 22) in all TAGs samples. Whereas, for lipid systems contained different concentrations of phloroglucinol, the dependency between the Ki and [AH]n was linear at n = − 0.5 denotes that their A⋅ participates in chain propagation side reaction 21 (Fig. 6). The Wi/f values for TAGs treatments with gallic acid and phloroglucinol were 5.2617 and 0.0012 (Ms−1), respectively. Considering to Wi/f, denotes the extent of participating AH in chain initiation reaction 17, less tendency was observed for phloroglucinol to participate in the side-chain initiation reactions than gallic acid in bulk oil systems.
The ORR can be considered to be in a direct ratio to the rate of chain initiation (17–19) and propagation (20, 21, and 22) reactions, and in a reverse ratio to the rate of chain termination (23, 24, and 25) reactions [32]. Based on ORR values (Table 3 and Fig. 4) the antioxidant strength increased as the concentration of gallic acid and phloroglucinol increased. However, the ORR decreased more steeply in TAGs containing gallic acid than in TAGs containing phloroglucinol. The increasing trend in the antioxidant power of gallic acid with concentration can be ascribed to the lower contribution of the side reaction of chain initiation 17 or the participation of antioxidant molecules and radicals in the main (23) and side (24 and 25) reactions of chain termination. This means that reactions 24 and, or 25 played a more prominent role than reactions 17 and 18 in the inhibitory performance of gallic acid. Reaction 24 is considered an inhibitory reaction of the reactive LOO⋅. In contrast reaction 25 has been reported to be a significant reaction in forming antioxidative acting products, e.g., dimmers [26]. The result showed that the activation energy of reaction 23 was higher in the presence of phloroglucinol. The O–H BDE of the phenolic –OH group and –COOH group is affected by activation energy. More potent antioxidants, which have higher capabilities of direct hydrogen transfer to oxidizing radicals, show lower values of the O–H BDE [33]. The O–H BDE value in gallic acid decreases owing to the electron-donating effect of the carboxylate anion, especially under the anhydrous conditions provided by bulk oil systems, which favors H-atom transfer and electron-donating-based radical scavenging [34]. On the whole, gallic acid showed significantly higher antioxidant potency due to having one more electron-donating –COOH group than phloroglucinol capable of establishing additional intramolecular hydrogen bonds.
Conclusions
In this work, a kinetic study was carried out to compare the antiradical potency and mechanism of action of phloroglucinol and gallic acid in bulk oil. The anti-radical and anti-peroxide activities of antioxidants were compared through β-carotene bleaching, DPPH radicals scavenging, and Rancimat assays. Results revealed that the gallic acid had a higher antiradical/antioxidant activity than the phloroglucinol in various oxidative environments. In general, the presence of carboxyl group, lower values of the O–H bond dissociation enthalpy, higher amphiphilic property, location in the actual site of oxidation, more participation in the main reaction of chain termination, less participation in the pro-oxidative side reactions of chain initiation and the anti-oxidative side reactions of chain propagation are the main reasons for the better antioxidative performance of gallic acid compared to phloroglucinol. Results also confirmed that the combinational kinetic model is a reliable method for determining oxidation kinetic parameters, including IP, CMC, and PVmax.
Data availability
The authors acknowledge the availability of data and materials as well as the transparency of the data.
Abbreviations
- A· :
-
Antioxidant radical
- AA:
-
Antioxidant activity
- AH:
-
Antioxidant molecule
- BCB:
-
β-Carotene bleaching method
- Cox:
-
Calculated oxidizability
- CMC:
-
Critical micelle concentration
- DPPH·:
-
2,2 Diphenyl-1-picrylhydrazyl radical
- FID:
-
Flame ionization detector
- F:
-
Effectiveness factor
- HPLC:
-
High-performance liquid chromatography
- IC50 :
-
The concentration of antioxidant required for 50% inhibition of the radicals
- IP:
-
Induction period
- Ki :
-
Oxidation rate
- LOOH:
-
Lipid hydroperoxides
- LOO·:
-
Peroxyl radicals
- log P:
-
Partition coefficient
- MUFA:
-
Monounsaturated fatty acids
- OSI:
-
Oxidative stability index
- ORR:
-
Oxidation rate ratio
- PGH:
-
Pistachio green hull
- PV:
-
Peroxide value
- PVmax :
-
The maximum concentration of lipid hydroperoxides
- PUFA:
-
Polyunsaturated fatty acids
- SFA:
-
Saturated fatty acids
- TAGs:
-
Soybean oil triacylglycerols
References
L. Yu, Y. Wang, H. Wen, M. Jiang, F. Wu, J. Tian, Food Chem. 16, 130384 (2021)
S. Zhang, S.A. Willett, J.R. Hyatt, S. Martini, C.C. Akoh, Food Chem. 334, 127584 (2020)
C. Chen, C. Zhang, Q. Zhang, X. Ju, Z. Wang, R. He, Food Chem. 354, 129534 (2021)
M.K. Ahmmed, A. Carne, F. Ahmmed, I. Stewart, H.S. Tian, A.E.D.A. Bekhit, Food Chem. 353, 130302 (2021)
M. del PilarGarcia-Mendoza, F.A. Espinosa-Pardo, R. Savoire, C. Harscoat-Schiavo, M. Cansell, P. Subra-Paternault, Food Chem. 341, 128234 (2020)
H. Mansouri, R. Farhoosh, M. Rezaie, Food Chem. 328, 127128 (2020)
W. Chaiyasit, R.J. Elias, D.J. McClements, E.A. Decker, Crit. Rev. Food Sci. Nutr. 47, 299–317 (2007)
M. del Pilar Garcia-Mendoza, F.A. Espinosa-Pardo, R. Savoire, C. Harscoat-Schiavo, M. Cansell, P. Subra-Paternault, Food Chem. 341, 128234 (2021)
E.S. Budilarto, A. Kamal-Eldin, Eur. J. Lipid Sci. Tech. 117, 1971–1977 (2015)
F. Shahidi, Y. Zhong, J. Agric. Food Chem. 59, 3499–3504 (2011)
R.A. Sarteshnizi, M.A. Sahari, H.A. Gavlighi, J.M. Regenstein, M. Nikoo, Food Biosci. 27, 37–45 (2019)
S. Lalegani, H.A. Gavlighi, M.H. Azizi, R.A. Sarteshnizi, Food Res Int. 105, 94–101 (2018)
N. Sadeghinejad, R.A. Sarteshnizi, H.A. Gavlighi, M. Barzegar, LWT. 102, 393–402 (2019)
S. Alavi Rafiee, R. Farhoosh, A. Sharif, Eur. J. Lipid Sci. Tech. 120, 1800319 (2018)
A. Rajaei, M. Barzegar, A.M. Mobarez, M.A. Sahari, Z.H. Esfahani, Food Chem. Toxico. 48, 107–112 (2010)
M. Delfanian, R.E. Kenari, M.A. Sahari, J. Food Sci. Technol. 53, 2244–2252 (2016)
N. Shahsavari, M. Barzegar, M.A. Sahari, H. Naghdibadi, Plant Food. Hum. Nutr. 63, 183–188 (2008)
M. Delfanian, R.E. Kenari, M.A. Sahari, Int. J. Food Prop. 18, 2813–2824 (2015)
M.H. Gordon, F. Paiva-Martins, M. Almeida, J. Agric. Food Chem. 49, 2480–2485 (2001)
H. Yoshida, I. Kondo, G. Kajimoto, J. Am. Oil Chem. Soc. 69, 1136–1140 (1992)
C. Capannesi, I. Palchetti, M. Mascini, A. Parenti, Food Chem. 71, 553–562 (2000)
M. Wong, R. Timms, E. Goh, J. Am. Oil Chem. Soc. 65, 258 (1988)
N.C. Shantha, E.A. Decker, J. AOAC Int. 77, 421–424 (1994)
S.H. Fatemi, E.G. Hammond, Lipids 15, 379–385 (1980)
R. Farhoosh, LWT. 98, 406–410 (2018)
E.M. Marinova, N.V. Yanishlieva, Food Chem. 81, 189–197 (2003)
E.T. Denisov, I. Khudyakov, Chem. Rev. 87, 1313–1357 (1987)
F. Garavand, A. Madadlou, S. Moini, Int. J. Food Prop. 20, 19–29 (2017)
R. Farhoosh, L. Nyström, Food Chem. 244, 29–35 (2018)
M. Asnaashari, R. Farhoosh, R. Farahmandfar, Food Sci. Nutr. 7, 4007–4013 (2019)
M. Leopoldini, N. Russo, M. Toscano, Food Chem. 125, 288–306 (2011)
E.M. Marinova, N.V. Yanishlieva, J. Sci. Food Agric. 60, 313–318 (1992)
H.Y. Zhang, Y.M. Sun, X.L. Wang, Chem. Eur. J. 9, 502–508 (2003)
H.F. Ji, H.Y. Zhang, L. Shen, Bioorg. Med. Chem. Lett. 16, 4095–4098 (2006)
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MD. The first draft of the manuscript was written by MD, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any authors.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delfanian, M., Sahari, M., Barzegar, M. et al. Structure–antioxidant activity relationships of gallic acid and phloroglucinol. Food Measure 15, 5036–5046 (2021). https://doi.org/10.1007/s11694-021-01045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01045-y