Abstract
Lucerne seeds (Medicago sativa L.) were germinated at different time-temperature regimes (24, 28, and 32 °C for 24, 48, and 72 h). The effect of germination was evaluated on physicochemical properties, techno-functional characteristics, antinutrients, bioactive constituents, antioxidant activity, and mineral profile of lucerne seeds to ascertain the best time-temperature regime for the germination. High germination capacity was observed in seeds germinated at 28 and 32 °C in comparison to 24 °C, however, lower germination losses were manifested at 28 °C. Time-temperature regime of 28 °C for 48 h was most effective for the germination of lucerne seeds. Germination at aforesaid conditions improved hydration properties, foaming capacity, and gelation ascribed to protein denaturation reflected as a change in the α-helix and β-sheets in ATR-FTIR spectra of germinated flours. Furthermore, reduction in tannins (32.91%), lectin (100%), and trypsin inhibitor activity (67.42%) were observed. Increased DPPH∙ RSA (6.90%), ABTS∙+ RSA (10.51%), metal chelating activity (14.64%), and zinc concentration were also manifested with germinated flour. The optimal balance of techno-functionality, reduced antinutrients, and high antioxidant capacity of germinated flour (28 °C for 48 h) was validated using Principle Component Analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Legumes are an integral part of our diet to obtain well balanced nutrition. In addition to being a rich source of protein, carbohydrates, dietary fibre, and micronutrients; their role in preventive nutrition has been empathized due to its associated bioactive constituents and antioxidants [1]. Legumes have been processed and consumed traditionally in the form of boiled, pressure cooked, curried, sprouted, and roasted legumes. However, with the advent of the trend in the consumption of convenience and healthy food products, legumes are also processed (extruded/fermented/germinated) to form flours for household consumption or utilization as a functional ingredient to improve the nutritive value of food formulations. In recent years, many food products including pasta, baked products, breakfast cereal, snacks, and beverages developed from germinated flours are making a significant place on the market shelves [2]. Furthermore, such products offer better market positioning as ‘more nutritious’, ‘healthier’, ‘natural’, and ‘tastier’ products to capture the wide demographics [3].
Germination or biological activation of legumes is a simple, cost-effective, and environment-friendly method of processing legumes to improve their nutritional composition, functional properties, and bioactive potential [4, 5]. Germination has been widely utilized for improving the nutritive value of legumes by reduction of antinutritional factors like tannins, phytic acid, lectins, and trypsin inhibitor [6,7,8]. Germination has also been manifested with the genesis of various secondary metabolites that confer improved antioxidant activity to germinated legumes [9]. Furthermore, germination also induces changes in the morphology and functional behaviour of macromolecules with consequent improvement in the techno-functional characteristics to allow better functionality in the food systems [1, 5].
Lucerne (Medicago sativa L.) is a widely cultivated perennial non-conventional legume. It is native to southwest Asia with its cultivated forms that arose from western Persia and eventually spread to Asia, Europe, and America. It has a deep root system and thus it can be grown in an area with scarcity of water [10]. It can also grow well on irrigated soils of arid and semi-arid tracts [11]. Sowing of the lucerne is done around the middle of October and the harvesting takes about 75 days, with the subsequent cutting of fodder at an interval of 30–40 days. After the last cutting of fodder around mid March, the irrigation is stopped to arrest vegetative growth to allow the good yield of seeds [11]. Lucerne is commonly known as rijka in northern India and different varieties of lucerne including Sirsa-8, Sirsa-9, Type-9, GAUL-1, GAUL-2, LL Composite-5, LL Composite-3, and Anand Lucerne-3, etc. have been developed in India [10]. Lucerne is popularly known as the “father of all foods” and is considered to be the green food of the millennium [12]. Lucerne seeds contain high amount of protein, dietary fibre, essential polyunsaturated fatty acids, vitamins, and total phenols [13,14,15]. Lucerne seeds also exert therapeutic potential by conferring antiatherosclerotic, anticancer and antioxidant activity, antidiabetic effect, and positive influence on hypercholesterolemia [16]. The potency of lucerne saponins in the reduction of cholesterol has been demonstrated in a study where diets containing lucerne seeds exhibited a positive influence on serum cholesterol of patients suffering from type II hyperlipoproteinemia [17].
Few studies have documented the development of sprouts with improved antioxidant activity from lucerne seeds [12, 4]. However, a detailed study on the germination of lucerne seeds at different time-temperature regimes and its effect on the composition and functional properties of lucerne flour has not been reported in the literature. Therefore, the present study was aimed to evaluate the germination behaviour of lucerne seeds at different time-temperature regimes. Furthermore, the effect of germination was evaluated on the techno-functional characteristics, proximate composition, antinutrients, bioactive constituents, antioxidant activity, and mineral profile to ascertain the best temperature-time combination for the germination of lucerne to obtain germinated flour with improved functionality.
Materials and methods
Material
Clean and damage free lucerne seeds (LL Composite-5) were obtained from Punjab Agricultural University, Ludhiana (India) and stored in airtight PET jars at 4 °C. All the chemicals used in the study are of analytical reagent grade.
Steeping behaviour
Steeping behaviour was evaluated as per Montanuci et al. [18] with some modifications. Seeds (100 g) were soaked in distilled water (1:10 w/v) at 24, 28, and 32 °C in a thermostat controlled incubator (Narang Scientific Works, New Delhi). Hydrated seeds were taken out after every 1 h, excess water was removed using filter paper and dried at 130 °C. The moisture content of the seeds was expressed on % dry basis and plotted against steeping time (h).
Germination of lucerne seeds
Germination was carried out as per Singh et al. [5] with some modifications. Lucerne seeds were soaked in distilled water (1:10 w/v) at different temperatures (24, 28, and 32 °C) in a thermostat controlled incubator (Narang Scientific Works, New Delhi). Soaking was carried out for 8 h, based on the optimum soaking time of lucerne seeds obtained after evaluating the steeping behaviour of the seeds. Soaked seeds were spread out thinly between the double layered wetted muslin cloths and covered with another wet muslin cloth. Germination temperatures were selected based on the preliminary trials carried out as per the observations and recommendations of Singh et al. [5] and Devi et al. [19]. Temperature lower than 24 °C resulted in the germination of very few seeds whereas germination at a higher temperature (35 °C) resulted in high germination losses as well as development of mild off flavour. Therefore, germination was carried out at 24, 28, and 32 °C for 24, 48, and 72 h and R.H. 90–95% in a thermostat controlled incubator (Narang Scientific Works, New Delhi). Muslin cloth was kept moist by sprinkling it with distilled water. Germinated seeds were dried at 50 °Cin a hot air oven (≈ 8% m.c.).
Germination characteristics
Germination characteristics viz. germination rate, germination capacity, and sprout length were determined as outlined by Pinzino et al. [20] by germinating 100 sound seeds as per aforesaid germination conditions. Sprout length was calculated by using digital vernier calliper by noting the length of the sprout and expressed in cm. Germination capacity was calculated by calculating the number of seeds germinated out of 100 and expressed in percentage. Germination rate was expressed in percentage and calculated as per the following equation.
where G1, G2 and G3 refer to the number of seeds germinated at 24, 48, and 72 h respectively.
Germination losses
Germinated seeds were devegetated manually by gentle rubbing. Dry matter loss was evaluated by determining 1000 kernel weight and germination (leaching, metabolic and vegetative) losses were determined as per Eqs. 1–3 [21]
Preparation of the flour
Flour was prepared from ungerminated and germinated seeds (≈ 8% m.c.) using cyclotec mill (Newport Scientific, Australia) and passed through 60 mesh sieve. Flours were packed in airtight PET jars and stored at 4 °C.
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR)
ATR-FTIR spectra of flours were recorded using Fourier transform infrared spectrometer (Thermo Scientific, Nicolet 67000) in 400–4000 cm−1 by directly placing onto ATR crystal.
Functional properties
Bulk density
Bulk density of flour was evaluated as per Singh et al. [5] by taking 20 g flour in a measuring cylinder and gently tapping ten times and noted the volume and expressed as g/cm3.
Water absorption capacity and water solubility index
Water absorption capacity was evaluated as per Singh et al. [5] by taking 3 g flour sample in a pre-weighed 50 mL centrifugation tube and added 30 mL deionised water to it and allowed the flour to absorb water for 30 min with gentle stirring at an interval of 10 min, followed by centrifugation at 3000 rpm for 15 min and decanting the water. The increase in weight was evaluated by weighing the centrifugation tube and expressed as g/g. Decanted water was taken in a pre-weighed petri plate and allowed to dry in a hot air oven at 100 °C till it gets completely dry to determine the water solubility index. Water solubility index was expressed in percentage.
Oil absorption capacity
Oil absorption capacity was evaluated as per Singh et al. [5] by taking 3 g flour sample in a pre-weighed 50 mL centrifugation tube and added 30 mL refined soybean oil to it and allowed the flour to absorb oil for 30 min with gentle stirring at an interval of 10 min, followed by centrifugation at 3000 rpm for 15 min and decanting the oil. The increase in weight was evaluated by weighing the centrifugation tube and expressed as g/g.
Swelling index
Swelling index was determined by the procedure of Awolu et al. [22] by taking 1 g sample in a measuring cylinder and noting the initial volume followed by the addition of 10 mL deionised water and left undisturbed for 1 h, followed by noting the volume of swelled sample and calculating using the following equation.
Swelling capacity and leaching loss
Swelling capacity was determined as per Singh et al. [5] by taking 0.5 g flour sample in a pre-weighed 50 mL centrifugation tube and added 15 mL deionised water to it and covered tubes were heated in a water bath at 90 °C for 30 min and shaken every 5 min to avoid lump formation. Tubes were cooled, followed by centrifugation at 3000 rpm for 25 min and decanting the water. The increase in weight was evaluated by weighing the centrifugation tube and expressed as g/g. Decanted water was taken in a pre-weighed petri plate and allowed to dry in a hot at oven at 100 °C till it gets completely dry to determine the leaching loss. Leaching loss was expressed in percentage.
Emulsification capacity and emulsion stability
Emulsification capacity and emulsion stability were determined as per Singh et al. [5] by taking 2 g flour in a 50 mL centrifugation tube and added 20 mL deionised water and 20 mL soybean oil, shaken well and followed by centrifugation at 4000 rpm for 10 min. After centrifugation, the height of the emulsion layer was noted and emulsification capacity was determined in percentage as per the following equation.
Emulsion stability was determined by heating the afore formed layer at 80 °C for 30 min on a boiling water bath, followed by cooling at room temperature and centrifugation as mentioned above. The height of the emulsion layer obtained after centrifugation is noted and emulsion stability was expressed in percentage as per the following equation.
Foaming capacity and foam stability
Foaming capacity and foam stability were determined as per Singh et al. [5] by taking 2 g flour sample in a 500 mL beaker and added 100 mL deionised water to it and suspension was blended using blender (Kalsi Company, Ambala, India) for 1 min and contents were transferred to 250 mL measuring cylinder and foaming capacity was determined in percentage as per the following equation. Foam appearance was visually assessed and described as dense foam, moderately dense foam, and foam with large bubbles based on the characteristics of the foam.
Foam stability was evaluated by noting the decline in the volume of foam after every 10 min interval of 60 min and calculated by the following equation.
Gel consistency
Gel Consistency was determined by following the procedure of Singh et al. [5] by taking 0.2 g flour in a screw cap tube and added 0.2 ml of ethanol and 3 mL 0.1 N acetic acid solution/deionised water and heating the suspension in boiling water bath for 8 min. Tubes were cooled at room temperature by keeping them in an upright position, followed by laying them down on the levelled surface for over 1 h and noted the distance travelled by the gel and reported in cm. The gel consistency is inversely proportional to the distance travelled by the gel.
Dispersibility
Dispersibility was determined by taking 10 g flour in a 200 mL measuring cylinder and adding deionised water up to the mark of 100 mL, mixed well, and allowed to stand for 3 h. The volume of settled particles was subtracted from 100 and dispersibility was expressed in percentage.
Gelation behaviour
Gelation behaviour of the flour was determined by evaluating the least gelation concentration and appearance of the resultant gel. Least gelation concentration was evaluated by the method described by Singh et al. [5]. 2–30% (w/v) of flour dispersions were made in distilled water. The suspension was vortex and heated in a boiling water bath in covered test tubes for 1 h, followed by cooling under running water. Test tubes were further cooled by keeping at 4 °C for an hour. Gelation was deemed when gelled suspension does not fall on inverting the tube whereas a slight flow of gelled suspension on inverting the tube was regarded as partial gelation. Gel appearance was recorded by visual assessment based on the firmness of gelled suspension as liquid, viscous, curdy, gel, firm gel, and solid gel.
Proximate composition
Crude protein (using the factor 6.25 × N), crude fat, crude fibre, and ash were evaluated using standard procedures [23].
Estimation of antinutrients
Tannins and phytic acid were evaluated by standard procedures using tannic acid and sodium phytate as standard respectively and results were expressed as mg/g [24]. For the estimation of trypsin inhibitor activity, the flour sample was extracted with 0.01 M phosphate buffer (pH 7.5). Trypsin inhibitor activity was estimated by enzyme assay using BAPNA (Nα-benzoyl-DL-Arginine-p-Nitroanilide) as a substrate [25] and expressed as trypsin inhibitor unit (TIU/mg protein). One trypsin inhibitor unit (TIU) refers to an increase of 0.01 absorbance units per 10 ml of the reaction mixture at 410 nm. Lectins were extracted using pre-cooled phosphate-buffered saline (pH 7.2). Lectin activity was determined using 2% suspension of trypsinised rabbit erythrocytes [26] and expressed as haemagglutinin unit (HU/g). One HU refers to as the reciprocal of the agglutination in the highest dilution.
Bioactive constituents
Total phenolic content [27], total flavonoids [28] and saponins [25] were estimated colorimetrically using standard procedures and results were expressed as gallic acid equivalent (GAE), quercetin equivalent (QE), and diosgenin equivalent (DE) mg/g respectively.
Antioxidant activity assays
DPPH∙ radical scavenging activity [28] and ABTS∙+ radical scavenging activity [29] was evaluated and expressed as trolox equivalent antioxidant capacity (μmol TE/100 g). Ferric-Ion Reducing Antioxidant Power (FRAP) was evaluated by the method of Thaipong et al. [29] and results were expressed as μmol TE/g. Reducing power was estimated as described by Gupta et al. [30] and expressed as ascorbic acid equivalent (mg AEE/g). Metal chelating activity was determined as per Chew et al. [31] and results were expressed as mmol EDTA equivalent/100 g.
Mineral profile
Macrominerals (Na, K, Ca, Mg & P) and microminerals (Fe, Cu, Mn & Zn) were estimated in unprocessed and germinated flours. One gram flour sample was digested using the mixture of nitric and perchloric acid (3:1) using microwave-assisted digestion. The volume of digested material was made to 50 mL using deionized water, followed by filtration and estimation using inductively coupled plasma mass spectrometry (X-Series2, Thermo Scientific). Results were expressed as mg Kg−1 [32].
Statistical analysis
The data obtained were analyzed statistically using SPSS software (Version 22.0, IBM Corporation) to determine statistical significance and expressed as mean ± standard deviation. ANOVA was performed and means were compared by post-hoc Tukey’s test. P-value < 0.05 was considered significant. Principle component analysis (PCA) was carried out using Statistica v.12 to understand the overall influence of germination on composition and techno-functional characteristics.
Results and discussion
Steeping behaviour
Hydration isotherms of lucerne seeds at different temperatures are shown in Fig. 1. Evaluation of steeping behaviour is crucial to assess the optimum time required for the soaking of the grains based on maximum hydration to allow the biological activation of seeds [33]. Initially, the water uptake was faster at all the temperatures; especially during the first 3 h of the steeping, followed by a gradual increase up to 8 h and no increase in the moisture content was observed after 8 h. Higher soaking temperature demonstrated better hydration ascribed to increased grain diffusivity [18]. However, no significant difference (p > 0.05) in the water uptake was observed for the seeds steeped at 28 and 32 °C after 7 h. Similar observations of increase in the water uptake were observed for barley and finger millet with the increase in the temperature of steeping [18, 21] Based on the steeping behaviour, 8 h soaking time was found to be optimum (Fig. 1).
Hydration isotherms of lucerne seeds at different temperatures. Values are expressed as mean and error bars represent standard deviation (n = 3). The means with different small superscripts (a, b, c) are significantly different at p < 0.05 for the effect of soaking temperature. The means with different capital superscripts (A–H) are significantly different at p < 0.05 for the effect of soaking time
Germination characteristics
Germination characteristics of lucerne seeds at different temperature-time regimes are shown in Table 1. Germination temperature is a crucial factor in the germination of seeds and optimum temperature favours germination by activation of metabolic processes as it affects the activity of enzymes required for the biological activation of grain [33]. Optimum temperature of germination refers to the temperature range where seeds exhibit highest germination rate and can vary depending upon seed species and quality [34]. High germination rate and capacity were observed in seeds germinated at 28 and 32 °C in comparison to 24 °C, however no significant difference (p > 0.05) was observed in seeds germinated at 28 and 32 °C. In addition, the germination capacity of seeds after 24 h of germination at 24 °C was markedly lower (39.20%) in comparison to seeds germinated at 28 and 32 °C that showed almost 95% germination capacity. The increase in the sprout length was observed with the increase in the temperature and time of germination. Heidari et al. [34] and Devi et al. [19] reported concomitant increase in the sprout length with the increase in the temperature and time of germination.
Germination losses
The total loss of dry matter due to germination was indicated by a concomitant decrease in the 1000 kernel weight of the seeds with an increase in the temperature and time of germination as a function of increased leaching, metabolic and vegetative loss (Table 2). The higher leaching loss at increased temperature can be ascribed to increased grain diffusivity [18]. Furthermore, the increase in the metabolic loss with the increase in time and temperature of germination was due to increase in the activity of the enzymes that in return dictated the vegetative loss as a function of sprout length. During the germination period of 72 h, the vegetative loss was found to be more as compared to the metabolic loss. Malleshi and Desikachar [21] documented a similar trend for the germination losses in malted barley. Based on the germination characteristics and germination losses, 28 °C was selected for the germination of the lucerne seeds due to good germination capacity and lesser germination losses.
ATR-FTIR spectra
ATR-FTIR spectras for ungerminated and germinated lucerne flours are given in Fig. 2. Amide I (C=O stretching) and amide II (N–H bending and C–N stretching) peak at 1600–1700 cm−1 and 1580–1480 cm−1 is associated with the secondary structure of proteins [35]. Major peak at 1635.1 and 1635.4 cm−1 in flours germinated for 24 and 48 h respectively represented no major conformational change in intermolecular β-sheets. However, the shift in the peak from 1635.7 to 1662.7 cm−1 after germination for 72 h represented major conformational change [35]. Small shift from 1544.3 cm−1 to 1543.2, 1538.4 and 1536.5 cm−1 after germination for 24, 48, and 72 h can be manifested with conformation changes in the α-helix. Furthermore, the peaks ranging from 3291.8 to 3275.5 cm−1 manifests with -OH stretching vibration and can be associated with the phenolic compounds.
Functional properties
The effect of germination on the functional properties of lucerne flour is presented in Table 3. Bulk density (BD) of the flour reduced (0.428 to 0.402 g/cm3) with progression in germination. Reduced bulk density is advantageous in the preparation of weaning foods [36]. Water absorption capacity (WAC) decreased after germination for 24 h due to the degradation of starch [5] whereas further germination increased the WAC due to the denaturation of protein that resulted in the exposure of polar side chains and peptide bonds [37]. However, no significant difference (p > 0.05) was observed after 48 and 72 h of germination. The dispersibility (D) of the flour improved after germination and maximum dispersibility of the flour was observed after 24 and 48 h of germination, followed by a slight reduction after 72 h of germination. Similarly, swelling capacity (SC) of the flour decreased after germination for 24 h, followed by an increase after 48 h and again a slight reduction after 72 h. Increase in the oil absorption capacity (OAC) was observed only after germination for 72 h. Aforesaid trends of WAC, D, SC, and OAC after 72 h germination can be ascribed to exposure of hydrophobic residues due to excessive denaturation of protein as observed in the FTIR spectra (Fig. 2). Elkhalifa and Bernhardt [36] documented a similar trend in the BD, WAC, and OAC of germinated sorghum flour. Swelling index is a function of lose particles of flour and exhibits the binding forces within the starch granules [22, 38]. Swelling index of the flour increased with the progression of the germination. Higher swelling index can be attributed to the weakening of the bond between starch and protein molecules due to germination. Water solubility index (WSI) decreased after germination for 48 h, followed by a further reduction in the WSI after germination for 72 h. Reduced WSI can be ascribed to the denaturation of water soluble albumin proteins. Furthermore, denatured proteins tend to entangle flour particles and reduce their leaching into the water. Leaching loss reduced up to germination for 48 h, followed by a slight increase due to amplified denaturation of protein during heating of flour paste that resulted in leaching of low molecular weight fractions of proteins.
Emulsification and foaming capacity of legume flours is majorly attributed to its storage proteins. These proteins exert surface activity by the formation of the viscoelastic film when an unfolded polypeptide chain interacts with the neighbouring molecules at air/water or oil/water interfaces [37]. Emulsification capacity and emulsion stability slightly decreased after germination and no significant (p > 0.05) variation was observed in flours germinated for different time durations. Foaming capacity (FC) of flour was significantly (p < 0.05) higher after germination and maximum FC (101%) was observed after germination for 24 h. Similar trend was observed for foam stability (Supplementary Fig. 1). Increase in the FC can be ascribed to easy unravelling and migration of partially denatured protein at the air-water interface [37]. Dobhal and Raghuvanshi [39] also observed a similar trend for the emulsification and foaming capacity of the germinated black soybean. The effect of germination was also evident on the foam appearance (Table 3). The bubble size of foam is proportional to the flexibility of the protein molecules [37]. Flour from ungerminated flour produced denser foam in contrast to germinated flours. Foam with very large bubbles was observed after germination for 24 h followed by gradual reduction. Initial phase of germination resulted in partial denaturation of protein and as the germination progressed, large polypeptide chains were broken down to smaller fractions and engendered smaller bubbles. Minimum value for gel consistency in deionised water was observed after a germination period of 48 h whereas ungerminated flour exhibited the lowest gel consistency value in acid. The increase in the gel consistency value in deionised water after germination period of 24 and 72 h can be ascribed to starch degradation and exposure of hydrophobic residues of protein respectively [5]. The disparity in the gel consistency under acidic conditions with a minimum value of gel consistency by ungerminated flour can be ascribed to the cumulative effect of gelation of starch as well as acid and heat mediated denaturation of proteins.
Gelation behaviour
The gel formation in lucerne flour is the cumulative function of gelatinization of starch and denaturation of protein and their relative interaction as a result of germination. Heat induced gelation of a protein involves the formation of a continuous three-dimensional network as a result of interaction of partially denatured proteins [37]. However, the presence of other macromolecules (e.g. starch) affects the gelation behaviour depending on the compatibility of the other macromolecule with proteins in mediating gel formation [37, 40]. Germinated flours exhibited significant reduction in the least gelation concentration (LGC) in comparison to ungerminated flour (Table 4). The least gelation concentration was in order; 0 h (20%) > 24 h (12%) > 48 h (8%) ≈ 72 (8%). High LGC of ungerminated flour can be ascribed to the presence of separate gel phase of protein and starch that resulted in competitive water binding by both the macromolecules. Shim and Mulvaney [40] also reported similar findings for corn starch/whey protein isolate mixed gels. The progression of germination manifests with the gradual degradation of starch and denaturation of proteins that resulted in better apolar interactions and consequently improved gelation. Furthermore, the formation of clotted particles at lower concentrations (2–6%) indicated the hydrophobic interaction of the proteins due to the apolar aggregation, and as the concentration increased there was the formation of single coagulum/gel. In addition, germination also discerned its effect on the opaqueness of the gel, and flours germinated for 48 and 72 h exhibited translucent gel from 8 to 14% and 8 to 10% flour concentration respectively. Elkhalifa and Bernhardt [36] also reported a reduction in the least gelation concentration of germinated sorghum flour (12%) in comparison to the ungerminated counterpart (18%).
Proximate composition
The proximate composition of unprocessed and germinated lucerne flour is presented in Supplementary Table 1. Germination exhibited a significant effect on the proximate composition of lucerne. The crude protein content of lucerne significantly (p < 0.05) increased from 34.50 to 41.23% over the germination period of 72 h. Increase in the protein content can be attributed to the loss in the dry weight due to the loss of carbohydrates and lipids in the respiration and germination process. Furthermore, the increase in the protein content can be attributed to the synthesis of amino acid during germination [41, 42]. Significant (p < 0.05) reduction was observed in the crude fat content of germinated lucerne flour (3.32%) in comparison to the unprocessed counterpart (7.13%). As aforesaid, fat gets hydrolyzed and oxidized during its utilization as a substrate for biochemical processes during germination and resulted in a significant decrease in the fat content as the germination progressed [41]. Similar trend of gradual reduction was also observed for the crude fibre and ash content. The reduction in the crude fibre can be ascribed to the activity of cell wall degrading enzymes and loss of seed coat during the removal of vegetative portion of the germinated seed. The reduction in the ash content can be partly due to the leaching of minerals during the process of steeping and partly as a result of the loss of mineral associated with the seed coat [43, 44]. Pandey et al. [45] documented a similar trend for the proximate composition after the malting of alfalfa seeds.
Antinutrients
Gradual decrease (36.05%) in the tannin content was observed with the progression of germination (Table 5). Pronounced decrease after 24 h can be ascribed to leaching of tannins in the steep water during soaking whereas a comparatively lower reduction afterward was due to oxidation of tannins during germination [46]. Maximum reduction (13.29%) in the phytic acid was observed after 48 h of germination due to the activity of endogenous phytase enzyme [7]. Higher reduction of tannin content (36.05%) in comparison to phytate (13.29%) was due to high concentration of phytates in the cotyledons of the seeds in contrast to tannins that are present in the seed coat [47]. Mittal et al. [8] also observed meagre reduction (3.46%) in phytic acid content after germination for 48 h in chickpeas. Furthermore, slight increase in the phytic acid content (10.04 to 10.34 mg/g) after germination period of 72 h in comparison to 48 h can be ascribed to the depletion of the endosperm portion of the seeds that resulted in an overall increase in the phytic acid content due to dominant phytic acid rich cotyledon fraction. Germination (72 h) resulted in a drastic reduction in the trypsin inhibitor activity (75.56%). Complete inactivation of lectin activity was observed after the germination period of 24 h. Germination resulted in the inactivation of lectin and trypsin inhibitor activity due to conformational changes in their native structures [48, 6].
Bioactive constituents and antioxidant activity
Germination resulted in a gradual reduction of total phenol content (65.35%) whereas total flavonoids were slightly higher after 48 h in comparison to 24 and 72 h, which showed no significant (p > 0.05) difference (Table 5). The reduction in total phenol and flavonoid content can be ascribed partly to leaching in steep water and partly to enzymatic degradation during germination [49]. However, an increase in the flavonoid content after germination for 48 h can be ascribed to the release of bound flavonoids due to germination. Gujral et al. [50] observed a similar decrease in the total phenol content of legumes after germination period of 12 and 24 h. Saponins showed an interesting trend of 17.55% reduction after 24 h (due to leaching of saponins in steep water) followed by an increase in saponin content (48 h) due to the biogenesis of saponins and their enhanced extraction from weakened cell structure [51]. However, further germination (72 h) decreased the saponin content by 5.34%. Guajardo-Flores et al. [52] observed a similar trend in the increase of saponin content in black beans after germination for 24 h, followed by a decrease in saponin concentration on further germination.
Different methods exhibited diversification in the antioxidant capacity of germinated lucerne based on the affinity of different antioxidant species formed during germination. DPPH radical scavenging activity showed a gradual increase (8.84%) with the progression of the germination whereas the maximum increase in the ABTS radical scavenging (10.51%) and metal chelating activity (14.64%) was observed after germination for 48 h. In addition, FRAP and reducing power of germinated lucerne flour was lower in comparison to ungerminated lucerne flour. The reduction in FRAP and reducing power was concomitant with the reduction in total phenol and flavonoids. Minimum reduction in FRAP (22.33%) and reducing power (15.66%) was observed after germinated for 48 h. The highest reduction of 34.40% FRAP and 30.19% reducing power was observed after germination for 72 h. Correlation of total phenol and flavonoids with antioxidant assays is highly specific to antioxidant species present in the food. Studies have demonstrated a positive correlation of total phenol and flavonoids with FRAP and reducing power assay in contrast to DPPH and ABTS radical scavenging activity [53, 54]. Furthermore, the increase in DPPH RSA, ABTS RSA, and metal chelating ability suggests the genesis and better extraction non-phenolic antioxidants after germination of seeds [55, 56]. Similar increase in DPPH RSA besides reduction in phenolic compounds was observed in germinated peas and beans [49].
Mineral profile
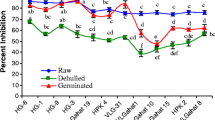
Germination exerted diverse effect on the mineral profile of lucerne flour (Fig. 3). Na content increased after germination for 24 h, followed by decrease in the Na. K content significantly (p < 0.05) decreased after germination. However, no significant (p < 0.05) variation was observed in K content of the flours germinated for 24, 48, and 72 h, speculating the loss of K primarily due to leaching in the steep water. Kajla et al. [57] reported a similar reduction in the Na and K in germinated flaxseeds. Ca content increased after germination for 24 h followed by a decrease and seeds germinated for 0, 48 and 72 h showed no significant (p > 0.05) variation in the Ca content. Mg and Mn content increased after germination with no significant (p > 0.05) variation between samples germinated for 24, 48, and 72 h. Fe content decreased after germination whereas Cu content only showed reduction after germination for 24 h. Minerals are differently localized in the legumes and trends for aforesaid minerals can be ascribed to the cumulative effect of solid loss, leaching, and remobilization of minerals during the germination. Sangronis and Machado [58] documented an increase in Ca and Mg, no variation in Cu, and a decrease in the Fe content after the germination of pigeon pea. An increase in the Ca, Mg, and Mn has been reported in germinated pearl millet [59]. P content decreased after germination for 24 h and increased after 72 h due to leaching and breakdown of phytic acid respectively [60]. Azeke et al. [60] reported an increase in P content of rice, maize, millet, sorghum, and wheat after germination. A pronounced increase in the Zn content was observed after germination. Zn is associated with the cotyledon fraction of legumes and usually interacts with proteins and other food constituents that ascribe to its low extraction [61, 62]. Germination modified the cellular structure of seeds and weakened the interaction of Zn with other food constituents and resulted in its better extractability [51]. However, the slight reduction in Zn after germination of 72 h in comparison to 24/48 h can be due to the complexation of Zn with the denatured protein. Atlaw et al. [63] observed a similar trend in the Zn content of germinated fenugreek seed flour.
Principal component analysis
PCA loading plot (Fig. 4a) elucidates the relationship between various characteristics of ungerminated and germinated lucerne flours. The characteristics in the same quadrant are positively correlated whereas the characteristics that lie in the opposite quadrants are negatively correlated. Total phenols (TP) and flavonoids (TF) were positively correlated with the Ferric Reducing Antioxidant Power (FRAP) and reducing power (RP) in contrast DPPH RSA, ABTS RSA, and metal chelating activity (MC). However, ABTS RSA and MC were positively correlated. PCA score plot (Fig. 3b) elucidated that germinated flours exhibited marked variation in their characteristics in comparison to ungerminated flour. Furthermore, germinated flours also depicted variations in their characteristics. Ungerminated flour was manifested with the high concentration of antinutrients (T, PA, TI, and LA) and bioactive constituents (TP and TF) and high LGC. Flours obtained after germination for 48 and 72 h showed a negative correlation with antinutrients. However, flour obtained after germination for 48 h showed better correlation with DPPH RSA, ABTS RSA, and MC along with an optimal balance of good functional properties (WAC, OAC, D, FC, and FS) and mineral concentration (particularly Zn, Mg, and Mn).
Principle component analysis (PCA) showing loading (a) and score plot (b) describing the relationship between the germination and characteristics of lucerne flour. WAC Water Absorption Capacity, WSI Water Solubility index, OAC Oil Absorption Capacity, SC Swelling Capacity, LL Leaching Loss, EC Emulsification Capacity, ES Emulsion Stability, FC Foaming Capacity, FS Foam Stability, D Dispersibility, LGC Least Gelation Concentration, T Tannins, PA Phytic acid, TI Trypsin Inhibitor Activity, LA Lectin Activity, TP Total Phenolic Content, TF Total Flavonoids, S Saponins, DPPH RSA DPPH Radical Scavenging Activity, ABTS RSA ABTS Radical Scavenging Activity, FRAP Ferric Reducing Antioxidant Power, RP Reducing Power, MC Metal Chelating Activity, Macrominerals (Na, K, Ca, Mg & P), Microminerals (Fe, Cu, Mn & Zn)
Conclusions
Germination temperature of 28 °C exhibited good germination capacity with lower associated germination losses. Germination at 28 °C for 48 h was an optimum time-temperature regime for the germination of lucrene to improve its functionality. Germination at aforesaid conditions exhibited an optimal balance of reduction of antinutritional factors, increase in the free radical scavenging (DPPH and ABTS), and metal chelating activity and improvement in the techno-functional properties. In view of the aforesaid improved techno-functionality and antioxidant capacity, germinated lucerne flour presents opportunities for utilization as a functional ingredient in food formulations.
References
M.D.S. López, P. Cortez, S. Rosales Martínez, M. Arellano Cárdenas, Cornejo, Mazón, in Antioxidants Properties and Effect of Processing Methods on Bioactive Compounds of Legumes in Grain Legumes. ed. by B.A.K. Goyal (InTech, Croatia, 2016), pp. 103–126
E. Lemmens, A.V. Moroni, J. Pagand, P. Heirbaut, A. Ritala, Y. Karlen, J.A. Delcour, Impact of cereal seed sprouting on its nutritional and technological properties: a critical review. Compr. Rev. Food Sci. Food Saf. 18(1), 305–328 (2019)
S. Mattucci, Sprouted grains add nutrition and new flavors to products. http://www.mintel.com (2015)
Ž Tarasevičienė, A. Viršilė, H. Danilčenko, P. Duchovskis, A. Paulauskienė, M. Gajewski, Effects of germination time on the antioxidant properties of edible seeds. CyTA-J. Food 17(1), 447–454 (2019)
A. Singh, S. Sharma, B. Singh, Influence of grain activation conditions on functional characteristics of brown rice flour. Food Sci. Technol. Int. 23(6), 500–512 (2017)
Y.S. Momonoki, M. Sugawara, T. Watanabe, Change in activity of soybean trypsin inhibitor by removal of C-terminal amino acid residues during seed germination. Plant Prod. Sci. 5(1), 51–57 (2002)
R. Greiner, U. Konietzny, Phytase for food application. Food Technol. Biotechnol. 44(2), 125–140 (2006)
R. Mittal, H.P.S. Nagi, P. Sharma, S. Sharma, Effect of processing on chemical composition and antinutritional factors in chickpea flour. J. Food Sci. Eng. 2(3), 180–186 (2012)
Y. Aguilera, M.F. Díaz, T. Jiménez, V. Benítez, T. Herrera, C. Cuadrado, M.A. Martín-Cabrejas, Changes in nonnutritional factors and antioxidant activity during germination of nonconventional legumes. J. Agric. Food Chem. 61(34), 8120–8125 (2013)
K.C. Pandey, A.K. Roy, Forage Crops Varieties. (IGFRI, Jhansi, 2011), pp. 58–59
J.S. Mahal, S. Kaur, Package of practices for crops of Punjab, Rabi 2020–21. Punjab Agric. Univ. 37(2), 74 (2020)
G. Zinca, C. Vizireanu, Impact of germination on phenolic compounds content and antioxidant activity of alfalfa seeds (Medicago sativa L.). J. Agroaliment. Process. Technol. 19(1), 105–110 (2013)
G. Giuberti, G. Rocchetti, S. Sigolo, P. Fortunati, L. Lucini, A. Gallo, Exploitation of alfalfa seed (Medicago sativa L.) flour into gluten-free rice cookies: nutritional, antioxidant and quality characteristics. Food Chem. 239, 679–687 (2018)
M. Márton, Z.S. Mándoki, J. Csapo, Evaluation of biological value of sprouts-I. Fat content, fatty acid composition. Acta Univ. Sapientiae Aliment. 3, 53–65 (2010)
J. Bhojak, M. Sharma, D. Sharma, E.T. McCarthy, V.J. Savin, R. Kamal, Determinants of antioxidant effects of alfalfa seeds. FASEB J. 20, A1022 (2006)
L. Cornara, J. Xiao, B. Burlando, Therapeutic potential of temperate forage legumes: a review. Crit. Rev. Food Sci. Nutr. 56(sup1), S149–S161 (2016)
J. Mölgaard, H. Von Schenck, A.G. Olsson, Alfalfa seeds lower low density lipoprotein cholesterol and apolipoprotein B concentrations in patients with type II hyperlipoproteinemia. Atherosclerosis 65(1–2), 173–179 (1987)
F.D. Montanuci, L.M.D.M. Jorge, R.M.M. Jorge, Kinetic, thermodynamic properties, and optimization of barley hydration. Food Sci. Technol. 33(4), 690–698 (2013)
C.B. Devi, A. Kushwaha, A. Kumar, Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). J. of Food Sci Technol. 52(10), 6821–6827 (2015)
C. Pinzino, A. Capocchi, L. Galleschi, F. Saviozzi, B. Nanni, M. Zandomeneghi, Aging, free radicals, and antioxidants in wheat seeds. J. Agric. Food Chem. 47(4), 1333–1339 (1999)
N.G. Malleshi, H.S.R. Desikachar, Influence of malting conditions on quality of finger millet malt. J. Inst. Brew. 92(1), 81–83 (1986)
O.O. Awolu, O.V. Oyebanji, M.A. Sodipo, Optimization of proximate composition and functional properties of composite flours consisting wheat, cocoyam (Colocasia esculenta) and bambara groundnut (Vigna subterranea). Int. Food Res. J. 24(1), 268 (2017)
A.A.C.C., Approved Methods of the American Association of Cereal Chemists, 10th Ed. (A.A.C.C., Washington, D.C. 2000)
P. Sharma, A. Kaur, S. Kaur, Nutritional quality of flours from guar bean (Cyamopsis tetragonoloba) varieties as affected by different processing methods. J. Food Sci. Technol. 54(7), 1866–1872 (2017)
S. Kaur, B.N. Dar, S. Pathania, S. Sharma, Reduction of antinutritional factors in cereal brans for product development. J. Food Process. Pres. 39(3), 215–224 (2015)
Y.W. Luo, W.H. Xie, Effect of different processing methods on certain antinutritional factors and protein digestibility in green and white faba bean (Vicia faba L.). CyTA-J. Food 11(1), 43–49 (2013)
F.P. Flores, R.K. Singh, W.L. Kerr, R.B. Pegg, F. Kong, Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 153, 272–278 (2014)
M. Kiranmai, C.M. Kumar, I. Mohammed, Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Res. J. Pharma. Bio. Chem. Sci. 2(3), 254–261 (2011)
K. Thaipong, U. Boonprakob, K. Crosby, L. Cisneros-Zevallos, D.H. Byrne, Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 19(6–7), 669–675 (2006)
A. Gupta, M. Naraniwal, V. Kothari, Modern extraction methods for preparation of bioactive plant extracts. Int. J. App. Nat. Sci. 1(1), 8–26 (2012)
Y.L. Chew, J.K. Goh, Y.Y. Lim, Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 116(1), 13–18 (2009)
B. Kaur, B. Singh, N. Kaur, D. Singh, Phytoremediation of cadmium-contaminated soil through multipurpose tree species. Agrofor. Syst. 92(2), 473–483 (2018)
A.S. Ali, A.A. Elozeiri, Metabolic processes during seed germination, in Advances in Seed Biology. ed. by B.J.C. Jimenez-Lopez (InTech, Croatia, 2017), pp. 141–166
Z. Heidari, B. Kamkar, J. Masoud, Sinaky, Influence of temperature on seed germination response of fennel. Adv. Plants Agric. Res. 1(5), 00032 (2014)
J. Kong, S. Yu, Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 39(8), 549–559 (2007)
A.E.O. Elkhalifa, R. Bernhardt, Influence of grain germination on functional properties of sorghum flour. Food Chem. 121(2), 387–392 (2010)
P. Sahni, B. Singh, S. Sharma, Functionality of proteins and its interventions in food. IFI Mag. 37(3), 41–52 (2018)
L.O. Sanni, A.A. Adebowale, W. Awoyale, G.O. Fetuga, Quality of gari (roasted cassava mash) in Lagos State, Nigeria. Niger. Food J. 26(2), 125–134 (2008)
N. Dobhal, R.S. Raghuvanshi, Physical characteristics and effect of germination on functional properties of black soybean (Glycine max). Asian J. Dairy Food Res. 37(1), 56–60 (2018)
J. Shim, S.J. Mulvaney, Effect of heating temperature, pH, concentration and starch/whey protein ratio on the viscoelastic properties of corn starch/whey protein mixed gels. J Sci. Food Agric. 81(8), 706–717 (2001)
R. Jan, D.C. Saxena, S. Singh, Physico-chemical, textural, sensory and antioxidant characteristics of gluten–Free cookies made from raw and germinated Chenopodium (Chenopodium album) flour. LWT-Food Sci. Technol. 71, 281–287 (2016)
M.P. Ongol, E. Niyonzima, I. Gisanura, H. Vasanthakaalam, Effect of germination and fermentation on nutrients in maize flour. Pakistan J. Food Sci. 23(4), 183–188 (2013)
R.A. Ghavidel, J. Prakash, Effect of germination and dehulling on functional properties of legume flours. J. Sci. Food Agric. 86(8), 1189–1195 (2006)
N.L. Tatsadjieu, F.X. Etoa, C.M.F. Mbofung, Drying kinetics, physico-chemical and nutritional characteristics of “Kindimu”, a fermented milk-based-sorghum-flour. J. Food Technol. Africa 9(1), 17–22 (2004)
S. Pandey, N. Chaturvedi, D. Gupta, Effect of malting on nutritional profile of alfalfa seeds and development of value added fermented products. Int. J. Ferment. Foods 8(2), 73–78 (2019)
F.H.M.G. Savelkoul, A.F.B. Van der Poel, S. Tamminga, The presence and inactivation of trypsin inhibitors, tannins, lectins and amylase inhibitors in legume seeds during germination. A review. Plant Foods Hum. Nutr. 42(1), 71–85 (1992)
R.K. Gupta, S.S. Gangoliya, N.K. Singh, Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci Technol. 52(2), 676–684 (2015)
F.H.M.G. Savelkoul, S. Tamminga, P.P.A.M. Leenaars, J. Schering, D.W. Ter Maat, The degradation of lectins, phaseolin and trypsin inhibitors during germination of white kidney beans, Phaseolus vulgaris L. Plant Foods Hum. Nutr. 45(3), 213–222 (1994)
M.L. López-Amorós, T. Hernández, I. Estrella, Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compost. Anal. 19(4), 277–283 (2006)
H.S. Gujral, M. Angurala, P. Sharma, J. Singh, Phenolic content and antioxidant activity of germinated and cooked pulses. Int. J. Food Prop. 14(6), 1366–1374 (2011)
Y. Kurosawa, H. Takahara, M. Shiraiwa, UDP-glucuronic acid: soyasapogenol glucuronosyltransferase involved in saponin biosynthesis in germinating soybean seeds. Planta 215(4), 620–629 (2002)
D. Guajardo-Flores, M. García-Patiño, D. Serna-Guerrero, J.A. Gutiérrez-Uribe, S.O. Serna-Saldívar, Characterization and quantification of saponins and flavonoids in sprouts, seed coats and cotyledons of germinated black beans. Food Chem. 134(3), 1312–1319 (2012)
A.A. Al-Laith, J. Alkhuzai, A. Freije, Assessment of antioxidant activities of three wild medicinal plants from Bahrain. Arab. J. Chem. 12(8), 2365–2371 (2019)
C. Liu, Y. Zhao, X. Li, J. Jia, Y. Chen, Z. Hua, Antioxidant capacities and main reducing substance contents in 110 fruits and vegetables eaten in China. Food Nutr. Sci. 5, 293–307 (2014)
J.V.V. Ribeiro, K.A. Batista, K.F. Fernandes, Potential iron and copper chelating activity of naturally occurring peptides and protein fractions from common bean (Phaseolus Vulgaris). Int. J. Biochem. Physiol. 4, 000161 (2019)
L. Plaza, B. de Ancos, P.M. Cano, Nutritional and health-related compounds in sprouts and seeds of soybean (Glycine max), wheat (Triticum aestivum. L) and alfalfa (Medicago sativa) treated by a new drying method. Eur. Food Res. Technol. 216(2), 138–144 (2003)
P. Kajla, A. Sharma, D.R. Sood, Effect of germination on proximate principles, minerals and anti nutrients of flaxseeds. Asian J. Dairy Food Res. 36(1), 52–57 (2017)
E. Sangronis, C.J. Machado, Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. LWT-Food Sci. Technol. 40(1), 116–120 (2007)
M.H. Badau, I. Nkama, I.A. Jideani, Phytic acid content and hydrochloric acid extractability of minerals in pearl millet as affected by germination time and cultivar. Food Chem. 92(3), 425–435 (2005)
M.A. Azeke, S.J. Egielewa, M.U. Eigbogbo, I.G. Ihimire, Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). J. Food Sci Technol. 48(6), 724–729 (2011)
T.L.L. Cambraia, R.L.F. Fontes, L. Vergütz, R.F. Vieira, J.C.L. Neves, P.S. Corrêa Netto, R.F.N. Dias (2019) Agronomic biofortification of common bean grain with zinc. Pesquisa Agropecuária Brasileira. https://doi.org/10.1590/s1678-3921.pab2019.v54.01003
S. Hemalatha, K. Platel, K. Srinivasan, Influence of heat processing on the bioaccessibility of zinc and iron from cereals and pulses consumed in India. J. Trace Elements Med. Bio. 21(1), 1–7 (2007)
T.K. Atlaw, J.Y. Kumar, N. Satheesh, Effect of germination on nutritional composition and functional properties of fenugreek (Trigonella foenum-graecum Linn) seed flour. Int. J. Nutr. Food Sci. 7(3), 110–115 (2018)
Acknowledgements
This work was supported by INSPIRE Fellowship (Sanction Order No. IF170737) by Department of Science and Technology, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, S., Sahni, P. Germination behaviour, techno-functional characteristics, antinutrients, antioxidant activity and mineral profile of lucerne as influenced by germination regimes. Food Measure 15, 1796–1809 (2021). https://doi.org/10.1007/s11694-020-00777-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00777-7