Abstract
Microencapsulation is employed to protect bioactive ingredients in foods and is also used for their controlled release at targeted sites. The objectives of this study were to examine the bio active properties and stability of microcapsules containing nutmeg oleoresin encapsulated with different ratios (2:1, 1:1 and 1:2) of gum arabic and sorghum starch (native and OSA modified) prepared by freeze-drying. The properties of nutmeg microcapsules, including oxidation stability, antioxidant activity, total phenolic content and total flavonoid content were evaluated after sixty days storage trial. The finding revealed that freeze drying method had a spectacular effect on the retention of bioactive compounds during storage that consequently improved the storage stability of the all nutmeg encapsulated powders. The sample composed of 1:2 ratio of gum arabic with OSA starch resulted to have lowest peroxide value after 60 days of storage. Furthermore, the microcapsules were also observed through Fourier Transform Infrared spectroscopy and Scanning electron microscopy for advance analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutmeg oleoresin (Myristica fragans Houtt) is derived from its powder by solvent extraction. It contains essential oils and bioactive compounds that act as ideal to utilized as flavoring material, also have carminative, antifungal, antidysentric, stimulant, narcotic, and anti-inflammatory activities and natural fixatives which tend to restrict volatilization of aromatic compounds more importantly in heat processed food [1, 2]. Due to the liquid nature of oleoresin and its bioactive extracts, nutmeg oleoresin is liable to high temperature, air and sunlight therefore, having a very short life span if not stored correctly. Furthermore, free radical and reactive oxygen species are responsible for lipid oxidation, which is the major chemical change involved to get inferior quality food products during processing, transportation, and storage [3]. In order to restrain these unwanted changes, encapsulation aids in order to extend the shelf life.
Encapsulation aims to protect the sensitive material components, reduce the loss of nutrients and change the components of food liquid materials to a solid form that is easier to be handled [4]. Microencapsulation techniques are typically sustained by drying processes like spray drying, fluid-bed coating and freeze drying etc. Among different encapsulated technologies, the technique of freeze drying is very appropriate for the food materials that are susceptible to heat as this process conserves the preliminary functional characteristics of those compounds. However, the retention capacity highly depends on the drying technique and the material used as covering material. Therefore, it is very much important to choose appropriate shell materials (their combinations and proportion) and procedure to encapsulate the core material in order to get high encapsulation efficiency and maximize the retention of bioactive compounds within the wall material matrix [5].
Natural exudation of tree Acacia creates arabic gum which is hydrocolloid by nature. There has been considerable enticement in recent years to replaces gum arabic totally or partially because gum arabic is produces in places having political turbulence and unpredictable climatical conditions which can break off the supply of the product [6]. Among protein and carbohydrate polymers, starches from different sources have the advantage of better interest due to its outstanding usefulness as controller and texture stabilizers in food sectors [7].
Sorghum is a very useful and vital cereal grain that ranked fifth worldwide owing to its drought resistance, little operating expense and constituting 68–70% of starch as the major component of grain sorghum. Starches and its derivatives like (modified starches or dextrins), have numerous important physicochemical, thermal, and rheological properties, widely used in the food manufacturing industries in order to conserve and protect volatile compounds. They can act as carriers for fat replacer, aroma encapsulation, and also emulsion stabilizers [8]. By different chemical alterations and adaptations of starches, process and storage permanence may be increased [9]. Furthermore, it is practicable to modify the basic structure of starch by inserting hydrophobic side chains in a original backbone of hydrophilic starch molecule. By this mode, the stabilization of dispersion enhances because of the lipophilic nature of starch that adsorb both water and oil and that modified starch termed as octenyl-succinate starch, or OSA starch, which is formed by esterification process of starch and anhydrous octenyl succinic acid when given alkaline conditions. The 3% OSA modification has been permitted by Food and Drug Administration (FDA) as food additive in the European Union. OSA starch has now been used fruitfully for several years as wall material in microencapsulation and for beverage emulsions stability. The valuable ingredients are most favorably protected when it is used in encapsulation processes and preserved them against oxidation [10].
The objective of this research study was to evaluate the stability and retention of bioactive compounds within freeze dried nutmeg capsules (composed of gum arabic in combination with native and OSA modified starches in different proportions) during sixty days of storage. The volatile oil compositional analysis of nutmeg oil was primarily evaluated through gas chromatography–mass spectroscopic analysis and gelatinization properties of starches were analyzed by Differential Scanning calorimetry. The feed emulsions were analyzed by light microscopy to observe their stability while the nutmeg freeze dried microcapsules were characterized for antioxidant, total phenolic content, total flavonoid content, internal morphology and oxidative stability.
Material and methods
Isolation of starch from sorghum grains was done by using the procedure illustrated by Mehboob, et al. [11]. Nutmeg oleoresin was extracted by ethanol from nutmeg seeds according to the process explained by Assagaf et al. [12]. Octenyl succinic anhydride modification of sorghum starch was done by the detailed procedure of Bhosale and Singhal [13] followed by the storage at 4 °C before further use. Gum arabic, aluminium chloride, chloroform, methanol, n-propanol, ethyl ether, sodium metabisulphite, ethanol, sodium hydroxide, hydrochloric acid, 2, 2-bipyridyl, 2, 2-diphenyl-1-picryhydrazyl radical (DPPH) and n-hexane utilized were of analytical grade and used without further purification.
Gas chromatography/mass spectrometric analysis of nutmeg oleoresin
GC–MS analyses of the essential oils were performed according to the method described by Meiling et al. [14] with modifications using Shimadzu gas chromatograph (GC-2014) system. Compounds were break up and separated on a ZB MS capillary column (100.0 m and 0.25 mm ID and Film thickness 0.50 μm). A sample of 1.0 mL at a pressure of 361.1 kPa was inserted in the split mode having split ratio of 10. Ionization energy of 70 eV was used in GC–MS detection in an electron ionization system. A flow rate of 1.5 mL/min was adjusted for Helium applied as gas carrier. The temperature adjustments of column oven were same as in GC analysis. The temperatures for MS transfer line were set at 220 °C and 290 °C, and mass range was 50–550 m/z.
DSC of starch samples
To study the gelatinization and retrogradation of sorghum starch (native and OSA modified), differential scanning calorimeter (DSC Q10, TA Instruments, USA) was performed by the method of Ali and Hasnain [15]. Briefly, 2 mg starch (dry basis) was measured in Tzero aluminium pans followed by addition of 6 mL deionize distilled water using a microliter syringe. The pan was then sealed using a T-zero hermetic lid and was then allowed to stand for 1 h at room temperature before heating. The sealed pan was then heated from 30 to 110 °C at a heating rate of 10 °C/min in DSC (Q10, TA Instruments, USA). After scanning, the pans were stored at 4 °C and rescanned after 14 days of cold storage in order to study the effect of modifications. Onset gelatinization temperature (To), peak gelatinization temperature (Tp), conclusion gelatinization temperature (Tc), enthalpy of gelatinization (Hgel) and enthalpy of retrogradation (Hret) were directly calculated using TA Universal Analysis software. Percent retrogradation (%R) was calculated by dividing enthalpy of retrogradation with enthalpy of gelatinization.
Composition of wall material
The wall material concentration was 30% (w/w) with respect to total solids and nutmeg oleoresin load was 10% (w/w) with respect to wall material mass. The wall materials used for freeze dried microencapsulation of nutmeg oleoresin were gum arabic, native sorghum starch and OSA modified sorghum starch in three different ratios 2:1, 1:1 and 1:2.
Microscopic examination of emulsions
The emulsions were examined visually with a light microscope (MBL-2000, KRUSS, Germany) at 10 × magnification.
Freeze drying process
The freeze dried microcapsules were prepared by freezing emulsions for 24 h at − 30 °C and then freeze dried at − 70 °C in open trays using pilot scale lyophilization system for 48 h. After drying, zip lock, sealed polyethylene bags were used to keep the freeze dried samples. The samples were stored in the desiccators having silica gel beads for further analyses. All tests were performed in triplicate.
Extraction of bioactive compound
For quantifying the antioxidant activity and phenolic compounds in freeze dried nutmeg microcapsules, the coating of the microcapsules were completely damaged according to the procedure described by Chatterjee et al. [16].
DPPH radical scavenging activity
The DPPH radical scavenging activity of nutmeg microcapsules were determined by the method of Marinova and Batchvarov [17].
Estimation of total phenolic content
Total phenolic content of freeze dried nutmeg powder was determined according to the method described by Wojdyło et al. [18]. About 100 µL extract was taken followed by adding 0.2 mL Folin- Ciocalteau reagent with 2 mL of distilled water. Incubated the extract for 3 min. Add 1 mL of 20% sodium carbonate solution. Again incubate the solution for about an hour and take absorption at 765 nm.
Total flavonoid content
The total flavonoid content was determined by the method of Choudhary et al. [19] using aluminium chloride colorimetric method. About 0.25 mL of bioactive extract was taken and add 1.25 mL of distilled water with 75µL of sodium nitrate solution. After 6 min, 150µL aluminium chloride solution was added. 500 mL of sodium hydroxide solution (1 M) was poured by making volume up to 2.5 mL and absorbance was taken at 510 nm. The calibration curve was prepared using Quercetin and expressed as mg/mL.
Internal morphology of microcapsules
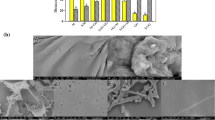
Morphological properties of nutmeg microcapsules composed with different ratios of gum arabic and sorghum starch (native and OSA modified) were observed by scanning electron microscopy (JSM, 6380A, Jeol Japan) operating at an accelerating voltage of 8 kV. The powdered sample was fixed on a stub, making cut with the help of blade and coated with the gold layer in a vacuum evaporator. The images of all microcapsules were studied at 5000 × magnifications.
Oxidative stability of oleoresin in microcapsules
In order to find out the oxidative stability of nutmeg encapsulated oil, the freeze dried powder samples as well as pure nutmeg oleoresin were stored at 4 °C and 25 °C in air tight plastic bags for 8 weeks. Samples were inspected on first week and again at eighth week with respect to lipid oxidation by evaluating the peroxide index of encapsulated oil. To determine the Peroxide value, the oil was extracted by the procedure described by Partanen et al. [20].
Lipid oxidation
Peroxide values were observed by standard method of IDF standard method as described by Frascareli et al. [21].
FTIR spectral analysis of freeze dried microcapsules
The Fourier transform infra red spectroscopy of nutmeg oil and freeze dried microcapsules were qualitatively observed using FTIR (Shimadzu, IR Prestige-21, Japan) by KBr Disc technique. The samples were oven dried at 70 °C for 24 h before analysis to prevent the hindrance due to presence of moisture. The spectra were recorded from 4000 to 600 cm−1 (mid infra red range) at the resolution of 8 cm−1.
Statistical analysis
All measurements were carried out in triplicate. The results were statistically analyzed using software, Statistical package for social sciences (SPSS version 17.0 USA). Analysis of variance (ANOVA) was performed using the Duncan’s multiple range tests to compare treatments means. Significance level was defined as p < 0.05. Whereas for comparing significant difference at p < 0.05 for two samples (n = 2), paired type t-test was used employing SPSS (version 20, SPSS, Inc, USA).
Result and discussion
Thermal analysis of native and OSA-modified starch
DSC results of sorghum starch (native and OSA modified) are given in Table 1. The values of To, Tp and Tc for OSA modified sorghum starch were found to be lower as compared to native sorghum starch. The gelatinization enthalpy for native sorghum starch was found to be 14.2 J/g while OSA starch resulted to have lower gelatinization enthalpy (10.3 J/g). To, Tp, and Tc values of both native and OSA sorghum starches were found to be analogous to the prior report [22]. In general, gelatinization temperatures in DSC thermograms are associated with the extent of perfection of crystallites within the starch granule while gelatinization enthalpy is related to the degree of crystallinity which indicates the amount of energy required to disrupt the structure of starch granule [23]. The lower values of gelatinization temperatures and enthalpy in OSA sorghum starch were due to the introduction of hydrophobic alkyl groups in starch structure that weaken the hydrogen bonding and swell the starch at low temperature [24].
The attachment of OSA group in the backbone of native starch makes the structure more flexible, thus contributes to the reduction of gelatinization temperature of OSA modified structure [25]. The variations in gelatinization temperatures of OSA starches depend upon the starch base, the difference in molecular alignment of starch origin and degree of substituted octenyl group. Moreover, the cleavage of existing hydrogen bonds in the backbone of starch granule supposed to involved due to low enthalpy values and making of new bonds involving water to give disrupted structure with increased entropy [26]. The postponement in the retrogradation behavior of sorghum starch was positively affected by OSA modification. Retrogradation percentage after two weeks of cold storage was found to be 25.4% and 26.2% for native sorghum and OSA sorghum starch respectively representing decline in long term retrogradation due to recrystallization of amylopectin chain as the insertion of octenyl succinic groups that disrupted the close rearrangement of starch chains [11].
GC–MS analysis of nutmeg oil
The volatile oil composition of the nutmeg was analyzed using GC–MS technique (Table 2). A total of twenty three different components peaks Fig. 1, with different retention times, were eluted from the GC column and were further analyzed with an electron impact MS voyager detector. Recognition of element was finished on the basis of their retention time and mass spectra library search. The relative amount of individual components was calculated based on GC peak area. GC–MS analysis of the nutmeg oil has led to the classification and quantification of 23 different compounds representing 99.92% of the total extract (Table 2). The major compounds were alpha-terpinolene (44.51%), β-pinene (11.29%), γ-terpene (9.56%), α-longipinene (6.54%) and safrole (6.60%). In addition, the nutmeg oleoresin was also contained considerable amounts of alpha- terpeneol, alpha- pinene, carane, myristicin and limonene. Such changes in the chemical composition of essential oil across countries might be endorsed to the varied agro-climatic (climatical, seasonal, geographical) conditions of the regions, maturity level and adaptive metabolism of plants [27].
Light microscopic images (at 100 × magnification) of emulsions of nutmeg oil in different wall material solutions containing a GA: NAT (1:2), b GA: NAT (1:1), c GA: NAT (1:2) and d GA: OSA (1:2); e GA: OSA (1:1) and f GA:OSA (1:2). GA gum Arabic, NAT native sorghum starch, OSA octenyl succinic anhydride
Microscopic analysis of emulsions
Light microscopy examinations of feed emulsions are shown in Fig. 1. All the feed samples showed stable and homogenized emulsions. It verifies that emulsion oil droplets were homogeneously and equally disseminated in the aqueous phase with no flocculation and separation in all cases. Due to modification, the emulsion batches of OSA starch showed dense and compact structures as compared to native starch batches. Similar clumping of OSA starch was more observed in Fig. 1. The stability of emulsions was not affected by different loads of gum arabic with native and modified sorghum starch as oil droplets were not observed in any batch. The stability of emulsion in feed solution comprising of gum arabic is due to its hydrophobic peptide chain and hydrophilic arabinogalactan blocks that formed an interfacial membrane to provide stability against droplet aggregation [28]. The stability of emulsion in the feed solution comprising of OSA starch is due to its anionic and non-polar side group that avoids the aggregation of droplets through steric repulsion.
Antioxidant property
As shown in Table 3, a significant difference was found in the values of antioxidant activity of microcapsules composed of different wall material ratios. The percentages for antioxidant activity ranged from 53.52 to 80.96% in samples composed of gum arabic and native starch. The samples comprised of OSA starch with gum arabic found to have DPPH radical scavenging activity in a range of 56.46–79.32%. The highest antioxidant activity was found in the samples composed of gum arabic with native and OSA modified starches in a ratio of 1:2 i.e., 80.96% and 79.32%. The higher values of DPPH radical scavenging activity of nutmeg microcapsules are due to the absorption of lignans and their glycosides that produce biologically active compounds having catechol structure that commenced high antioxidant capability in nutmeg. The addition of arabic gum produced higher retention of reducing power, polyphenol and radical scavenging propolis compounds [29].
Gum arabic, which is a charged molecule, could have a strong association with the more polar antioxidant compounds by providing a protective effect when they subjected to poor environmental hazards. There were slightly decreased in antioxidant values of freeze dried microcapsules during 60 days of storage. The reduction of antioxidant activity during storage is associated with a reduction in phenolic compounds. Usually in plants, in the very beginning, secondary metabolism produced phenolic antioxidants. The redox characteristics and chemical structure (i.e. number and position of hydroxyl group) which participated in scavenging free radical activity, chelating transitional metals and inhibiting lipoxygenase are main features on which their antioxidant activity depends [30].
Total phenolic and flavonoid content
In Table 4, Regarding the total phenolic content, the values were significantly different for all microencapsulated powders comprised of different ratios of gum arabic and sorghum starch (native and OSA modified). The retention percentages ranged from 0.74 to 1.17 GAE mg/L for all the samples. The freeze-dried treatment containing gum arabic with native sorghum starch in 2:1 ratio presented the highest retention of phenolic compounds. The results showed that the samples comprising of gum arabic with native starch in a ratio 2:1 and 1:2 found to have high phenolic content values as compared to microcapsules comprising of OSA starch with gum arabic. It was reported by Laine et al. [31] that freeze dried encapsulates were able to stable during long term storage conditions. The type of encapsulating shell material and core to coating fractions are the most fundamental variable for the polyphenols encapsulation. The sample composed of 1:1 ratio gum arabic with native starch resulted to have lower phenolic contents among all the samples. Phenolic losses during the storage can occur due to a number of reasons such as enzymatic or non-enzymatic oxidation, hydrolysis or polymerization with other molecules etc.[32]. These changes may be induced by different factors including the chemical structure and dispersive matrix of phenolic compounds, physicochemical properties of microcapsules, processing and storage conditions of food product. After 60 days of storage, all the microcapsules showed better retention of phenolic content. The reduction in the phenolic compound during storage also associated with the reduction of DPPH scavenging activity of microcapsules.
According to Table 4, total flavonoid content of nutmeg microcapsules ranged from 891.8 to 1475.7 QE mg/mL. All samples showed high retention of flavonoid content. The highest flavonoid content was observed in samples comprised of gum arabic with sorghum starch (native and OSA modified) in a ratio of 1:2. The same samples also found to have the highest antioxidant activity. After 60 days of storage, better retention of flavonoid content was observed. The modified starch, when used as an encapsulating agent of bioactive compounds, contributed to delay their release in microcapsule formulations. They may assume to increase the gastrointestinal transit time, acting as a carrier and thus boost the intestinal absorption. These experiments show that OSA modified capsule could also act as a potential carrier of compounds with potential health benefits [33].
A minor decrease in flavonoid content was observed in all the samples during 60 days of storage. The flavonoid retention values after 60 days of storage were in a range of 794.97–1434.87 QE mg/mL. Ballesteros et al. [34] reported that oxygen, light and moisture could cause the oxidation and degradation of flavonoids, owing to the existence of unsaturated bonds in their molecular structures. In the lyophilization process, the crushing of the freeze-dried product may result in deterioration of compounds, since the product’s disclosure may provoke the occurrence of oxidation reactions [35].
Internal morphology of microcapsules
Figure 2, represents the internal SEM microstructures of nutmeg microcapsules comprised of different wall material ratios of gum arabic in combination with native and OSA modified sorghum starch. The SEM images confirmed that the internal cut surfaces of nutmeg microcapsules were perforated while some vacant spaces were also observed. The external surface of the freeze-dried matrix was smooth, free of dents and shrinkage to restrict the entrapped molecules from exposure to heat and oxygen. Similar morphological characteristics of freeze dried limonene microcapsules were also found by Kaushik and Roos [36] by using gum arabic- sucrose- gelatin system. According to Aguilera and Stanley [37], the structural rigidity and stiffness provided by the frozen surface where the sublimation takes place, and the lack of water in the liquid state results in a porous structure with no shrinkage, which is the main quality characteristics of freeze-dried microcapsules.
Peroxide value
The peroxide values for encapsulated nutmeg oil were measured under storage conditions at 25 ± 1 °C for 60 days in order to check lipid oxidation in microcapsules (Fig. 3). Peroxide values for the microcapsules composed of gum arabic with native sorghum starch GA: NAT (2:1), GA:NAT (1:1) and GA:NAT (1:2) were found to be 0.0012, 0.0459 and 0.0131 meqv/Kg respectively at ambient temperature. The microcapsules comprised of OSA starch with gum arabic GA: OSA (2:1), GA: OSA (1:1) and GA: OSA (1:2) were found to have peroxide values 0.0257, 0.0151and 0.0049 meqv/Kg for freshly prepared microcapsules. After 60 days of storage, peroxide values were slightly increased except in the case of GA: OSA (1:2). The peroxide values for microcapsules GA:NAT (2:1), GA:NAT (1:1), GA:NAT (1:2), GA: OSA (2:1), GA:OSA (1:1) and GA:OSA (1:2) were found to be 0.0265, 0.0761, 0.0302, 0.0457, 0.0403 and 0.0019 meqv/Kg respectively. Peroxide value measures the primary products of oxidation (hydroperoxides) of the reaction between oxygen and unsaturated fatty acids. All samples showed stability of nutmeg oleoresin during 60 days of storage within the microcapsules composed of gum arabic with native and modified OSA starch in different ratios. Peroxide values lower than 5 mEqv/Kg are at a low oxidation state and between 5 and 10 mEqv/Kg are at average oxidation state [38]. Oxygen is the major concern in the initiation of primary oxidation products and controlling these reactions is important to avoid off flavor formation in foods. In bulk fats and oils, the mechanism of oxidations is well known as compared to the dispersed liquid phase which is complicated due to different compositional and structural behavior. Numerous factors like porosity of wall material, biopolymer composition, moisture content and glass transition temperature of biopolymers which affected the oxygen permeability of the wall matrix and determined the oxidative stability of the core material. The effectiveness of encapsulation at inhibiting oxidation varies tremendously in the literature depending on the type and sensitivity of the core material, properties of the wall matrix, and the encapsulation process. Minemoto and coworkers compared freeze-drying with hot-air drying at 50 °C, finding freeze-dried samples more resistant to oxidation even though they showed lower microencapsulation efficiency [39].
FTIR analysis
FTIR analysis of microcapsules provided information about the chemical bond and structural changes in the core material and shell material after encapsulation. Figure 4 shows the Fourier transform infra red spectra of both nutmeg oleoresin and freeze dried nutmeg microcapsules. Figure 4a, shows the FTIR spectra of microcapsules made by mixtures of gum arabic and native sorghum starch while 4b shows FTIR spectra of microcapsules comprised of gum arabic with OSA sorghum starch. In Fig. 4a, in comparison with nutmeg oleoresin, the spectra show the same peaks at 603, 750, 1380, 1535, 2926 and 3429 cm−1. The peak at 3429 cm−1 is related to O–H stretching while 1380 cm−1 shows the ester C–O group in structure. The FTIR peaks of encapsulated powders showed more stretching behavior from 2800 cm−1 that was not observed in nutmeg oleoresin spectra. The FTIR results show the change in absorption at a certain number position so there must be a chemical interaction between the wall material and nutmeg oleoresin after freeze drying process. The reactive groups of materials are remaining the same, indicating that the arabic gum and sorghum starch does not form a cross-linking but only the physical interaction between them. In Fig. 4b, the spectral peaks at 603, 1033, 1413, 2925 and 3400 cm−1 were same in all spectra showing presence of nutmeg oleoresin in mixtures of gum arabic and OSA modified sorghum starches. In views of the above facts, it is established that nutmeg oleoresin as the core material has been successfully encapsulated in gum arabic and sorghum starch mixtures (native and OSA modified) in different ratios.
a FTIR analysis of (a) nutmeg oleoresin (b) gum arabic (c) sorghum native (d) mixture of GA:NAT 2:1, (e) mixture of GA:NAT 1:1 (f) mixture of GA:NAT 1:2. b FTIR analysis of (a) nutmeg oleoresin (b) gum arabic (c) OSA sorghum (d) mixture of GA:OSA 2:1, (e) mixture of GA:OSA 1:1 (f) mixture of GA:OSA 1:2
Conclusion
The present study observed that bioactive components of nutmeg oleoresin were successfully encapsulated through freeze drying procedure within the different matrices of gum arabic, native and modified OSA sorghum starches. The microcapsules resulted in better retention of phenolic, and flavonoid contents with high antioxidant activity. The microcapsules possessed remarkable oxidative stability during storage for 60 days. It may conclude that freeze drying procedure supposed to be an outstanding technique in order to protect bioactive compounds within the matrices of gum and sorghum starch.
References
S.P. Piaru, R. Mahmud, A.M.S.A. Majid, S. Ismail, C.N. Man, J. Sci. Food Agric. 92(3), 593 (2012)
G. Sonavane, V. Sarveiya, V. Kasture, S. Kasture, Pharm. Biochem. & Behav. 71(1–2), 239 (2002)
F. Shahidi, Y. Zhong, Chem. Soc. Rev. 39(11), 4067 (2010)
J.-H. Ahn, Y.-P. Kim, Y.-M. Lee, E.-M. Seo, K.-W. Lee, H.-S. Kim, Food Chem. 107(1), 98 (2008)
M. Martins, M.F. Barreiro, M. Coelho, A.E. Rodrigues, Chem. Eng. J. 245, 191 (2014)
Y. Dror, Y. Cohen, R. Yerushalmi-Rozen, J. Polym. Sci. 44(22), 3265 (2006)
R. Hui, C. Qi-He, F. Ming-liang, X. Qiong, H. Guo-qing, Food Chem. 114(1), 81 (2009)
A. Boutboul, P. Giampaoli, A. Feigenbaum, V. Ducruet, Carbohydr. Polym. 47(1), 73 (2002)
Y. Bai, Y.-C. Shi, Carbohydr. Polym. 83(2), 520 (2011)
E. Domian, A. Brynda-Kopytowska, J. Cenkier, E. Świrydow, J. Food Eng. 152, 72 (2015)
S. Mehboob, T.M. Ali, F. Alam, A. Hasnain, LWT-Food Sci. Technol. 64(1), 459 (2015)
M. Assagaf, P. Hastuti, C. Hidayat, S. Yuliani, S. Supriyadi, J. Agritech (2013). https://doi.org/10.22146/agritech.9562
R. Bhosale, R. Singhal, Carbohydr. Polym. 66(4), 521 (2006)
M. Qi, D.W. Armstrong, Anal. & Bioanal. Chem. 388(4), 889 (2007)
T.M. Ali, A. Hasnain, Int. J. Polym. Anal. & Charact. 17(3), 227 (2012)
D. Chatterjee, P. Bhattacharjee, J. Food Eng. 117(4), 545 (2013)
G. Marinova, V. Batchvarov, Bulg. J. Agric. Sci. 17(1), 11 (2011)
A. Wojdyło, J. Oszmiański, R. Czemerys, Food Chem. 105(3), 940 (2007)
S. Choudhary, B.S. Tanwer, T. Singh, R. Vijayvergia, Int. J. Pharm. & Pharm. Sci. 5(1), 296 (2013)
R. Partanen, J. Raula, R.S.E.P.P. Anen, J. Buchert, E. Kauppinen, P. Forssell, J. Agric. & Food Chem. 56(14), 5717 (2008)
E. Frascareli, V. Silva, R. Tonon, M. Hubinger, Food bioprod. Process 90(3), 413 (2012)
D. Thirathumthavorn, S. Charoenrein, Carbohydr. Polym. 66(2), 258 (2006)
J. Silverio, H. Fredriksson, R. Andersson, A.-C. Eliasson, P. Åman, Carbohydr. Polym. 42(2), 175 (2000)
Y.-F. Chen, L. Kaur, J. Singh, Starch Food (Elsevier, Amsterdam, 2018), p. 283
F. Zhu, Y.-Z. Cai, M. Sun, H. Corke, Food Chem. 112(4), 919 (2009)
D. Paton, Cereal Chem. 64(6), 394 (1987)
A.D. Gupta, V.K. Bansal, V. Babu, N. Maithil, J. Genet Eng. Biotech. 11(1), 25 (2013)
T. Harnsilawat, R. Pongsawatmanit, D.J. McClements, J. Agric. Food Chem. 54(15), 5540 (2006)
V. Busch, A. Pereyra-Gonzalez, N. Šegatin, P. Santagapita, N.P. Ulrih, M. Buera, LWT-Food Sci. Technol. 75, 227 (2017)
N. Balasundram, K. Sundram, S. Samman, Food Chem. 99(1), 191 (2006)
P. Laine, P. Kylli, M. Heinonen, K. Jouppila, J. Agric. food Chem. 56(23), 11251 (2008)
X. Cao, X. Bi, W. Huang, J. Wu, X. Hu, X. Liao, Innov. Food Sci. & Emerg. Technol. 16, 181 (2012)
M.C.P. de Araujo Santiago, R.I. Nogueira, D.R.S.F. Paim, A.C.M.S. Gouvêa, R.L. de Oliveira Godoy, F.M. Peixoto, S. Pacheco, S.P. Freitas, LWT-Food Sci. Technol. 73, 551 (2016)
L.F. Ballesteros, M.J. Ramirez, C.E. Orrego, J.A. Teixeira, S.I. Mussatto, Food Chem. 237, 623 (2017)
P.H. van Golde, M. van der Westelaken, B.N. Bouma, A. van de Wiel, Life Sci. 74(9), 1159 (2004)
V. Kaushik, Y.H. Roos, LWT-Food Sci. Technol. 40(8), 1381 (2007)
J.M. Aguilera, D.W. Stanley, Principles of Food Processing and Engineering (Springer, New York, 1999)
L. Maguire, S. O’sullivan, K. Galvin, T. O’connor, N. O’brien, Int. J. Food Sci. Nutr. 55(3), 171 (2004)
Y. Minemoto, S. Adachi, R. Matsuno, J. Agric. Food Chem. 45(12), 4530 (1997)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arshad, H., Ali, T.M. & Hasnain, A. Bioactive properties and oxidative stability of nutmeg oleoresin microencapsulated by freeze drying using native and OSA sorghum starches as wall materials. Food Measure 14, 2559–2569 (2020). https://doi.org/10.1007/s11694-020-00502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00502-4