Abstract
Solid state fermentation (SSF) is a technological tool to improve the functional and nutritional properties of bio resource products. The present investigation was carried out to evaluate the significance of SSF by means of Aspergillus oryzae on enzyme activity, functional properties and mineral contents of peanut oil cakes (POC). Effect of SSF on the functional properties of POC sample was evaluated and significant improvement was observed in all the tested functional properties except bulk density where significant (p < 0.0.5) decrease was observed. Significant improvement in enzyme activity was also contributed by SSF during different enzyme assays. Microstructural analysis of the fermented POC samples showed a progressive development in the smoothness of granule surface with increasing time of fermentation. Fourier transform infrared spectroscopic analysis was done to evaluate the composition of POC samples at the macromolecular level. The results of FTIR showed a significant increase in protein content with advancing fermentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a remarkable rise in consumer awareness for the health benefits of the functional foods. Increasing demand for the development of novel functional foods necessitates the use of innovative ingredients with scientifically sustained claims by the food industries [1] of these, ingredients of natural origin have better acceptability by the international community over their synthetic counterparts [2]. Use of food industry related by-products which are well known for their substantial amounts of bioactive compounds are gaining importance as their usage would allow considerable reduction in food wastes thereby making these by-products valuable from health as well as environmental point of view [3]. Therefore, there is a great interest in the effective use of plant based bio-resources as ingredients to improve the functional properties and another health promoting properties [4].
Oil cakes are produced during oil processing of seeds and categorized in two types i.e. edible and non-edible cakes [5]. Oil cakes have been in use for feed applications to poultry, fish and swine industry [6]. Press cakes and meals originating from oil extraction are promising source of food proteins due to their widespread availability, high protein content, low amounts of antinutritive compounds and no toxicity [7]. Being rich source of protein, oil cakes also have immense potential in several food based application as an ideal candidate for food supplementation.
Peanut (Arachis hypogaea L.) is a widely utilized, highly valuable source of edible oil and protein meal in human and animal nutrition [8]. Apart from having these major nutrients, peanut seeds are also rich in various micro as well as macro nutrients that possess functional properties like emulsifying, foaming, water absorption properties etc., well known and reported by several authors [9, 10]. Peanut oil cakes (POC) obtained as a result of oil processing is major byproduct that needs to be judiciously utilized for their concentrated form nutrients.
Solid-state fermentation (SSF) processes can be defined as “the growth of microorganisms (mainly fungi) on moist solid materials in the absence of free-flowing water” [11]. SSF is used to produce the product in a controlled way. It represents a technological alternative for a great variety of legumes and cereals, or combination of them, to improve their nutritional quality, to obtain edible products with palatable sensorial characteristics and formation of shorter chain compounds with lower molecular mass [12] Therefore, SSF has been applied to many cereals, legumes and their byproducts to increase their functional properties as well as food applications [11]. The application of modern biotechnical knowledge and process control technologies can lead to significant productivity increases from this ancient process. Polyphenolic compounds are characterized by the existence of more than one phenolic group per molecule, are common dietary phytochemicals found in fruits, vegetables and grains. Several studies already reported the increased value of phenolic compounds in different substrates after fermentation [13,14,15].
Aspergillus oryzae is a filamentous fungus, which has an ability to secrete large amounts of hydrolytic enzymes including protease, amylase, cellulase, and phytase [16, 17]. It is widely used in the manufacture of traditional fermented food products all over the world. Moreover, A. oryzae is genomically well characterized and considered to be a safe organism for producing food enzymes because it lacks expressed sequence tags for the genes responsible for aflatoxin production [17].
However, limited attempts have been made so far to access the enzyme extraction, functionality, mineral content and food application of solid state fermented POC. Therefore, present investigation was undertaken to study the effect of fermentation on the enzyme extraction, functional properties and mineral content from POC during the incubation period of 144 h by the fungi (A. oryzae).
Materials and methods
Materials
Peanut (HNG-10) was obtained from Pilimandori, Fatehabad, Haryana (India). A. oryzae (MTCC 3107) was procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technologies Chandigarh, India. Xylan (from Birchwood), α-amylase, p-nitrophenol-β-d-glucoside, β-glucosidase, potato dextrose agar and Czapek-dox medium were procured from Sigma Aldrich Co. (St. Louis, USA). Potassium di-hydrogen phosphate, potassium chloride, sodium carbonate, magnesium sulphate, nitric acid, sulphuric acid and perchloric acid were procured from Thermo Fisher Scientific India Pvt. Ltd., (Mumbai, India). Hexane, ethanol, α-amylase, human pancreatic lipase, colipase, cholesterol esterase, phospholipase A2, mucin, bovine serum albumin, pepsin (2950 units mg/l of protein), pancreatin, glucuronic acid, glucoseamine hydrochloride, ammonium sulphate and taurocholic acid sodium salt hydrate were purchased from Sigma Chemical Co (Madrid, Spain). All chemicals used were of AR grade. Triple distilled water and acid washed glassware were used throughout the experiments.
Methods
Preparation of peanut oil cakes (POC)
Peanut variety HNG-10 samples were brought to the expeller of local market of Sirsa, India for preparation of POC. Peanut cakes were grinded using blender (Braun AG Frankfurt A.M. Mx 32, Germany) to obtain fine powder and stored in air tight container at 4–7 °C temperature.
Microbial strain and substrate
The Fungal strain A. oryzae (MTCC 3107) was used for solid state fermentation (SSF) in present study. This fungal strain is generally recognized as safe (GRAS), was cultivated and maintained on potato dextrose agar (PDA) plates. Spore suspension was prepared with a spore count of 1 × 106 spores/ml. POC of variety HNG-10 were used as the substrate for the fungi.
Preparation of inoculum
The fungal culture of A. oryzae, maintained on slants of potato dextrose agar were transferred to fresh PDA plates before starting of each experiment. The inoculated plates were incubated at 30 °C for 144 h. Spore suspension was prepared in sterilized cellular grade water having a spore count of approximately 1 × 106 spores/ml.
Fermentation conditions
Substrate was first washed and dried for 1 h in a hot air oven (Narang Scientific Instruments, NSW 143, Ambala, India) at 30 °C before use, then it was grinded to fine powder in a grinder to make suitable for fermentation. Fifty gram of grinded sample was taken in 500 ml Erlenmeyer flasks and then soaked in 50 ml Czapek-dox medium [NaNO3 (2.5 g/l), KH2PO4 (1.0 g/l), KCl (0.5 g/l) and MgSO4. 2H2O (0.5 g/l)] at room temperature (30 °C) overnight. After decanting the excess media if any, the substrate was autoclaved (Vertical autoclave, Calton, NSW-227, India) and then subsequently cooled at room temperature before inoculation. The autoclaved substrate was inoculated with 5.0 ml spore suspension (1 × 106 spores/ml) of fungal strain, mixed properly and incubated in BOD Incubator (Calton, NSW-152, India) for 0, 48, 72, 96, 120 and 144 h, respectively at 30 °C. The non-fermented substrate as raw material was prepared without the addition of spore suspension.
Extraction of enzymes and enzyme assays
Fermented samples were taken after 0, 48, 72, 96, 120 and 144 h of interval during fermentation respectively and the enzymes were extracted from fermented substrate with triple distilled water (1:10 w/v). Extracted enzyme samples were filtered through Whatman filter paper No. 1. The supernatants were used for different enzyme assay by following the methods of Miller, [18].
Alpha-amylase assay
Alpha-amylase activity of non-fermented and solid stated fermented POC samples was determined by mixing 0.25 ml of appropriately diluted enzyme (1:5 v/v) with 0.5 ml of 0.2 M acetate buffer (pH 5.0) and 1.25 ml of soluble starch (1.0%). After 10 min of incubation at 50 °C, the concentration of glucose liberated from starch by the action of α-amylase was estimated using UV/VIS spectrophotometer (Systronic 106 UV–Vis spectrophotometer) at 575 nm. One unit of amylase activity was calculated as the amount of enzyme that liberates one micromole of reducing sugar (glucose) per min under the assay conditions. Results were expressed as EU (µM/ml).
β-Glucosidase assay
β-Glucosidase activity was assayed by using 1.0 ml reaction mixture containing 5 mM p-nitrophenyl-β-d-glucoside (pNPbG), 100 mM acetate buffer (pH 5.0), and an appropriately diluted enzyme extract. After 30 min of incubation at 50 °C, the reaction was terminated by adding 2.0 ml of 1 M Na2CO3, and the p-nitrophenol release was monitored at 400 nm. One unit (U) of β-glucosidase activity was calculated as the amount of enzyme that released one micromole of p-nitrophenol per min under the assay conditions.
Xylanase assay
It was determined by mixing 0.025 ml of appropriately diluted enzyme source with 0.475 ml of 0.2 M acetate buffer (pH 5.0) and 0.5 ml of 1.0% (w/v) xylan. After 10 min incubation at 50 °C, the concentration of xylose liberated from xylan by the action of xylanase was estimated spectrophotometrically at 575 nm. One unit of enzyme activity was calculated as the amount of enzyme that liberates one micromole of xylose per minute under the assay conditions.
Functional properties of samples
Bulk density
Bulk density was determined by following the method of Kaur and Singh [19]. Non-fermented and fermented POC powders were gently filled in 10.0 ml graduated cylinder with least count of 0.5 ml. The bottom of the cylinder was then gently tapped five times until there was no further diminution of the sample level after filling to the 10.0 ml mark. Bulk density was calculated as mass of sample per unit volume of sample (g/cm3).
Water binding and oil binding
Water and oil binding capacities of non-fermented and fermented POC were determined according to the method described by Adeleke and Odedeji [20]. Water and oil absorption was determined by mixing 1.0 g of POC with 15.0 ml deionized water and soya bean oil, respectively in a pre-weighed centrifuge tubes. After holding for 30 min, the tubes were centrifuged for 10 min at 3000×g (24 °C). The supernatant was discarded and tubes were then weighed again. Water absorption capacity and oil absorption capacity were expressed as g of water and g of oil retained per g of sample, respectively.
Emulsifying activity and stability
Emulsifying activity and stability were determined using the method of Neto et al. [21] with slight modifications (probe sonicator was used instead of homogenizer for emulsion preparation). Non-fermented and fermented samples (1.0%, 50.0 ml) solutions were sonicated with 25.0 ml soybean oil using a probe sonicator (Sonics and Materials Inc. New Town, CT, USA) with 5 s pulse rate for 15 min. The emulsions were centrifuged at 1100×g for 5 min. The height of the emulsified sample was then measured. The emulsifying activity (EA) was calculated as:
Emulsion stability (ES) was determined by heating the emulsion at 80 °C for 30 min before centrifuging at 1100×g for 5 min and calculated as follows:
Foaming properties
Foam capacity (FC) and foam stability (FS) were measured for the non-fermented and fermented POC samples. The method proceeds by whipping 100 ml of 3% (w/v) sample solution in triple distilled water. The suspensions were mixed using a magnetic stirrer for 45 min, followed by mixing in an auto-mix blender (230V AC Remi Anumpam Mixie Ltd., Bombay, India) at its maximum speed for exactly 10 min. The sample was then immediately transferred to 250 ml graduated cylinder. The volume was measured before and after stirring. FC was expressed as the volume (%) increased due to stirring. For the determination of FS, foam volume changes in graduated cylinder were recorded after 30 min (30 °C). FC and FS were then calculated by the following formula, respectively [22].
Scanning electron microscopy
The microstructure and surface characteristics of non-fermented and fermented POC samples were examined by scanning electron microscopy (SEM) (EV018, 18th special edition, Zeiss, Tokyo, Japan). Dry powdered samples of raw and fermented POC were sprayed on double adhesive tape mounted on aluminium stub. Mounted samples were coated with gold (20 nm thickness) on ion coater (IB-3 Ion coater, Eiko, Tokyo, Japan) at 0.05–0.07 Torr for 4 min maintain the ion current at 6 mA. The samples were finally examined by SEM at an acceleration voltage of 15 KV under high vacuum (9.0 × 10−5 Torr) and micrographs were recorded.
Fourier transform infrared (FTIR) spectroscopy
Spectra of non-fermented and fermented samples were acquired using FTIR spectrometer (Model—IR Affinity-01, Shimadzu, Japan) with diamond crystal cell ATR and inbuilt IR-Solution software at 4 cm−1 resolution. Forty five scans of each sample in the mid-infrared region 4000–800 cm−1 against air as a background were acquired. Spectra were acquired in terms of absorption and each measurement was repeated at least five times. After scanning of each sample, the ATR crystal was cleaned with ethanol and wiped using a soft tissue paper to remove impurities, if any.
Mineral content of POC samples
Calcium, iron, zinc, copper content of POC samples were estimated in atomic absorption mode and sodium and potassium contents of POC samples were estimated in emission mode using atomic absorbance spectrophotometer (AAS) (AA-7000, Shimadzu, Tokyo, Japan) as described by AOAC [23]. Samples were subjected to ashing (at 550 °C for 8 h), solubilised in tri acid mixture and heated for complete dissolution. All the samples were diluted to a suitable dilution before analysis by AAS.
Statistical analysis
Means and standard error mean was calculated using Microsoft Excel, 2007 (Microsoft Corp., Redmond, WA, USA). Significant difference between values was verified by one-way analysis of variance (ANOVA) and comparison between means was made by critical difference value [24].
Results and discussion
Enzyme production and enzyme assay
Agro industrial residues and wastes are the best suited substrates in SSF for the production of enzymes. Results of enzyme production and assay are depicted in Fig. 1. Pandey et al. [25] reported in their study that SSF is the most technically advantageous economic process to produce different variety of enzymes from fungal strains. These fungal strains releases various enzymes during fermentation [26, 27]. Cho et al. [28] observed that fermentation also led to mobilization of bioactive phenolic compounds as a result of enzyme activity by hydrolyzing the phenolic glycosides to release free phenolic acids. The enzyme activities were calculated from the equation obtained from the standard used for different enzymes and results were expressed as Enzyme Unit i.e. EU (µg/ml). Results revealed that fermented POC samples showed significantly increased (p < 0.05) enzyme activity in comparison with non-fermented POC samples i.e. 7.34 ± 1.45 EU (µg/ml) and 7.07 ± 0.40 EU (µg/ml). In case of α-amylase and β-glucosidase enzyme assays the highest activity i.e. 11.37 ± 0.45 EU (µg/ml) and 62.70 ± 2.41 EU (µg/ml) respectively, was observed at 120 h of incubation during fermentation with filamentous fungi A. oryzae and then the activity of both enzyme assays became significantly low (p < 0.05), respectively. This could be due to the depletion of essential nutrients required for the growth and enzyme production by the fungus. In case of xylanase, fungi produce several auxiliary enzymes required for the degradation of substituted xylan [29]. However, findings obtained from xylanase assay revealed that the enzyme activities got significantly increased (p < 0.05) up to 72 h of incubation [13.56 ± 0.69 EU (µg/ml)] and then a gradual decrease was observed with increasing incubation period. Increase in enzyme activity through fermentation was observed in all the fermented POC samples. These results were in accordance with Bhanja et al. [30] and Duhan et al. [31] who observed increased enzyme activities in solid stated fermented samples than that of non-fermented samples [32, 33].
Enzyme activity (with different assays) of control and fermented POC samples during solid state fermentation. Data are presented as means ± SEM (n = 3). A–EMeans within row with different uppercase superscript are significantly different (p < 0.05) from each other. a−fMeans within column with different lowercase superscript are significantly different (p < 0.05) from each other
Functional properties
Functional properties of different food components and ingredients play a vital role during processing of food as well as for their application in novel food product development. These functional properties help in improving the processing conditions during the fabrication of various innovative food products [34]. In this direction, SSF technology has come up with numerous end products that can eventually improve the functional behavior of foodstuffs. Therefore, a detailed study on the effect of SSF on functional properties viz. bulk density, water and oil absorbance capacity, emulsifying properties and foaming capacity of non-fermented and fermented POC was carried out during 144 h of fermentation.
Bulk density
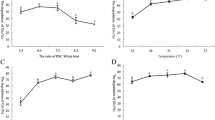
Bulk density, a complex product property encompasses particle density (occluded air and density of the solid) and the interstitial air present in a food material. The values for bulk density of raw (non-fermented) and fermented samples, ranging from 0.31 to 0.52 g/cm3 as shown in Fig. 2a. SSF significantly (p < 0.05) decreased the bulk density of POC samples during fermentation in comparison with non-fermented samples. However, samples with fermentation time of 120 and 144 h showed slight but significant difference from each other. The bulk density of a powder sample influences the texture, mouth feel and the amount and strength of packaging material required for its distribution [35]. Therefore, decrease in bulk density of fermented POC sample would be an advantage in the packaging as well as preparation of different low bulk food materials [36, 37].
a Bulk density, b water and oil binding capacity, c emulsifying properties, d foam property of control and fermented POC samples during solid state fermentation of control (non-fermented) and fermented POC samples during solid state fermentation. Data are presented as means ± SEM (n = 3). a–fMeans within column with different lowercase superscript are significantly different (p < 0.05) from each other
Water and oil binding capacity
Water and oil binding capacity of food products are considered as important functional properties from processing point of view. Results of water and oil binding capacity of non-fermented and fermented POC samples are shown in Fig. 2b. Significant increase (p < 0.05) in water holding capacity was observed in all the fermented POC samples in comparison with non-fermented POC samples. However, non-significant (p < 0.05) difference was observed in between the fermented POC samples with increasing time of fermentation, respectively [12]. Oloyede et al. [37] reported that fermentation causes unfolding and modification of macromolecules of the products. This unfolding exposes the hydrophilic domains of the amino acid residues of proteins and other macromolecules which have higher affinity for aqueous medium. In the present study, higher values of oil binding capacity indicates that fermentation process resulted in increased number of exposed hydrophobic interaction sites of fermented POC samples as compare to the raw samples. In addition, an improvement in oil binding capacity after fermentation is due to physical entrapment of oil on the surface of POC samples. According to Oloyede et al. [37] OBC of the oil cakes is widely affected by their origin and the method of processing employed for their extraction. These factors significantly influence the composition, physical structure, porosity, and particle size of the dried cake powder. This parameter is of special interest, particularly for the industrial application where the holding of fat during processing and storage is of significance.
Emulsifying activity and foam properties
Results revealed that emulsifying properties of POC samples were improved by SSF with A. oryzae. Emulsion capacity indicates the maximum amount of oil that can be emulsified by dispersion, while emulsion stability indicates the ability of an emulsion with a certain composition to remain unchanged [38]. Emulsifying activity including emulsion ability and emulsion stability of raw and fermented POC samples are presented in Fig. 2c. Fermentation of POC led to significant increase (p < 0.05) in emulsifying properties with the progression of fermentation. Prinyawiwatkul et al. [39] reported that fungal proteolytic activity might have exposed hydrophobic groups which resulted in change of hydrophilic–lipophilic balance that eventually favoured emulsification. Moreover, according to Lim et al. [40] enzymatic hydrolysis during fermentation process generally results in improved emulsifying activity by producing lower molecular weight peptides that easily migrate into the oil water interface.
Foam formation and stability are dependent on properties like pH, viscosity, surface tension and the processing methods employed. Foaming properties of non-fermented and fermented POC samples are shown in Fig. 2d. SSF of POC significantly improved the foam capacity and foam stability with fermentation time. The increase in water holding capacity might have resulted into significant improvement in the foam capacity of POC samples [41]. The electrostatic property of macromolecules present in an environment marks the stability of that system. Fermentation process led to an increase in electrostatic charge which finally improved the foam forming capacity and foam stability [42]. Therefore to conclude, increase in foam stability would be the combined effect of increased WHC and electrostatic charge.
Microstructural properties (scanning electron microscopy)
Scanning electron microscopy was used to elucidate the microstructural properties of the fermented POC samples during fermentation period of 144 h, respectively. Micrographs of scanning electron microscopy of fermented POC samples with different magnification as compared to non-fermented (control) are shown in Fig. 3. Micrographs of non-fermented POC powder showed collapsed structure with coarse and rough surface with visible aggregates however, smooth surfaced structure of POC was also visible (Fig. 3a). Micrographs of fermented POC sample (Fig. 3c–f) clearly indicated that fermentation led to smooth surface structures of POC with increasing time of incubation, respectively. This could be due to small particle size attributed by POC sample with increasing time of fermentation [43]. However, fermented POC sample of 48 h (Fig. 3b) showed slightly collapsed and coarse appearance.
Fourier transform infrared spectroscopy (FTIR)
FTIR analysis was conducted to elucidate the chemical structure of non-fermented and fermented POC samples during SSF by A. oryzae. FTIR spectra of all POC samples are shown in Fig. 4a–f, respectively. Result of FTIR signifies that there were clear differences in control and fermented POC throughout the spectra. Specific functional groups of POC components exhibited typical bands in the region 4000–800 cm−1 with the strong and broad O–H stretching modes of water absorbing in the region 3700–3000 cm−1 [44]. The peak at 1200–900 cm−1 was probably associated with the phosphate group (O=P–O) of the POC proteins. It was noted that spectra of fermented samples were more distinct in the region 2300–1700 cm−1 with clear differences in their absorbance values. This region associated with the stretching and bonding of CH2 and CH3 of fat molecules. Absorptions in this region include contributions from complex interacting vibrations rising to the generally unique fingerprint. Crude protein increased after fermentation and the loss of dry matter during fermentation could be a reason for the increase in protein content in POC samples. According to Shi et al. [45] after 72 h of SSF with A. oryzae, free amino nitrogen production was increased to 34.5 mg/g which is equivalent to 55% conversion from the total protein in rape seed meal. An increased amount of small peptide in POC might be due to the digestion of large-size peptides by proteases secreted by Aspergillus niger [43].
Comparative ATR-FTIR spectra at a 0 h, b 48 h, c 72 h, d 96 h, e 120 h and f 144 h of non-fermented and fermented POC samples during fermentation. Here (I) O=P–O of protein, (II) C–N stretching and N–H bonding, (III) C=O stretching vibrations, (IV) stretching and bonding of CH2 and CH3 of fat, (V) N–H stretching vibrations, (VI) N–H group and (VII) hydroxyl group
The most widely used modes in protein structural studies are amide I, amide II and amide III. The amide I band arises principally from the C=O stretching vibration of the peptide group. Secondary structures of protein is always associated with the amide I bands. Amide I is the most intense absorption band of the polypeptides (C=O). There are also some in-plane NH bending contributions to amide I. The secondary structure of proteins are imitated by the following bands i.e. 1610–1640 cm−1 for the β-sheet; 1640–1650 cm−1 for the random coil; 1650–1658 cm−1 for the α-helix; 1660–1700 cm−1 for the β-turn [46]. Surface hydrophobicity is related with the content of α-helix and in case of fermented POC improved WHC and OBC significantly revealed the balance of hydrophilic and hydrophobic domains. Furthermore the number of ionizable and hydrophobic groups on the protein surface could be increased due to change in physical or chemical interactions with other proteins of substrates. The amide II band is primarily N–H bending with a contribution from C–N stretching vibrations. The amide III absorption is normally very weak in the infrared, arising primarily from N–H bending and C–N stretching vibrations [46]. Spectra of non-fermented and fermented POC sample revealed that the amide I absorption occurs in the region 1600–1700 cm−1. Hydrogen bonding and the coupling between transition dipoles are amongst the most important factors governing conformational sensitivity of the amide bands.
Mineral content
Six minerals viz., iron, zinc, calcium, sodium, potassium and copper of control (non-fermented) and fermented samples were estimated using atomic absorbance spectrophotometer (Table 1). The mineral content of all the samples showed significant difference from each other however, non-significant (p < 0.05) differences were observed in the values of mineral content with increasing time of fermentation. Similar findings were proposed by Joshi and Sandhu [47] where, they noticed significant increase in mineral content of apple pomace during SSF by yeast species.
Conclusions
Effect of SSF on the different functional properties, enzyme activity and mineral content of POC has been studied. Fungal strain incubated with substrate for 144 h at 30 °C and findings from functional properties of fermented flour revealed the decrease in bulk density and significant increase in other functional properties than that of non-fermented sample. Enzyme activity in different enzyme assays was also significantly increased due to SSF. SSF of POC also attains part of credit in improving the morphological characteristics and mineral content. FTIR revealed the significant increase in protein content during fermentation.
Abbreviations
- SSF:
-
Solid state fermentation
- OBC:
-
Oil binding capacity
- WHC:
-
Water holding capacity
- EA:
-
Emulsion activity
- ES:
-
Emulsion stability
- FC:
-
Foam capacity
- FS:
-
Foam stability
- AAS:
-
Atomic absorption spectrophotometer
- FTIR:
-
Fourier transform infrared spectroscopy
- SEM:
-
Scanning electron microscopy
- POC:
-
Peanut oil cakes
References
K. Nyemb, C. Guerin-Dubiard, S. Pezennec, J. Jardin, V. Briard-Bion, C. Cauty, S.M. Rutherfurd, D. Dupont, F. Nau, The structural properties of egg white gels impact the extent of in vitro protein digestion and the nature of peptides generated. Food Hydrocolloid 54, 315–327 (2016)
P. Gornas, M. Rudzinska, Seeds recovered from industry by-products of nine fruit species witha high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind. Crops Prod. 83, 329–338 (2016)
M. Herrero, A.D.P. Sánchez-Camargo, A. Cifuentes, E. Ibanez, Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. Trends Anal. Chem. 71, 26–38 (2015)
S. Karboune, N. Khodaei, Structures, isolation and health-promoting properties of pectic polysaccharides from cell wall-rich food by-products: a source of functional ingredients. Curr. Opin. Food Sci. 8, 50–55 (2016)
A.K. Sarker, D. Saha, H. Begum, A. Zaman, M.M. Rahman, Comparison of cake compositions, pepsin digestibility and amino acids concentration of proteins isolated from black mustard and yellow mustard cakes. AMB Express 5, 22 (2015)
J. Zhao, T. Zhou, Y. Zhang, Y. Ni, Q. Li, Optimization of arachin extraction from defatted peanut (Arachis hypogaea) cakes and effects of ultra-high pressure (UHP) treatment on physiochemical properties of arachin. Food Bioprod. Process. 95, 38–46 (2015)
C. Pickardt, P. Eisner, R.D. Kammerer, R. Carle, Pilot plant preparation of light coloured protein isolates from de-oiled sunflower (Helianthus annuus L.) press cake by mild-acidic protein extraction and polyphenol adsorption. Food Hydrocolloid 44, 208–219 (2015)
B.S. Zhang, In vitro antithrombotic activities of peanut protein hydrolysates. Food Chem. 201, 1–8 (2016)
M.P. Martin, V. Nepote, N.R. Grosso, Chemical, sensory, and microbiological stability of stored raw peanuts packaged in polypropylene ventilated bags and high barrier plastic bags. LWT-Food Sci. Technol. 68, 174–182 (2016)
S.S. Arya, A.R. Salve, S. Chauhan, Peanuts as functional food: a review. J. Food Sci. Technol. 53(1), 31–41 (2016)
C.H. Chi, S.J. Cho, Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT-Food Sci. Technol. 68, 619–625 (2016)
Yu.. Xiao, G. Xing, X. Rui, W. Li, X. Chen, M. Jiang, M. Dong, Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. Food Sci. Technol. 63, 1317–1324 (2015)
J.S. Duhan, P. Saharan, S.K. Gahlawat, Surekha, Antioxidant potential of various extracts of stem of Thuja orientalis: in vitro study. Int. J. Appl. Biol. Pharm. 3(4), 264–271 (2012)
J.S. Duhan, A. Rana, P.K. Sadh, P. Saharan, Surekha, Antimicrobial and free radical scavenging activity of selective medicinal plants combination. World J. Pharm. Sci. 4(3), 1202–1216 (2015)
A. Rana, P. Saharan, P.K. Sadh, Surekha, J.S. Duhan, Free radical scavenging and antimicrobial potential of mixture of selective medicinal plants. Asian J. Pharm. Clin. Res. 7(4), 27–32 (2014)
G. Guan, Z. Zhang, H. Ding, M. Li, D. Shi, M. Zhu, L. Xia, Enhanced degradation of lignin in corn stalk by combined method of Aspergillus oryzae solid state fermentation and H2O2 treatment. Biomass Bioenergy 81, 224–233 (2015)
C. Chancharoonpong, P.C. Hsieh, C.S. Sheu, Enzyme production and growth of Aspergillus oryzae S. on soybean koji fermentation. APCBEE Procedia 2, 57–61 (2012)
G.L. Miller, Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–429 (1959)
M. Kaur, N. Singh, Relationships between various functional, thermal and pasting properties of flours from different Indian black gram (Phaseolus mungo L.) cultivars. J. Sci. Food Agric. 87, 974–984 (2007)
R.O. Adeleke, J.O. Odedeji, Functional properties of wheat and sweet potato flour blends. Pak. J. Nutr. 9(6), 535–538 (2010)
V.Q. Neto, N. Narain, J.B. Silva, P.S. Bora, Functional properties of raw and heat processed cashew nut (Anarcarduim occidentale, L.) kernel protein isolate. Nahrung Food 45, 258–262 (2001)
J.N. DeWit, E. Hontelez-Backx, M. Adamse, Evaluation of functional properties of whey protein concentrates and whey protein isolates 3 functional properties in aqueous solution. Neth. Milk Dairy J. 42, 155–172 (1988)
AOAC, Official Methods of Analysis. The Association of Official Analytical Chemists, 18th edn. (North Fredrick Avenue Gaithersburg, Maryland, 2005), p. 481
G.W. Snedecor, W.G. Cochran, Statistical Methods, 8th edn. 1989 (Affiliated East-West Press, Iowa State University Press, Ames, 1994)
A. Pandey, P. Selvakumar, C.R. Soccol, P. Nigam, Solid state fermentation for the production of industrial enzymes. Curr. Sci. 77, 149–162 (1999)
A. Kumar, J.S. Duhan, Production and characterization of amylase enzyme isolated from Aspergillus niger MTCC-104 employing solid state fermentation. Int. J. Pharm. Biol. Sci. 2, B250–B258 (2011)
A. Kumar, J.S. Duhan, S.K. Tanwar, Screening of Aspergillus spp. for extra cellular α-amylase activity, in Impact of global climate change on earth ecosystem, ed. by D.R. Khanna, A.K. Chopra, G. Matta, V. Singh, R. Bhutiani (Biotech Books, New Delhi, 2013), pp. 205–214
K.M. Cho, S.Y. Hong, R.K. Math, J.H. Lee, D.M. Kambiranda, J.M. Kim, Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. Food Chem. 114, 413–419 (2009)
M.L. Polizeli, C.S. Rizzatti, R. Monti, H.F. Terenzi, J. Jorge, D.S. Amorim, Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67, 577–591 (2005)
T. Bhanja, A. Kumari, R. Banerjee, Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour. Technol. 100, 2861–2866 (2009)
J.S. Duhan, K. Mehta, P.K. Sadh, P. Saharan, Surekha, Bio-enrichment of phenolics and free radicals scavenging activity of wheat (WH-711) fractions by solid state fermentation with Aspergillus oryzae. Afr. J. Biochem. Res. 10(2), 12–19 (2016)
P.K. Sadh, P. Saharan, J.S. Duhan, Bio-augmentation of antioxidants and phenolic content of Lablab purpureus by solid state fermentation with GRAS filamentous fungi. Res. Eff. Technol. 3, 285–292 (2016). doi:10.1016/j.reffit.2016.12.007
P.K. Sadh, P. Saharan, S. Duhan, J.S. Duhan, Bio-enrichment of phenolics and antioxidant activity of combination of Oryza sativa and Lablab purpureus fermented with GRAS filamentous fungi. Res. Eff. Technol. 3, 347–352 (2017). doi:10.1016/j.reffit.2017.02.008
L. Bhandari, N.S. Sodhi, P. Chawla, Effect of acidified methanol modification on physico chemical properties of Black-eyed pea (Vigna unguiculata) starch. Int. J. Food Prop. (2016). doi:10.1080/10942912.2016.1171236
I. Amadou, M.E. Gounga, Y.H. Shi, G.W. Le, Fermentation and heat-moisture treatment induced changes on the physicochemical properties of foxtail millet (Setaria italica) flour. Food Bioprod. Process. 92(1), 38–45 (2013)
A.E.O. Elkhalifa, B. Schiffler, R. Bernhardt, Effect of fermentation on the functional properties of sorghum flour. Food Chem. 92, 1–5 (2005)
O.O. Oloyede, S. James, B.O. Ocheme, C.E. Chinma, V.E. Akpa, Effects of fermentation time on the functional and pasting properties of defatted Moringa oleifera seed flour. Food Sci. Nutr. 4(1), 89–95 (2016)
V.N. Enujiugha, A.A. Badejo, S.O. Iyiola, M.O. Oluwamukomi, Effect of germination on the functional properties of African oil bean (Penthaclethra macrophylla Benth) seed flour. J. Food Agric. Environ. 1, 72–75 (2003)
W. Prinyawiwatkul, K.H. McWatters, L.R. Beuchat, R.D. Phillips, Functional characteristics of cowpea (Vigna unguiculata) flour and starch as affected by soaking, boiling, and fungal fermentation before milling. Food Chem. 58, 361–372 (1997)
J.Y. Lim, J.J. Kim, D.S. Lee, G.H. Kim, J.Y. Shim, I. Lee, Physicochemical characteristics and production of whole soymilk from Monascus fermented soybeans. Food Chem. 120(1), 255–260 (2010)
Y.F. Cheng, R. Bhat, Functional, physicochemical and sensory properties of novel cookies produced by utilizing underutilized jering (Pithecellobium jiringa Jack.) legume flour. Food Biosci. 14, 54–61 (2016)
P. Chawla, L. Bhandari, P.K. Sadh, R. Kaushik, Impact of solid state fermentation (Aspergillus oryzae) on functional properties and mineral bioavailability of black eyed pea (Vigna unguiculata) seed flour. Cereal Chem. 94(3), 437–442 (2016). doi:10.1094/CCHEM-05-16-0128-R
C. Shi, J. He, J. Yu, B. Yu, X. Mao, P. Zheng, Z. Huang, D. Chen, Physicochemical properties analysis and secretome of Aspergillus niger in fermented rapeseed meal. PLoS ONE 11(4), e0153230 (2016)
L.W. Yoon, G.C. Ngoh, A.S. Chua, Simultaneous production of cellulase and reducing sugar through modification of compositional and structural characteristic of sugarcane bagasse. Enzym. Microb. Technol. 55, 250–256 (2013)
C. Shi, J. He, J. Yu, B. Yu, X. Mao, P. Zheng, Z. Huang, D. Chen, Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol. 6(1), 13 (2015)
C. Wang, L. Jiang, D. Wei, Y. Li, X. Sui, Z. Wang, D. Li, Effect of secondary structure determined by FTIR spectra on surface hydrophobicity of soybean protein isolate. Procedia Eng. 15, 4819–4827 (2011)
V.K. Joshi, D.K. Sandhu, Effect of solid state fermentation and yeast species on composition of apple pomace: application of PCA. Int. J. Food Ferment. Technol. 2(1), 87–91 (2012)
Acknowledgements
The support by Department of Biotechnology, Chaudhary Devi Lal University, Sirsa, Haryana, India is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadh, P.K., Chawla, P., Bhandari, L. et al. Bio-enrichment of functional properties of peanut oil cakes by solid state fermentation using Aspergillus oryzae . Food Measure 12, 622–633 (2018). https://doi.org/10.1007/s11694-017-9675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9675-2