Abstract
Tea is commonly known to be rich in certain micro- and macroelements. The objective of the study was to evaluate and compare the percentage transfer to the infusion of some essential elements (Ca, K, F, Mg, Na, and P) and determine concentrations of macroelements available in the bagged and leaf black teas of the same brand as well as to assess whether drinking tea could contribute to the daily nutritional mineral requirements of the consumers. The results showed that regardless of the tea type, the most abundant elements in both dry teas and infusions were K and Ca, while F concentrations were the lowest. The PCCA showed that content of elements in dry teas was high while in infusions low. The content of elements was similar in the dry bagged and leaf teas of the same brand, except for F which content was higher in the bagged teas (the U Mann–Whitney test, p < 0.05). Whereas, in case of the infusions, bagged teas contained higher amounts of Ca, F, K, Mg and Na (the U Mann–Whitney test, p < 0.05). The solubility of F, K and Na was high, while in case of Ca, Mg and P moderate. For K, Mg and Na the percentage release from dry tea to the infusion was significantly higher in the bagged than in leaf teas (the U Mann–Whitney test, p < 0.05). The results showed that the tea consumption may substantially contribute to F and Na daily intake (up to 37 and 77.5% of human dietary reference intake, respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tea-drinking habit is worldwide spread [1], as evidenced by the fact that except for water, tea infusion is the most widely consumed drink [2]. As about 18–20 billion cups of tea are consumed in the world each day, there is both the economic and social interest in this product [3]. Stimulant beverages such as coffee or tea are commonly known to be a source of certain micro- and macroelements. The concentration of these elements in the beverage depends on many factors: the species, the genetics of the plant, the age of the plant when harvested, soil conditions, rainfall and geographical location of the plantation, as well as the conditions during infusion preparation [4]. Seeing that tea is consumed in a considerable amount, and therefore becomes a part of our daily diet, the assessment of the nutrient and mineral composition of this product is of great importance in order to judge its nutritional value and assess its possible health effects on the consumers. Furthermore, the information about the amounts of elements in tea increasingly attracts the attention of consumers and producers [1, 5, 6]. Many studies that explored the possible medicinal properties of the tea have focused on its organic chemical components. Considerably less research has been conducted on the nutritional value of tea, especially rare are comparative studies for the same minerals in the dry tea material and the infusion [5].
Tea is made from the leaves and young shoots of Camellia sinensis (L.) Kuntze [2, 6, 7]. About 80% of produced tea is manufactured as the black tea, which is the most popular in Western countries [8]. The production of black tea involves five steps: withering, rolling (crushing and macerating of leaves), fermentation (resulting in significant compositional changes), firing and sorting into appropriate grades. The commercially available teas differ in respect of the quality, flavour, size of leaves, chemical composition, color and many other features. The differences are related mostly to the particular methods of production and the localization of cultivation [6, 9]. The leaf tea, considered a good quality tea, is produced from a bud and two to four young top leaves [10]. Other types of tea, classified with respect to the size, are: brokens, fannings and dust. They have a less coarse texture than the whole leaf tea and are considered less valuable. Broken tea leaves are usually sold as medium grade loose teas or manufactured together with fannings and dusts for the use in tea bags [11].
The chemical composition of tea is very complex and consists of tannin substances, alkaloids, flavonoids, proteins, enzymes, aroma forming substances, vitamins, minerals and trace elements [1, 7]. Tea leaves may contain a substantial amount of metals such as Cu, Fe, Mn, and Zn [12]. Also Ca, F, K and Mg contents were proved to be very high in both tea leaves and beverages [8, 13, 14]. These minerals play an important role in human organism. They are found in the structure of teeth (Ca, P, and F) and bones (Ca, Mg, Mn and F), play a vital role as a structural part in many enzymes (Cu, Fe, Mn and Zn) and in the formation of erythrocyte cells (Fe) as well as the control of blood pressure (Ca and K). Some minerals are also involved in immune (Ca, Mg, Cu and Zn) and brain (Mn) systems [15].
While brewing, elements included in tea leaves are extracted into water, so the infusion can be a reliable dietary source of some minerals and nutrients, which must be supplied in the human diet in order to meet the requirements for optimal functioning [12, 16]. The actual amount of elements that can get into the human body, are affected by many factors including: the total element content in dry tea, the amount of tea used to prepare an infusion, characteristics of water used for brewing, the brewing time, fraction of the total content extracted to the infusion and the bioavailability of elements when drinking the beverage [6, 8, 13, 17]. Since the manufacturing process has a strong influence on many features of the tea, the type of tea chosen by consumers is important in view of the intake of certain elements and hence on the health benefits or harmful effects of tea. The potential impact of macro- and microelements contained in tea to the human organism can be estimated by determining their amount extracted to the tea infusion [13]. The knowledge about nutrients and metals content in beverages is important due to the possibility of estimating their contribution to the nutrition requirements [17, 18].
In the present study the concentrations of Ca, F, K, Mg, Na and P in leaf and bagged black tea from the same brands and in their infusions were determined and compared. The objective of the present investigation was to evaluate the percentage transfer of the elements to the tea infusion and determine concentrations of macroelements and F in bagged and leaf black tea of the same brand and to assess whether tea drinking could contribute to the daily nutritional mineral requirements of consumers.
Materials and methods
A selection of ten leaf black teas and ten bagged black teas of the same brand was purchased from the local stores in Wrocław, Poland. The teas were chosen among worldwide commercialised brands to ensure a wider range of the consumer’s interest. Table 1 presents information about a type of each tea, its name, the producer, the geographic origin and the recommended time of steeping. The procedure fully described in Dambiec et al. [19] was followed for the preparation of samples of dry teas and tea infusions as well as the quality control of experiment.
Prior to analysis, five infusion bags were randomly selected from each box of tea and their contents were mixed [6, 13, 17]. Results of Cao et al. [20] study proved that the paper bag did not affect the content of elements in the tea infusion so further analyses were performed on teas without bags. Samples of both bagged and leaf teas were dried at 50 °C to constant weight and stored in a desiccator at room temperature.
Analysis of dry tea material
Prior to analysis of the total alkali metal and phosphorus concentrations in dry teas, the material was milled into fine powder in a laboratory mill IKA Labortechnik M20 to obtain a representative sample. Then samples (1.0 g) were digested in an open system with concentrated nitric acid (HNO3) (65%, analytical grade) and hydrogen peroxide (H2O2) (30%, analytical grade). During the process, the temperature was raised to 95 °C until the evolution of nitrous oxide gas stopped and the clear solutions were achieved. Subsequently, the digests were cooled and diluted to 100 mL with deionized water in volumetric flasks and then filtered to remove any turbidity or particles. Magnesium concentration was determined using FAAS (AVANTA PM by GBC Scientific Equipment, Braeside, Australia); Ca, K and Na concentrations using a flame photometer (JENWAY Ltd. PFP7, Stone, UK) and P concentration by FIA (MLE GmbH, Dresden). The total F content was analysed with the potentiometric detection using the combination fluoride electrode from Sentek (Braintree, UK) and microprocessor-pH-meter HI 9107 from HANNA Instruments (Kehl am Rhein, Germany) after extraction from dry pulverized samples with HNO3 followed by aqueous potassium hydroxide (KOH) according to AOAC method for fluoride determination in plants [21].
Analysis of infusions
The tea infusions were prepared by pouring boiling deionized water (80 mL) over broken tea leaves (2.0 g to represent the typical quantity used by tea drinkers to prepare one cup of tea) in a 250 mL beakers and stirred using a glass rod to ensure proper wetting of leaves and extraction of organic and inorganic matter. Each tea infusion was covered with a watch-glass to minimize evaporation and allowed to steep for 5 min (recommended brewing time, according to the tea industry’s). Then, the solution was filtered, cooled and diluted with deionized water to 100 mL in a volumetric flask. Calcium, K, Mg, Na, and P concentrations were determined using the methods described above. For F determination, 25 mL of the tea infusion was added to 25 mL of TISAB (total ionic strength adjustment buffer) solution and the potentiometric detection of F was carried out as described above.
Chemicals and reagents
HNO3, KOH, H2O2, HCl and TISAB were of analytical grade and supplied by Chempur® (Piekary Śląskie, Poland). Atomic absorption standard solutions of Ca, Mg, K, Na at a concentration of 1.000 g L−1 and P at a concentration of 1.000 mg L−1 were obtained from Sigma-Aldrich® (Poznań, Poland).
Quality control
For all segments of the experiment, plastic and laboratory glassware were cleaned by rinsing with a 10% HCl and next with distilled water. All solutions in the experiment were prepared using deionized water. All analyses were performed on three separate weighted portions of each sample in order to determine the precision of measurements. Blank samples were prepared by following the same protocol but without dry tea material or infusion. The results were calculated on a dry weight basis. The reproducibility of the methods used was compared to the results of an inter-laboratory study through analysing reference material Bush Branches and Leaves, NCS DC73348 LGC standards (China National Analysis Center for Iron & Steel, Beijing, China). The average recovery rate, calculated as a ratio: (the measured element content in analysed certified reference material/content reported by producer)*100%, was found to be 100 ± 3 (percent ± standard deviation) (Table 2).
Data analysis
For each tea sample the mean concentration was calculated based on the results of analysis of the three separate weighted portions. In order to determine differences between bagged and leaf teas of the same brand as well as between their infusion in respect of element concentrations, results were analysed using the U Mann–Whitney test [22]. The differences between teas from various origins was checked using non-parametric Kruskal–Wallis ANOVA [23]. The normality of the features was checked by Shapiro–Wilk’s test [23]. Statistical confidence was set at p = 0.05.
The matrix of concentrations of elements in dry teas and infusions was subjected to ordination to detect possible gradients of element levels by means of the principal component and classification analysis (PCCA). The principal components were computed using the active variables only. A plot of PCCA ordination of the tea samples and projection of the concentrations of elements in teas on the factor plane gives information about similarities among samples and shows correlations between the original variable and the first two factors [24].
The percentage extraction of each element from the dry tea to the infusion was determined by the following ratio: (element concentration in tea infusion/element concentration in dry tea) × 100%. The differences between the element’s percentage extraction from bagged and leaf teas were established using the U Mann–Whitney test [22].
Linear correlations between concentrations of the elements in teas and their infusions were evaluated using Spearman’s correlation coefficients.
The CSS-Statistica Statsoft (StatSoftPolska Ltd., Kraków, Poland, http://www.statsoft.com) [25] was used to perform all statistical calculations.
Results and discussion
The ranges of total element contents for individual dry tea samples and element concentrations in tea infusions examined in the present study are summarized in Tables 3 and 4, respectively.
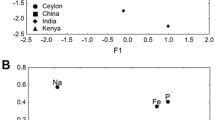
The PCCA ordination of dry tea samples and infusions (Fig. 1) showed that dry teas were correlated positively and infusions negatively with the first principal factor. The projection of the variables on the factor plane showed that dry teas were correlated with a high content of elements while infusions with a low content of elements. Most of the bagged teas were correlated positively with the second principal factor and consequently with the high F content while leaf teas were correlated negatively with the second factor and contained less F. These observations have been confirmed by the statistical analysis. There were no significant statistical differences between bagged and leaf teas of the same brand in respect of the content of macroelements. Only F contents in the bagged teas were significantly higher than in the leaf ones (the U Mann–Whitney test, p < 0.05). It may be associated with the lower quality of tea used in tea bags [10]. Moreover, as suggested by Kumar et al. [26], different element concentrations in different types of tea (e.g. granular tea leaves, powder and tea bags) are result from differences in processing methodologies. The concentration of elements studied were similar in teas from various origins (China, Sri Lanka, mixture) (Kruskal–Wallis ANOVA, p < 0.05).
Ordination plot of the dry tea samples and infusions based on the content of six elements (Ca, F, K, Mg, Na, P) and projection of element contents on the factor plane. Numbers refer to numbers of tea sample in Table 1; full triangle—dry leaf teas, full circle—dry bagged teas, empty triangle—leaf tea infusions, empty circle—bagged tea infusions
In general, the mean concentrations of studied elements could be arranged according to their levels in both dry bagged and leaf teas in the following order: K > Ca > Mg > P > Na > F. This scheme corroborates findings given by many authors [1, 5, 19, 27], who stated that the most abundant element in tea was K. This element may be specifically incorporated within a binding ligand in the tea leaves [1]. The sequence of concentrations in the infusion made from leaf tea was the same as above, but the order slightly differed in the bagged tea infusions and was as follows: K > Ca > P > Mg > Na > F.
The infusions of bagged and leaf teas differed significantly in respect of Ca, Na, K, Mg and F concentrations (the U Mann–Whitney test, p < 0.05). On average, a higher content of these elements in the infusions made with bagged teas than in infusions made of leaf teas was observed. Also, the average extraction depended on the type of tea. For the bagged teas the extraction rate was the highest for K, followed by F, Na, Ca, Mg and P. While for the leaf teas the sequence was as follows: F > K > Na > Ca > P > Mg. The percentage extraction of Mg, K and Na from dry tea to the infusion was statistically significantly higher for bagged than for leaf teas (the U Mann–Whitney test, p < 0.05). Generally, tea bags contain finer particles than loose leaf teas, which may result in a better solubility of the elements [11]. The sequences of extraction and percentage transfer of the elements differed from those noted for different types of commercially purchased tea by Gallaher et al. [28], who observed that K and P were extracted in high amounts while Ca and Na were least soluble.
Calcium is an essential but controversial nutrient: there is no consensus on the level of human calcium requirement or the significance of calcium deficiency. The negative calcium balance, which may be the result of an inadequate calcium intake, is the major cause of osteoporosis. Inadequate intakes of this element have been associated with nephrolithiasis, colorectal cancer, hypertension and stroke, coronary artery disease, insulin resistance and obesity [29, 30]. Calcium consumption varies greatly worldwide, ranging from 300 mg in some developing countries to 800 mg per day or more in industrialized countries [30]. The calcium content in the teas studied (Table 3) were similar to obtained by Gebretsadik and Chandravanshi [8] (3821–4419 mg kg−1) and Malik et al. [4] (4326–6678 mg kg−1) in black tea as well as Carr et al. [31] (3560 ± 364 mg kg−1) for young tea leaves. The average total Ca rate leached from tea to the infusion was 44.1% for bagged teas and 36.6% for leaf teas and was significantly higher than noted by Gebretsadik and Chandravanshi [8] (12.5%), Malik et al. [4] (1.8–3.3%), Szymczycha-Madeja et al. [6] (ND-17.0%) and Brzezicha-Cirocka et al. [14] (7–15%).
Sodium is the principal cation in extracellular fluid in the body and an essential nutrient, necessary for the maintenance of plasma volume, acid–base balance, transmission of nerve impulses and normal cell functions. Sodium is found naturally in a variety of food and is the chemical component of common table salt [32]. According to Szymczycha-Madeja et al. [6], it is also one of the major constituents in black teas. The minimum intake level necessary for the proper functioning of the body is estimated to be as little as 200–500 mg day−1. In many countries the sodium consumption is above the recommended value of 2.0 g sodium day−1, which is associated with the increased blood pressure and less clearly with cardiovascular diseases [32]. The sodium concentrations found in present study (Table 3) were slightly higher than reported by Yemane et al. [1] (84.9–265 mg kg−1) and Dambiec et al. [19] (156–189 mg kg−1) as well as Kumar et al. [26] (21–118 mg kg−1). The average percentage transfer of the total Na content to the infusion (57.2 and 52.5% for bagged and leaf tea, respectively) was lower compared to Soomro et al. [33] (65%) and Szymczycha-Madeja et al. [6] who classified Na as highly extractable (> 55%).
Potassium is an element essential to maintain the total body fluid volume, acid and electrolyte balance as well as normal cellular functions. The consumption of large amounts of K from food is generally safe, because the body is able to successfully adapt and excrete the excess of this nutrient. While too low K consumption is associated with hypertension and many cardiovascular diseases, particularly coronary heart disease and stroke. Some studies suggest that K can help in mitigating the negative effects of excessive Na consumption on the blood pressure [34]. Potassium contents in teas studied (Table 3) were higher than noted in old leaves of Camellia sinensis studied by Carr et al. [31] (15,239 ± 2552 mg kg−1) as well as in different black trade labeled teas (17,250 ± 1235 and 14,235 ± 1146 mg kg−1) [5, 27]. The average total K content leached from the dry tea to the infusion was 64.1% for leaf teas and 73.4% for bagged teas, so this metal can be classified as highly extractable (> 55%) [6]. Gallaher et al. [28] noted similar excellent K extraction rates from the herbal teas (61.1–80.7%), while Olivier et al. [5] reported higher than our concentrations in the infusions in spite of lower K content in dry teas.
Magnesium is the fourth most abundant cation building the human body. Low Mg status was proved to cause some severe disturbances e.g. hypertension, coronary heart disease, type 2 diabetes mellitus and metabolic syndrome [30]. Too low Mg amount in a diet may cause increased insulin resistance which leads to accelerated atherosclerosis and premature death. Consumption of large doses of Mg, e.g. in drinking water, was inversely associated with the risk of hypertension and cardiovascular diseases. Moreover, it is considered as an important protective factor for acute myocardial infarction [35]. The magnesium contents in teas on Polish market (Table 3) were higher than concentrations noted in the black tea leaves by Ramdani et al. [12] (1726 ± 167 mg kg−1) but corresponded well to the data of Malik et al. [4] (2033–2648 mg kg−1) and Salahinejad and Aflaki [18] (1861–2029 mg kg−1). The present contents in dry teas (Table 3) as well as in infusions (Table 4) were slightly lower than in bagged teas available in Poland [19] but similar to results of Olivier et al. [5]. The percentage transfer of the total Mg content to the infusion (mean 39.2% for bagged teas and 32.0% for leaf teas) was in ranges of 15.2–53.1% reported by Gallaher et al. [28] and corresponded well with the value of 34% noted by Brzezicha-Cirocka et al. [14]. According to classification of Szymczycha-Madeja et al. [6] this element is moderately extractable (20–55%).
Phosphorous is a basic structural element of animal and plant bodies, building i.e. phospholipids, cell membranes, nucleic acids, a number of enzymes, hormones and intracellular signaling molecules. It is found in most food products, but mainly the protein-rich food groups, including dairy products, meat and fish. A number of studies have shown a correlation between high levels of P in serum and increased death risk for patients with kidney diseases [36]. Consumption of too high rates of P causes metastatic calcification, skeletal porosity and interference with calcium absorption [37]. The phosphorous contents in teas studied (Table 3) were higher than in trade labeled teas studied by Desideri et al. [27], which ranged between 1010 and 1499 mg kg−1. The percentage transfer of total P from dry teas to tea infusions (mean 37.5% for bagged teas and 35.2% for leaf teas) were higher than noted previously for bagged teas in Poland [19], however it fitted well in ranges observed by Gallaher et al. [28] for various herbal teas (12.4–57.9%). Our results are in agreement with Szymczycha-Madeja et al. [6] who classified phosphorous as a moderately extractable element.
Fluorine is considered an essential element for humans, but while low F concentrations provide protection against dental caries and promote healthy bone growth, F can also have an adverse effect—concentrations above 1.5 mg/l in drinking water carry an increasing risk of dental fluorosis, and much higher concentrations lead to skeletal fluorosis [38]. Concentrations of F in the teas studied (Table 3) were similar to obtained for black teas by Koblar et al. [9] (68–435 mg kg−1) but higher than reported by Cao et al. [20] (96.9–223 mg kg−1). However, Chan et al. [13] and Shu et al. [39] found F content in black tea higher than presented (ranging between 93 and 820 mg kg−1and between 590 and 708 mg kg−1, respectively). The percentage transfer of the total F content to the infusion was 70.8% for bagged tea and 73.4% for leaf tea. These findings are similar to obtained by Koblar et al. [9] who reported the efficacy of the F leaching 67–90% in the black teas and found no significant difference between percentage transfer of F for bagged and stick-shaped tea. Fluorine concentrations in the tea infusions in the present study (Table 4) were similar compared to Malinowska et al. [40] (0.32 and 4.54 mg L−1) and Chan et al. [13] (0.43–8.85 mg L−1). Malinowska et al. [40] reported higher fluorine levels in extracts prepared from granulated and powdered tea wrapped in bags compared to loose leaf tea.
In the present study statistically significant correlations, expressed by Spearman’s correlation coefficients (p < 0.05), were found between some elements content in dry teas and their infusions. Particularly, strong positive correlations were found between Na, P and F content in the dry teas and their concentration in infusions (r = 0.66, r = 0.80, r = 0.89 for leaf teas, and r = 0.85, r = 0.84, r = 0.99 for bagged teas, respectively), which suggest that the mineral content of the infusions was directly related to the content of elements in the dry tea.
Generally, tea infusions are considered to contain little amounts of minerals (Fig. 1), and therefore they do not make a valid contribution to meeting the mineral requirements of the human organism [37]. Low intakes of essential minerals from food and water are common in many parts of the world [36]. The present results showed that the daily contribution from drinking three cups of tea to the total exposure of most of the essential elements cannot be regarded as considerable. Tea provides only 1.28% of Ca, 1.84% of K 1.78% of Mg and below 1% of P daily recommended intakes (DRI) (Table 5). These conclusions are consistent with results of Kalantar-Zadeh et al. [36] who claimed that one cup of tea provides less than 1% of the K DRI for adults. Szymczycha-Madeja et al. [6] reported similar percentage contribution of tea to Mg and K daily doses. Also, Brzezicha-Cirocka et al. [14] stated that consumption of black tea is not likely to contribute significantly to Ca and Mg daily intake. However, in case of Na and F the contribution can be substantial because tea provides up to 77.5% of sodium and 37% of fluorine DRI [41].
The composition of tea infusion is strongly related to the water composition because it affects chemical extraction from tea leaves [28, 42]. In the present experiment, distilled water was used to prepare tea infusions. However, consumers prepare tea beverages with water from various sources and hence containing different elemental concentration, which may affect element concentrations in the infusions. Moreover, the element content in infusion is determined by conditions of extraction e.g. the amount of tea per volume of water, temperature, an area of extraction and the brewing time [43]. Consumers concerned with minimizing of the intake of certain minerals, e.g. patients on a low Na diet or suffering from renal difficulties, who have dietary restrictions on Na, P or K, should know the mineral content of their local tap water to assess the complete daily intake of elements [28]. Moreover, the present results suggest that black tea consumption may significantly contribute to the F intake, especially when the infusion is prepared using bagged teas. DRI for fluorine is only 3.00 mg day−1 [41] and this safe level can be easily exceeded by tea drinkers additionally exposed to other sources of fluoride (drinking water fluoridation, fluoride-containing toothpaste).
References
M. Yemane, B.S. Chandravanshi, T. Wondimu, Food Chem. 107, 1236–1243 (2008)
L. Yao, X. Liu, Y. Jiang, N. Caffin, B. D’Arcy, R. Singanusong, N. Datta, Y. Xu, Food Chem. 94, 115–122 (2006)
C.S. Yang, J.M. Landau, J. Nutr. 130, 2409–2412 (2000)
J. Malik, J. Szakova, O. Drabek, J. Balik, L. Kokoska, Food Chem. 111, 520–525 (2008)
J. Olivier, E.A. Symington, C.Z. Jonker, I.T. Rampedi, T.S. van Eeden, S. Afr. J. Sci. 108, 1–7 (2012)
A. Szymczycha-Madeja, M. Welna, P. Pohl, Trends Analyt. Chem. 35, 165–181 (2012)
G. Chen, C. Yang, S. Lee, C. Wu, J.T.C. Tzen, J. Food Drug Anal. 22, 303–309 (2014)
D. Gebretsadik, B. Chandravanshi, B. Chem. Soc. Ethiopia 24, 339–349 (2010)
A. Koblar, G. Tavčar, M. Ponikvar-Svet, Food Chem. 130, 286–290 (2012)
M.H. Wong, K.F. Fung, H.P. Carr, Toxicol Lett. 137, 111–120 (2003)
A. Hicks, AU J. Technol. 12, 251–264 (2000)
D. Ramdani, A.S. Chaudhry, C.J. Seal, J. Agric. Food Chem. 61, 4961–4967 (2013)
L. Chan, A. Mehra, S. Saikat, P. Lynch, Food Res. Int. 51, 564–570 (2013)
J. Brzezicha-Cirocka, M. Grembecka, P. Szefer, Eur. Food Res. Technol. 242, 383–389 (2016)
S.M.T. Gharibzahedi, S.M. Jafari, Trends Food Sci. Technol. 62, 119–132 (2017)
A. Pękal, M. Biesaga, K. Pyrzynska, Food Sci. Biotechnol 22, 925–930 (2013)
I.A. Hakim, U.M. Weisgerber, R.B. Harris, D. Balentine, C.A.J. van-Mierlo, I. Paetau-Robinson, Nutr. Res. 20, 1715–1724 (2000)
M. Salahinejad, F. Aflaki, Biol. Trace Elem. Res. 134, 109–117 (2010)
M. Dambiec, L. Polechońska, A. Klink, J. Food Comp. Anal. 31, 62–66 (2013)
J. Cao, S.F. Luo, J.W. Liu, Y. Li, Food Chem. 88, 233–236 (2004)
W. Horowitz, AOAC Official Methods of Analysis, 17th edn. (Association of Official Analytical Chemists International, Gaithersburg, 2000), pp. 51–56
A. Stanisz, Statistics Course with STATISTICA PL Based on Medicine Example, 3rd edn. (StatSoft Polska, Kraków, 2006), pp. 369–381
R.R. Sokal, F.J. Rohfl, Biometry: The Principles and Practice of Statistics in Biological Research, 4th edn. (W.H. Freeman, New York, 2012), pp. 220–228
P. Legendre, L. Legendre, Numerical Ecology, Developments in Environmental Modeling, 2nd edn. (Elsevier, Amsterdam, 1998), pp. 391–423
Inc StatSoft, 2009. STATISTICA (Data Analysis Software System), Version 9.0. http://www.statsoft.com
A. Kumar, A.G.C. Nair, A.V.R. Reddy, A.N. Garg, Food Chem. 89, 441–448 (2005)
D. Desideri, M.A. Meli, C. Roselli, L. Feduzi, Microchem. J. 98, 186–189 (2011)
R.N. Gallaher, K. Gallaher, A.J. Marshall, A.C. Marshall, J. Food Comp. Anal. 19, 53–57 (2006)
L. Fishbein, Regul. Toxicol. Pharm. 39, 67–80 (2004)
S.A. Atkinson, R. Costello, J.M. Donohue, Overview of global dietary calcium and magnesium intakes and allowances, Calcium and Magnesium in Drinking-Water: Public Health Significance, ed. by J. Cotruvo, J. Bartram (World Health Organization, Geneva, 2009)
H.P. Carr, E. Lombi, H. Küpper, S.P. Mcgrath, M.H. Wong, Agronomie 23, 705–710 (2003)
WHO, Guideline: Sodium Intake for Adults and Children (World Health Organization, Geneva, 2003), pp. 11–14
M.T. Soomro, E. Zahir, S. Mohiuddin, A.N. Khan, I.I. Naqvi, Pak. J. Biol. Sci. 11, 285–289 (2008)
WHO, Guideline: Potassium Intake for Adults and Children (World Health Organization, Geneva, 2012), pp. 10–12
C.N. Ong, Minerals from Drinking Water: Bioavailability for Various World Populations and Health Implications. Rolling Revision of the WHO Guidelines for Drinking-Water Quality (World Health Organization, Geneva, 2004)
K. Kalantar-Zadeh, L. Gutekunst, R. Mehrotra, C.P. Kovesdy, R. Bross, C.S. Shinaberger, N. Noori, R. Hirschberg, D. Benner, A.R. Nissenson, J.D. Kopple, Clin. J. Am. Soc. Nephrol. 5, 519–530 (2010)
National Research Council, Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride (The National Academies Press, Washington, 1997), pp. 146–190
WHO; Fluoride in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality (World Health Organization, Geneva, 2004), pp. 29–35
W.S. Shu, Z.Q. Zhang, C.Y. Lan, M.H. Wong, Chemosphere 52, 1475–1482 (2003)
E. Malinowska, I. Inkielewicz, W. Czarnowski, P. Szefer, Food Chem. Toxicol 46, 1055–1061 (2008)
Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes (DRIs): Recommended Dietary Allowances and Adequate Intakes. Vitamins (National Academies, Washington, 2011), pp. 2–6
A. Mossion, M. Potin-Gautier, S. Delerue, I. Le Hecho, P. Behra, Food Chem. 106, 1467–1475 (2008)
M. Długaszek, Ż. Kurpiewska, J. Mierczyk, Eur. Food Res. Technol. 241, 289–293 (2015)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Klink, A., Dambiec, M., Polechońska, L. et al. Evaluation of macroelements and fluorine in leaf and bagged black teas. Food Measure 12, 488–496 (2018). https://doi.org/10.1007/s11694-017-9662-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9662-7