Abstract
Wild almond (Amygdalus scoparia) is a drought-resistant, non-cultivated plant rich in protein. The extraction of protein isolates from wild almond was carried out using NaOH, buffered saline borate (BSB), and Tris–HCl and the isolates were characterized by their physico-chemical, thermal and emulsifying attributes. Use of BSB solution resulted in the maximum recovery of protein (56%, w/w) from wild almond. The amino acid compositions of wild almond protein isolates (WAPIs) were identified by high-performance liquid chromatography after acid hydrolysis. Aspartic and glutamic acids were the most abundant amino acids found in the WAPIs. Differential scanning calorimeter showed that WAPI is denatured above 80 °C. It was also found that the surface hydrophobicity values of the NaOH- and BSB-extracted proteins were 375 ± 6 and 359 ± 5, respectively. Water absorption capacities of isolates extracted by NaOH and BSB were 2.3 ± 0.4 and 2.6 ± 0.3 g water/g protein, respectively. In addition, oil absorption capacities for the isolates extracted using NaOH and BSB were 3.5 ± 0.7 and 3.1 ± 0.4 g oil/g protein, respectively. Moreover, the solubility levels of the extracted isolates increased up to 85.3% under alkaline conditions. The emulsion-activity indices of isolates increased at pH <4 and also at pH >4.5. In addition, the highest foaming capacity was obtained at pH 2 for the extracted isolates. In conclusion, the study has shown that WAPIs can enhance the oil absorption capacity, emulsifying and foaming properties. Thus, they could be considered for application as an ingredient for functional foods processed in alkaline conditions at temperatures below 80 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Apart from their nutritional values, proteins are important in the development and processing of food products. It has been reported previously that when products are made with similar organoleptic characteristics, consumers tend to prefer those made with plant-based proteins to those made with animal-based proteins [1]. Several studies on the physicochemical properties of proteins from bitter melon [2], oat seed [3], cashew nut [4], sesame seed [5], and mustard seed [6] have been reported. Among the nuts, almonds are considered as good sources of high-quality proteins with 16–22% protein contents (on a dry basis) [7]. A few studies have reported on the nutritional and functional properties of common species of edible almond (Prunus dulcis L.) [7,8,9]. Apart from the common variety of cultivated almond, around 20 species of wild almond (Amygdalus scoparia) have been reported in Iran [10]. Wild almond, which is abundant in several Iranian provinces, is a highly valuable and cost-effective plant [11]. Several studies have focused on different aspects of oil extraction from wild almond and have found that oil can contribute ~50% (w/w) of the kernel’s weight [11,12,13]. Consequently, the amount of protein in the residue after oil removal can reach up to 42% of the kernel’s weight [14] making wild almond a good candidate for protein supplementation in food and nutraceutical applications. Surface hydrophobicity (SH) and thermal behaviour have been among the parameters most commonly investigated in protein characterization. SH can demonstrate the extent of hydrophobic molecules on the surface of proteins. Thermal behaviour is important for determining the heat resistance of the protein in a food product [2]. Following previous studies, the current work aimed to assess the effects of commonly used solvents such as NaOH, buffered saline borate (BSB) and Tris–HCl on the extraction efficiency of wild almond protein, characterization of the protein isolates and the evaluation of the functional properties (solubility, emulsifying and foaming properties, and water- and oil-absorption capacities) of the extracted isolates.
Materials and methods
Materials
Seeds of a specific almond variety (A. scoparia) were collected from the forests of Sirjan (Kerman, Iran). The reagent 1-anilino-8-naphthalene sulphonate (ANS) and Tris–HCl buffer were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). All other solvents and chemicals used in this study were of analytical grade and purchased from Merck Chemical Co. (Darmstadt, Germany). Sunflower oil used in the emulsifying experiments was purchased from a local store.
Preparing almond flour for protein extraction
To prepare almond flour, kernels were first debittered by soaking in water for 24 h, during which the water was refreshed several times. In the next step, the brown skin of the almond kernels was removed manually and the remaining parts were dried in an oven (W. C. Hanau Co, FT420, Germany) at 40 °C and ground using a Moulinex grinder (AR1044, France). The ground samples were then passed through a 40-mesh sieve (ASTM-E:11, Damavand, Iran) and defatted for 4 h with n-hexane as solvent using a Soxhlet apparatus. The protein contents of the defatted samples were determined using a Kjeldahl apparatus in three replicates and a 5.18 conversion factor was applied for final determination purposes [15].
Preparation of wild almond protein isolates
To prepare wild almond protein isolates (WAPIs), the defatted wild almond flour from the previous stage was mixed at 1:10 ratio, w/v, for 1 h with different extraction solvents including BSB (1.0 M H3BO3, 0.025 M Na2B4O7, and 0.075 M NaCl adjusted to pH 8.45); 0.02 M Tris–HCl (adjusted to pH 8.1) and 1.0 N NaOH (adjusted to pH 9.1) [16]. The mixtures were then magnetically stirred under ambient temperature. The suspensions were then centrifuged at 12,000×g (Sigma- 3–18 K, Osterode am Harz, Germany) for 20 min, subsequently the supernatants were adjusted to an isoelectric point (IP) of 3.8 by 0.1 N HCl solution and the suspension was centrifuged again at 10,000×g for 15 min. Using deionized water, the recovered precipitates from the previous stage were washed and centrifuged again at 10,000×g, adjusted to pH 7.0 (using a 1.0 N NaOH solution), freeze-dried (Alpha 1-2 Lo Plus, Christ, Germany), and stored at 4 °C for the analytical measurements. All measurements were carried out in triplicate. Protein recovery was defined as the amount of extracted protein from 100 g original crude protein present in wild almond and was determined using the following equation:
Characterization of WAPIs
Determination of amino acid composition and content
A preliminary hydrolysis reaction was carried out to determine the amino acid compositions of wild almond proteins. Briefly, 200 mg of substrate was mixed with 10 mL HCl (6 N) and later incubated in a laboratory oven at 110 °C for 24 h, after which the samples were filtered and diluted to 25 mL. The solutions were then passed through a 0.45-µm filter and specified amounts (10, 20 and 40 µL) were pipetted into separate glass vials and dried using a vacuum oven at 40 °C. Ethanol-water-trimethylamine-phenyl iso-thiocyanate mixture (at 1:1:1:7 ratios, v/v/v/v) was used as the derivatization solvent. Twenty microliters of the solvent was added to the samples and the solutions were held for 20 min to complete the derivatization process. These steps were also used for standard amino acids. The amino acid composition was determined by a high performance liquid chromatograph system (Beckman Instruments Inc., Palo Alto, California, USA) equipped with a Waters 2487 dual-absorbance UV detector (Gilson Inc., Middleton, Wisconsin, USA). The calibration curve was obtained by injecting various concentrations of each amino acid standard.
Analysis of thermal properties
The thermal properties of the WAPIs were determined using a differential scanning calorimeter (DSC) (2010 Modulated DSC, TA Instrument, New Castle, DE) equipped with a thermal analysis software (version 8.0.0.0172). For this purpose, the WAPIs were rehydrated with deionized water to produce 20% protein solution (w/v) and were then left for 30 min in order to reach full thermal equilibrium. Fifty microliters of the protein solutions were then transferred into the aluminum pans and heated from 25 to 140 °C at a rate of 10 °C/min. An empty pan, used as the reference, was also heated in the same way. The instrument was calibrated using indium and zinc (Perkin-Elmer standards) prior to the measurement.
Surface hydrophobicity measurement
SH was determined by the method described by Horax et al. [2]. Protein solutions were prepared using a 0.01 M phosphate buffer (pH 7.0) to obtain concentrations ranging from 0.001 to 0.015% (w/v). Then, 1.6 mM ANS solution was prepared in a 0.01 M phosphate buffer (pH 7.0). Four milliliters of the diluted protein solutions were mixed with 50 µL of ANS. The fluorescence intensities were recorded using a spectrofluorimeter (Cary Eclipse, Varian, Austria) at 390 nm for the excitation and 470 nm for the emission wavelengths [2]. The fluorescence readings were made for triplicate samples. The SH was determined as the slope of the linear regression between the fluorescence intensities and protein concentrations.
Water absorption capacity
Experiments to determine water absorption capacity (WAC) were conducted according to the method described by Ogunwolu et al. [4]. For this purpose, 100 mg protein isolates were mixed with 1000 µL distilled water. The protein suspension was then centrifuged at 1800×g for 20 min at ambient temperature. The supernatant was discarded and for the purpose of better separation the tubes were placed at 45° angles. WAC was determined using the following equation [4]:
Oil absorption capacity
Oil absorption capacity (OAC) was determined according to the method described by Zhong et al. [17]. Briefly, 100 mg of each protein isolate and 1000 µL of sunflower oil were mixed using a vortex (Pole Ideal Pars, LP8809, Iran) for 30 s. The suspension was then incubated for 30 min at ambient temperature followed by 10 min centrifugation at 13,000×g. Finally, the supernatant was discarded and the OAC was determined using the following formula:
Solubility behaviour
To determine the effect of pH on the solubility of WAPIs, 100 mg of the protein isolates was mixed with 10 mL of deionized water and the pH levels of the obtained suspensions were adjusted to 2.0, 4.0, 6.0, 8.0, and 10.0 by adding 1.0 M HCl or 1.0 M NaOH. The samples were then stirred for 30 min at ambient temperature with pH re-adjustment every 10 min followed by centrifugation at 12,000×g for 20 min. Finally, the protein content was determined by the Kjeldahl method in triplicate and solubility was determined as follows [2]:
Emulsion properties
The emulsion properties of the protein isolates were determined using the method described by Pearce and Kinsella [18]. In brief, WAPIs were dispersed in deionized water in order to prepare 0.1 and 1.0% protein solutions. Then, the pH levels of the solutions were adjusted to 2.0, 4.0, 6.0, 8.0 and 10.0, and 10 mL sunflower oil was added. The prepared suspensions were homogenized for 1 min at 20,000 rpm using a homogenizer (Wise Tis, Daihan Scientific Co. Ltd., Korea). In the next step, 50 µL of the emulsion was sampled from the bottom of the container at 0 and 10 min after homogenization and mixed with 5.0 mL 0.1% sodium dodecyl sulfate. The absorbance at 500 nm was determined using a UV–Visible spectrophotometer (Unico, S2100SVIS, Plainfield, NJ). The emulsion-activity index (EAI) and emulsion-stability index (ESI) were determined as follows [18]:
where A0 is the absorbance measured immediately after homogenization, A10 is the absorbance after 10 min time lapse, ΔT is the time interval (10 min), and ΔA = A0─A10.
Foaming properties
Foaming capacity (FC) and foaming stability (FS) were determined by the methods described by Sze-Tao and Sathe [7] with slight modifications. Briefly, WAPIs were mixed with deionized water to produce 1 and 4% (w/v) of protein-isolate solutions and the pH levels were adjusted at 2.0, 4.0, 6.0, 8.0, and 10.0. The suspensions were then whipped with a blender system (Panasonic, Deluxe Super Blender MX-T7GN, Japan) for 3 min and immediately poured into a cylinder gradually. Three samples were prepared at each pH value. The following equation was used to determine FC [7]:
where v1 is the volume of the suspension before whipping and v2 is its volume after whipping stage. FS was calculated according to the following formula [7]:
where v3 is the volume of the foam after 60 min.
Statistical analysis
The experiments were conducted in three replicates and results are presented as the mean ± standard deviation. Analysis of variance (ANOVA) procedure followed by Duncan’s multiple-range tests using the SAS statistical computer package (version 9.2, SAS Institute Inc., Gary, NC) was applied to determine the significant differences (P < 0.05) among the means.
Results and discussion
Isolation of wild almond protein
The protein content of wild-almond flour was 20% (g protein/g wild almond) before defatting and 38% (g protein/g defatted wild almond) after that stage. Results revealed that the maximum yield of WAPIs (56%, w/w) was obtained by using BSB solution followed by NaOH (51%). On the other hand, WAPIs obtained by using Tris–HCl had the lowest amount of yield (37%) and therefore the samples obtained by using this solution were not considered for further analysis. The higher yield of recovery obtained by BSB could be due to the attraction of globulin, a salt-soluble and dominant fraction of wild almond proteins by the sodium chloride in BSB [2]. These results support previous findings by Sathe et al. [16], who reported significantly higher protein solubility in BSB than that in Tris–HCl. In addition, the skin of wild almond was removed to enhance the extraction efficiency of the proteins [19]. The phenolic compounds (in the almond husk) are sensitive to oxidation especially in alkaline medium and can react with available proteins by covalent and non-covalent bonds resulting in a decreased protein solubility [20].
Characteristics of WAPIs
Amino acid composition
The amino acid compositions of WAPIs and the levels of essential amino acids recommended by FAO/WHO [21] are presented in Table 1. In the present study, the amino acid compositions of WAPIs were investigated from two points of view. First, nutritional values and, second, their impacts on the physicochemical properties. According to the recommended levels by FAO/WHO [21] for adults and children, the first and the second limiting amino acids detected in the WAPIs were sulfur-containing amino acids (cysteine + methionine) and lysine. Based on the recommendation by FAO/WHO [21] for children, threonine was the third limiting amino acid in WAPIs. It should be noted that the amounts of essential amino acids such as valine, leucine, phenylalanine and threonine, in WAPIs are similar to the amounts reported for soybean protein [2]. Apart from their nutritional values, the isolates were also characterized by high contents of Glx (mixture of glutamic acid and glutamine) and Asx (mixture of aspartic acid and asparagine). These amino acids formed about 47% of total amino acids of the protein. Total protein contents of the extracted isolates by BSB and NaOH are also shown in Table 1.
Thermal properties
Protein thermal analysis offers accurate information about the denaturation temperature required in food processes especially in thermal treatments [22]. Thermal properties of the extracted isolates are shown in Table 2. Although the denaturation temperature of BSB-extracted isolate was higher than that of the NaOH-extracted isolate, BSB isolate required less energy for the denaturation (identified by ΔCp, heat capacity). The denaturation temperature is affected by the amino-acid compositions and the structures of the proteins [23]. The obtained data for the denaturation temperatures of WAPIs in the current study were very close to that reported by Horax et al. [2] for soy-protein isolate. Based on the obtained information, WAPIs can be recommended for use in the products subjected to thermal processes of lower than 80 °C. High levels of Glx and Asx (47% in the current study) can result in thermodynamically less-stable protein isolates [2]. On the other hand, total amounts of hydrophobic amino acids such as alanine, phenylalanine, valine, leucine, iso-leucine, and tyrosine in WAPIs were around 25%. These amino acids can participate in the thermal stability of the proteins due to the formation of a compact core [24]. As a consequence, overall thermal behavior of WAPI can be justified by the combination of relatively higher amounts of hydrophobic amino acids (25%) and also those of Glx and Asx (47%) in wild almond in comparison with soy-protein isolate [2].
Surface hydrophobicity
SH is one of the major parameters in determining the extent of hydrophobic regions on the surface of the protein molecules influencing their interfacial tension and emulsion properties [6]. Therefore, amino acid composition on the surface of the protein isolates can impact the SH values. Table 2 shows the SH values of the isolates extracted by NaOH and BSB. The differences between SH values may be due to the differences in the efficiencies of buffers applied in the extraction process. The SH values of most protein isolates have been shown to be within the range 133–832 [23]. It is well known that hydrophobicity levels of proteins are related to the amount of non-polar components they contain. When proteins enter the aqueous environment, the hydrophobic amino acids aggregate on the interior side of the protein and the polar residues align to the outer sections [7]. Thus, only a limited amounts of hydrophobic amino acids remains on the surface of the protein [7]. The obtained values in the present study were lower than the SH value for the soy-protein isolate (650–793) reported by Shevkani et al. [23]. According to Deng et al. [25], a combination of charged amino acids and low hydrophobicity was found to promote the solubility of protein.
Water absorption capacity
The WAC values of BSB- and NaOH- extracted isolates in the current study were found to be significantly different (Table 2). BSB-extracted isolate was found to possess higher WAC than that extracted by NaOH, which is due to the fact that BSB-extracted isolate exhibit higher solubility and charged amino acids at the working pH. According to Moure et al. [26], WAC is improved by the presence of charged amino acids and the application of water treatments such as soaking. Thus, the soaking treatment in debittering process could be considered as an effective factor for increasing the tendency of WAPIs to absorb water. The obtained results were comparable with the reported WAC values for Lathyrus clymenum (2.4 g water/g protein) [27] and cashew-nut isolate (2.2 g water/g protein) [4].
Oil absorption capacity
The OAC levels for the WAPIs extracted by NaOH and BSB are presented in Table 2. The reported data in the current study falls within a range of 3.1 (for BSB-) and 3.5 (for NaOH-extracted WAPIs) and are in agreement with the results of Sze-Tao and Sathe [7] for almond protein isolate (3.5 g of oil/g protein isolate). In addition, the OAC levels of the extracted isolates were significantly lower than that of the soy protein isolate reported by Sharma et al. [28]. Since OAC improves the sensory attributes and affects the emulsifying capacities of food products, WAPIs could potentially be utilized as an ingredient in bakery products and cold meat industry [25].
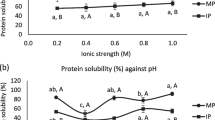
Solubility behavior
The effects of pH on the solubility levels of WAPIs extracted by NaOH and BSB are shown in Fig. 1. Minimum solubilities of NaOH (7.5%) and BSB isolates (3.2%) were obtained at pH 4.0, which was near the determined IP (3.8) of wild almond protein. Since electrostatic repulsion is minimum around IP, aggregation of proteins occurs at this point and thus results in the lowest solubility [29]. On the other hand, at alkaline conditions, the solubility levels of extracted isolates were increased up to 85.3 and 87.8% for NaOH and BSB isolates, respectively. These findings are in agreement with a number of studies including those by Kumar et al. [22] and Malomo et al. [29], which show that, due to their acidic properties, most proteins demonstrate low solubility levels around the pH range of 4–5 and high solubility levels at alkaline pH levels. In addition, the presence of high amounts of charged amino acids (Asx and Glx) results in an increase in the protein solubility [2]. WAPIs exhibited good solubility levels over the alkaline pH ranges making them potentially good candidates for food formulations such as meat and dairy analogues and protein-rich beverages [30].
Emulsion properties
Effects of pH and protein concentration on the emulsion properties of WAPIs are shown in Fig. 2. The results showed that EAI increased at pH <4 and also at pH >4.5. The pH-EAI curves were similar to the pH-solubility profiles of the protein isolates (Fig. 2a). The highest EAI was found at pH 2.0 for BSB-extracted isolate at 0.1% protein concentration. According to Majzoobi et al. [31], low solubility and weak hydratability of proteins at their IP’s lead to less migration to the oil–water interface and decreases emulsion activity. In addition, the obtained data showed that as the protein concentration increased to 1%, the emulsifying properties of WAPI were decreased at all pH levels (Fig. 2). Ogunwolu et al. [4] justified that when low concentrations of proteins are added to the emulsions, the greater amounts of unfolded polypeptide chains could be formed during the homogenization. As a result, the unfolded peptides containing hydrophobic parts would be able to stand better on the oil–water interface resulting in an enhancement in the emulsion properties [4]. As shown in Fig. 2b, the maximum ESI was found at pH 4 near the IP values of all extracted isolates. In addition, the emulsion stability of the suspension in the presence of 0.1% (w/v) protein isolate was higher than that at 1.0% (w/v). According to Mwasaru et al. [32], at the IP of proteins, the distance between the molecules was at its minimum due to the reduction of repulsion forces, while maximum protein adsorption and viscoelasticity were reported at the oil–water interface resulting in an increase in the emulsion stability at pH 4.
Foaming properties
The effects of pH on the foaming properties of protein isolates extracted by BSB and NaOH are shown in Fig. 3. One of the effective parameters on the FC and FS is solubility [4]. Besides, pH variations can transform the proteins conformations resulting into an alteration in the FC and FS values [25]. As shown in Fig. 3a, FC trends of the WAPIs were similar to the solubility curves at different pH values. Both BSB- and NaOH-extracted isolates presented low FC values of 8.1% (v/v) at pH 4.0. The highest FC value was obtained when using 1.0% NaOH solution at pH 2.0 (42.3%), while BSB-extracted isolate showed the highest value at pH 10 (34.5%). FC increased at pH values above 5.0 and below 4.0. An increase in the foaming properties was due to the hydrophilic interactions in the aqueous phase that caused unfolding in the protein structure [31].
The FS values of the WAPIs at various pH levels were studied over the period of 1 h. As shown in Fig. 3b, over a pH range of 2.0–10.0, the highest FS for the WAPIs were found at pH levels of 2.0, while the lowest stability was obtained at pH ~4.0. According to Deng et al. [25], the coagulation of protein in the isoelectric range reduced the stability of polypeptide chains at the air–water interface. Finally, it was found that at pH 2, increasing the concentrations of BSB protein isolates to 4% resulted into an increase in the FC and FS values up to their maximum values of 70 and 100%, respectively.
Conclusion
In the present study, three alkaline solvents (NaOH, BSB, Tris–HCl) were employed to extract protein from wild almond. Methionine and cysteine were the first limiting amino acids, while aspartic and glutamic acids were the most abundant amino acids found in the WAPIs. The results of thermal analysis indicated that the denaturation of the WAPIs occurs at a temperature above 80 °C. Since OAC is important in improving the mouth feel and flavor retention of the final product, the study results suggest that WAPIs could be useful ingredients for bakery industry [25]. WAPIs exhibited good solubility levels over the alkaline pH ranges making them potentially good candidates for food formulations. There was a negative relationship between the protein concentration and EAI. Both BSB- and NaOH-extracted protein isolates had the lowest FC at pH values between 4.0 and 5.0, which were around the IPs of both BSB- and NaOH- extracted isolates. Based on the obtained data, the FC values of the isolates were acceptable especially in the presence of 4.0% WAPI. Moreover, the stability of the foam was considerable in extreme alkaline and acidic conditions. The extracting solvents (NaOH and BSB) had no significant impact on the physicochemical properties of the obtained isolates. This study also suggests that BSB as an extracting solvent presented higher recovery for the preparation of WAPI. Generally, it can be concluded that WAPIs can provide desirable oil absorption capacity as well as emulsifying and foaming properties especially in alkaline conditions as a functional ingredient in food applications. The production of WAPI could also be considered as a suitable cost-effective alternative to commercial almond protein isolates.
References
J. Berghout, R. Boom, A. van der Goot, The potential of aqueous fractionation of lupin seeds for high-protein foods. Food Chem. 159, 64–70 (2014)
R. Horax, N. Hettiarachchy, A. Kannan, P. Chen, Protein extraction optimisation, characterisation, and functionalities of protein isolate from bitter melon (Momordica charantia) seed. Food Chem. 124, 545–550 (2011)
A. Mohamed, G. Biresaw, J. Xu, M.P. Hojilla-Evangelista, P. Rayas-Duarte, Oats protein isolate: thermal, rheological, surface and functional properties. Food Res. Int. 42, 107–114 (2009)
S.O. Ogunwolu, F.O. Henshaw, H.P. Mock, A. Santros, S.O. Awonorin, Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut. Food Chem. 115, 852–858 (2009)
A. Cano-Medina, H. Jiménez-Islas, L. Dendooven, R.P. Herrera, G. González-Alatorre, E.M. Escamilla-Silva, Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Res. Int. 44, 684–692 (2011)
M. Aider, D. Djenane, W.B. Ounis, Amino acid composition, foaming, emulsifying properties and surface hydrophobicity of mustard protein isolate as affected by pH and NaCl. Int. J. Food Sci. Tech. 47, 1028–1036 (2012)
K. Sze-Tao, S. Sathe, Functional properties and in vitro digestibility of almond (Prunus dulcis L.) protein isolate. Food Chem. 69, 153–160 (2000)
S. Ahrens, M. Venkatachalam, A.M. Mistry, K. Lapsley, S.K. Sathe, Almond (Prunus dulcis L.) protein quality. Plant Foods Hum. Nutr. 60, 123–128 (2005)
S. Yada, K. Lapsley, G. Huang, A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 24, 469–480 (2011)
K. Sorkheh, B. Shiran, V. Rouhi, E. Asadi, H. Jahanbazi, H. Moradi, T. Gradziel, P. Martínez-Gómez, Phenotypic diversity within native Iranian almond (Prunus spp.) species and their breeding potential. Genet. Resour. Crop Evol. 56, 947–961 (2009)
A. Moayedi, K. Rezaei, S. Moini, B. Keshavarz, Chemical compositions of oils from several wild almond species. J. Am. Oil Chem. Soc. 88, 503–508 (2011)
M. Balvardi, J.A. Mendiola, P. Castro-Gómez, J. Fontecha, K. Rezaei, E. Ibáñez, Development of pressurized extraction processes for oil recovery from wild almond (Amygdalus scoparia). J. Am. Oil Chem. Soc. 92, 1503–1511 (2015)
M. Balvardi, K. Rezaei, J.A. Mendiola, E. Ibáñez, Optimization of the aqueous enzymatic extraction of oil from Iranian wild almond. J. Am. Oil Chem. Soc 92, 985–992 (2015)
M. Mirzapour, K. Rezaei, M.A. Sentandreu, A.A. Moosavi-Movahedi, In vitro antioxidant activities of hydrolysates obtained from Iranian wild almond (Amygdalus scoparia) protein by several enzymes. Int. J. Food Sci. Technol. 51, 609–619 (2016)
D. Firestone, Official methods of analysis of the Association of Official Analytical Chemists. (Association of Official Analytical Chemists, Arlington, 1990)
S.K. Sathe, M. Venkatachalam, G.M. Sharma, H.H. Kshirsagar, S.S. Teuber, K.H. Roux, Solubilization and electrophoretic characterization of select edible nut seed proteins. J. Agric. Food Chem. 57, 7846–7856 (2009)
C. Zhong, R. Wang, Z. Zhou, S.-R. Jia, Z.-L. Tan, P.-P. Han, Functional properties of protein isolates from Caragana korshinskii Kom. Extracted by three different methods. J. Agric. Food Chem 60, 10337–10342 (2012)
K.N. Pearce, J.E. Kinsella, Emulsifying properties of proteins: evaluation of a turbidimetric technique. J. Agric. Food Chem. 26, 716–723 (1978)
A.J. Sfahlan, A. Mahmoodzadeh, A. Hasanzadeh, R. Heidari, R. Jamei, Antioxidants and antiradicals in almond hull and shell (Amygdalus communis L.) as a function of genotype. Food Chem. 115, 529–533 (2009)
Y.W. Sari, M.E. Bruins, J.P. Sanders, Enzyme assisted protein extraction from rapeseed, soybean, and microalgae meals. Ind. Crops Prod. 43, 78–83 (2013)
F.A.O/W.H.O. Protein and amino acid requirements in human nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation, WHO technical report series 935. (Food and Agriculture Organization/World Health Organization, Geneva, Switzerland, 2007)
K.S. Kumar, K. Ganesan, K. Selvaraj, P.S. Rao, Studies on the functional properties of protein concentrate of Kappaphycus alvarezii (Doty) Doty–An edible seaweed. Food Chem. 153, 353–360 (2014)
K. Shevkani, N. Singh, A. Kaur, J.C. Rana, Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 43, 679–689 (2015)
H. Zuber, Temperature adaptation of lactate dehydrogenase structural, functional and genetic aspects. Biophys. Chem. 29, 171–179 (1988)
Q. Deng, L. Wang, F. Wei, B. Xie, F. Huang, W. Huang, J. Shi, Q. Huang, B. Tian, S. Xue, Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 124, 1458–1465 (2011)
A. Moure, J. Sineiro, H. Domínguez, J.C. Parajó, Functionality of oilseed protein products: a review. Food Res. Int. 39, 945–963 (2006)
E. Pastor-Cavada, R. Juan, J.E. Pastor, M. Alaiz, J. Vioque, Protein isolates from two Mediterranean legumes: Lathyrus clymenum and Lathyrus annuus. Chemical composition, functional properties and protein characterisation. Food Chem. 122, 533–538 (2010)
G.M. Sharma, M. Su, A.U. Joshi, K.H. Roux, S.K. Sathe, Functional properties of select edible oilseed proteins. J. Agric. Food Chem. 58, 5457–5464 (2010)
S.A. Malomo, R.E. Aluko, A comparative study of the structural and functional properties of isolated hemp seed (Cannabis sativa L.) albumin and globulin fractions. Food Hydrocoll. 43, 743–752 (2015)
E. Akintayo, E. Adebayo, L. Arogundade, Chemical composition, physicochemical and functional properties of akee (Bilphia sapida) pulp and seed flours. Food Chem. 77, 333–336 (2002)
M. Majzoobi, E. Abedi, A. Farahnaky, M. Aminlari, Functional properties of acetylated glutenin and gliadin at varying pH values. Food Chem. 133, 1402–1407 (2012)
M.A. Mwasaru, K. Muhammad, J. Bakar, Y.B.C. Man, Influence of altered solvent environment on the functionality of pigeonpea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. Food Chem. 71, 157–165 (2000)
Acknowledgements
The authors would like to acknowledge the support provided by “Ministry of Science, Research and Technology,” “Center of Excellence for Application of Modern Technologies for Producing Functional Foods and Drinks” and “Research Council of University of Tehran” (Tehran, Iran) and also “the Research Council of College of Agriculture and Natural Resources of University of Tehran” (Karaj, Iran).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Amirshaghaghi, Z., Rezaei, K. & Habibi Rezaei, M. Characterization and functional properties of protein isolates from wild almond. Food Measure 11, 1725–1733 (2017). https://doi.org/10.1007/s11694-017-9553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9553-y