Abstract
Antioxidant activity of phenolic extract isolated from Tunisian walnut (Juglans regia L.) fruits has been investigated. Preliminarily, composition of walnut kernels was determined. Fat content varied from 53.5 to 66.9% (w/w), while total phenol ranged from 1.2 to 5.2 g/100 g of kernel as gallic acid equivalent (GAE). High amounts of ellagic acid (EA) and its derivatives were evidenced by HPLC analysis of the phenolic extract. Walnut fat showed induction time ranging from 2.5 to 3.7 h at 110 °C (rancimat test) and these values unchanged by adding 300 mg/kg of kernel phenolic extract which so showed to have no antioxidant effect. In addition, activities of the ellagic and gallic acids and trolox were compared in vitro by using the Folin–Ciocalteu’s, rancimat and DPPH radical scavenging methods. EA exhibited an antioxidant and radical scavenging activity significantly lower than other tested substances. In conclusion, EA showed to have a negligible role in preventing walnut fat oxidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Walnut (Juglans regia L.) is native to Asia and is one of the oldest cultivated nut trees [1]. Walnut fruit (WF) is consumed worldwide, fresh or toasted, alone or in other food products; moreover, walnut oil is industrially important in several sectors. World production of walnuts was around 3.4 million tons in 2013 and currently, the major producing countries are China, Iran, USA, Turkey, Ukraine and Mexico [2].
Antifungal, antimicrobial and antioxidant activities of different part of the walnut tree were evidenced [3, 4], while a regular consumption of WF has associated with many health benefits [5, 6]. Walnut kernel contains fat, proteins, vitamins and minerals. Kernel fat content may be up to 74% and the walnut oil is considered a good source of essential fatty acids, especially linoleic and α-linolenic acids [7, 8]. Nevertheless, the high content of polyunsaturated fatty acids makes WF prone to oxidation with formation of unpleasant odors and flavor during the storage [9]. WF is also a rich source of bio-phenols, responsible for the typical slightly astringent flavor [10]. Several phenolic acids, such as gallic, ellagic, syringic, 5-O-caffeoylquinic, caffeic, p-coumaric, ferulic and sinapic acids, have been identified [11]. However, the ellagic acid (EA), EA derivatives and water-soluble ellagitannins (ETs) have been found to be the major constituents of the WF phenolic fraction [12]. Recently, these molecules have generated commercial interest for their potential benefits to human health as antioxidant, antimutagenic, anticancer and apoptosis inducing activities [13].
Despite of the potential adaptation of walnut to the Tunisia environmental conditions, this crop is rather limited in the country and located principally in the North–West region [14]. However, walnut cultivation is increasing being part of Tunisian national programs of crop diversification; for this reason, most of the cultivated walnut trees belong to imported cultivars. Nutritional and compositional characteristics of walnut cultivars grown in Tunisia have been presented in previous studies [15, 16]. In this study, fat oxidative stability and antioxidant potential of biophenols extracted from new Tunisian walnut samples have been investigated. Walnuts were analyzed for basic composition (moisture, fat, crude protein, ash), fatty acid profile and phenolic composition. Finally, the rancimat test and the scavenging DPPH (2,2-diphenyl-1-picrylhydrazy) assay were carried out to study the mechanism of fat oxidation and the antioxidant power of EA.
Materials and methods
Chemicals and walnut samples
All reagents were of analytical grade. Gallic acid (GA), EA, trolox (TX) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Co (St. Louis, MO, USA). Sunflower oil was purchased from a local market; this oil, analyzed before use, was characterized by 3.4 meqO2/kg peroxide value, 0.1% free acidity as oleic acid and 7.1% palmitic, 2.8% stearic, 30.3% oleic, 58.3% linoleic, 1.5% other fatty acids.
The walnut (J. regia L.) samples used in this study consists of six zones of Tunisia (Maktar, Zaghouan and Mateur, located in northern Tunisia; Tunis and Menzel Bouzelfa in the northeast; Ain Draham in northwestern). The harvested plants were identified by Dr Ali Albouchi [National Institute of Research Rural Engineering, Water and Forest (INRGREF)]. The identification numbers of Maktar, Zaghouan, Mateur, Tunis, Menzel Bouzelfa, Ain Draham were WMK06, WZ06, WMT06, WT06, WMN06 and WA06, respectively. These voucher specimens were deposited in the Herbarium of (INRGREF). The WFs were harvested in Autumn 2015 and air-dried and stored in a fresh room. At the time of analysis (January 2016), the kernels of about 20 walnuts for each cultivar were ground in a coffee miller and then, treated three times with 50 mL n-hexane. Collected solvent was evaporated by rotary vacuum evaporator at 40 °C obtaining the walnut oil (WO), while the residue defatted ground kernels were air-dried for 24 h.
Basic composition
Moisture, ash, total fat, protein content (N × 6.25) and carbohydrates were determined in accordance with the methods described in Amaral et al. [17]. Fatty acid composition was determined by a gas-chromatography with flame ionization detector as described in De Leonardis et al. [18].
Preparation and analysis of walnut phenolic extract
Phenolic extract was obtained in accord to John and Shahidi [19] with the following modifications. One gram of defatted ground kernel was extracted three-times with 10 mL of 80:20 (v/v) acetone:water for 2 h at room temperature. The mixture was centrifuged at 4000g for 15 min by recovering the supernatant. Collected supernatants were dried by rotary vacuum evaporator at 40 °C and then, the residue was dissolved in 3 mL of 80:20 (v/v) methanol:water; this first walnut phenolic extract was named ‘WPE’. One mL of WPE was treated with equal volume of 20% HCl at 100 °C for 90 min by recovering three-times the hydrolyzed phenols with ethyl acetate. Evaporated the solvent, the dry residue was dissolved in 1 mL of 80:20 (v/v) methanol:water, obtaining the second phenolic extract named ‘WPE-HCl’. Total phenols were determined using the Folin–Ciocalteu’s reagent by using gallic acid as reference standards (GAE) [20]. HPLC analysis was carried out by a Varian ProStar 330 instrument (Mulgrave, AUS), equipped with a column Kinetex 5u C18 100 Å (150 × 4.6 mm) (Phenomenex, USA), using the same solvent gradient reported in De Leonardis et al. [20]. Detection was made at 280, 253 and 368 nm. EA was identified by comparison of retention time and spectral data with those of standard. The other compounds having spectra similar to that of pure EA were recognized as ellagic acid derivatives (EAD). Quantification of EA and its derivatives was performed on the EA’s calibration curve by expressing the results as ellagic acid equivalent (EAE).
Rancimat test
The induction time (hour) was measured by a rancimat apparatus mod. 730 (Metrohm AG, Herisau, Switzerland) under 110 or 120 °C temperature and 20 L/h air flow. WPE and WPE-HCl were added to the corresponding cold-extracted walnut oil (WO) at a concentration of 300 mg/kg GAE. Pure GA, EA and TX, dissolved in 80% methanol:water, were added (0.5–3.0 mM) to the sunflower oil. Equal volumes of solvent was added in the corresponding control oil sample.
DPPH scavenging capacity
Capacity to scavenge the DPPH free radical was determined in according to Pereira et al. [21], with modification. An aliquot of 0.2 mL of individual standard was added to 2.0 mL of 5 × 10−2 mM DPPH methanol solution, while an equal volume of alone solvent was added to the DPPH blank solution. Reaction solution decoloration was measured after 30 min with the spectrophotometer fixed at 517 nm. The DPPH scavenging effect was calculated by the following equation: DPPH inhibition percentage = [(Abs DPPH − Abs sample)/Abs DPPH] × 100, where Abs DPPH and Abs sample represent the absorbance of the DPPH blank and sample solutions. Standard compound concentration providing 50% inhibition (IC50) was calculated from the graph plotting inhibition percentage against standard concentration.
Statistical analysis
The SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used. All analyses were carried out in triplicate; significant differences between the means were evaluated by using ANOVA LSD test at p < 0.01.
Results and discussion
Basic walnut composition
Cultivar, origin and basic composition of the Tunisian walnut samples are reported in Table 1.
Walnut composition resulted in agreement with those published previously [15, 16]. Total fat was the highest constituent, followed by protein and carbohydrates. Specifically, the total fat ranged from 53.5% (n. 6) to 66.9 (n. 1). The cv. Franquette stood out having the lowest fat content (53.5%) and the highest values of protein (19.1%) and carbohydrates (24.7%). However, total fat and protein content resulted to be respectively lower and higher than those obtained with walnuts grown in Portugal [21], Italy [22] and New Zealand [23], while are similar to Turkish walnuts [24]. As regards the fatty acid composition, unsaturated fatty acids (UFA) were found to be significantly higher than saturated (SFA). Palmitic and stearic acids were the major SFA, while monounsaturated (MUFA) were composed substantially of oleic acid. Polyunsaturated fatty acids (PUFA) ranged from 67.2 to 78.0%; actually, PUFA presented only the linoleic and linolenic acids, with the marked dominance of the first. A C18:2/C18:3 ratio of 4 is considered a good nutritional balance; in general, the WF samples evidenced a C18:2/C18:3 ratio ranged from 4.4 to 5.4 with exception of the cv. Parisienne that showed a value of 8.3 and 6.8 for the sample 4 and 5, respectively.
Walnut phenolic extract composition
Walnut kernels contain a complex mixture of phenolic substances and the major components fall into the category of ellagitannins [12]. However, data on the walnut phenol composition could vary significantly in depending on the extraction methodologies [21].
In Fig. 1 the HPLC chromatogram of both the WPE and WPE-HCl extracts of the sample 2 (chosen as an example) were compared to that of pure standard. The EA eluted at about 20 min and showed absorbance maxima at the 253 and 368 nm wavelengths. ETs were not detectable directly by HPLC analysis. EA was the major peak both in the WPE and WPE-HCl and in this last the EA clearly increased. Conversely, GA was found in negligible amount as in WPE and WPE-HCl, by evidencing a limited presence of this compound as in free and bound form. Other unidentified peaks with spectrum equal to that of EA (shown in Fig. 1) has been recognized by naming them overall EADs.
Total phenols, EA and EADs content determined in the WF samples are reported in Table 2.
Total phenols varied from 1.2 to 5.2%, in line with data reported in literature [21]. The cv. Parisienne (n. 4 and 5) showed the highest total phenol content with values of 5.2 and 3.4%, respectively. Contrasting results were obtained for the cv. Hartley due to the samples 1 and 2 had approximately 2% of total phenols, while a 3.2% value was obtained for the sample 3. Finally, the cv. Franquette showed the lowest total phenol content (1.2%). EA and EADs represented only a part of the total phenols and, with exception of sample 1, total phenol content was linked generally to EADs content. EA varied from 113 (n. 6) to 396 (n. 1) mg/100 g as EAE.
Walnut fat oxidative stability
Rancimat test has been used frequently to study antioxidant role of phenolic substances [25, 26]. Induction time of the oils extracted from the kernels (WO) was compared with that of the same oils enriched with 300 mg/kg GAE of the WPE or WPE-HCl corresponding extracts (Fig. 2).
WO samples showed homogeneous results with induction times ranging from 2.5 to 3.7 h at 110 °C. WO of the cv. Parisienne (n. 3), characterized by low PUFA and high total phenol content, appeared to be the most stable. Induction time of the fortified WO samples unchanged with respect to the untreated oils evidencing any significant antioxidant effect for WPE and WPE-HCl. Phenolic concentration of the extracts was evaluated by using the Folin–Ciocalteu’s method, that is certainly one of the most used assay for determination of total phenols in plant beverages or extracts. This assay is based on the reduction, in alkaline medium, of phosphomolybdic-tungstic chromogen per share of several types of reducing substances. Actually, this reagent is not selective for the phenols and reacts also with several interfering substances as sugars, proteins and organic acids. Consequently, reducing capability of plant beverages or extracts is not necessarily predictive of its antioxidant effect [27]. Indeed, also the rancimat results given in Fig. 2 confirm that the Folin–Ciocalteu’s assay may be not fit to predict the antioxidant potential of a plant extract.
Antioxidant potential of the ellagic acid
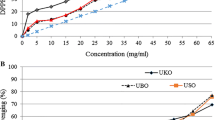
The antioxidant potential of pure EA, GA and TX was evaluated by using three different methods: Folin–Ciocalteu’s assay, rancimat test and DPPH radical scavenging activity. All these methods depend on the propensity of the tested substances to donate hydrogen. The graph reported in Fig. 3a shows that GA exhibited more potent reactivity with the Folin–Ciocalteu’s reagent than EA and TX. However, contradictory evidence about the relationship between the Folin–Ciocalteu’s assay and rancimat test has been obtained (Fig. 3b). Indeed, as regards GA, like other phenols, a close relationship between reactivity with Folin–Ciocalteu’s reagent and antioxidant effect in preventing oil oxidation was confirmed [25, 28]. Antioxidant effect of TX (Fig. 3b) was comparable to that of GA and for both compounds, into 0.5–2.0 mM concentration range, a similar linear relationship was found between induction time and concentration. Conversely, TX exhibited low reactivity with the Folin–Ciocalteu reagent (Fig. 3a). Finally, at the same concentrations, neither reducing ability nor antioxidant effect were obtained for EA.
The radical scavenging activity of the above-said pure compounds is shown in Fig. 4. GA exhibited DPPH activity higher than TX and EA. Specifically, the EC50 was 0.011, 0.020 and 0.029 mM for GA, TX and EA, respectively. Thus, both the rancimat and DPPH tests evidenced that EA plays an unsubstantial role in preventing oil oxidation and scavenging radicals. These results are apparently in contrast with those of Zhang et al. [12], that obtained a radical scavenging capacity of GA and EA higher than TX. In this study, radical scavenging capacities of WPE were not assayed. Other studies had evidenced a strong concentration-dependent radical scavenging capacity of walnut phenol extract higher than individual pure low molecular phenolic compounds [12, 21].
Conclusion
Tunisian walnuts have been studied, giving particular emphasis on oxidative stability of fat and antioxidant role of extracted biophenols. Chemical characterization of walnuts may help in selecting cultivars for future walnut commercial production in Tunisia. In this regard, cv. Franquette has distinguished negatively having lowest fat and total phenol content. Walnut oil evidenced a weak oxidative stability confirming to be a potential key factor affecting the shelf life of walnuts. Although Tunisian walnuts showed an abundance of phenolic substances, a scarce antioxidant activity of walnut phenolic extracts was highlighted. Pure EA revealed to be ineffective in preventing the oxidation of both the walnut and sunflower tested oils. Moreover, EA evidenced also a scarce reducing power and radical scavenging activity in vitro.
Abbreviations
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- EA:
-
Ellagic acid
- EAE:
-
Ellagic acid equivalent
- EADs:
-
Ellagic acid derivatives (EADs)
- ETs:
-
Ellagitannins
- GA:
-
Gallic acid
- GAE:
-
Gallic acid equivalent
- MUFA:
-
Monounsaturated fatty acids
- PUFA:
-
Polyunsaturated fatty acids
- WF:
-
Walnut fruit
- WPE:
-
Walnut phenolic extract
- WPE-HCl:
-
Hydrolyzed walnut phenolic extract
- WO:
-
Walnut oil
- SFA:
-
Saturated fatty acids
- TX:
-
Trolox
- UFA:
-
Unsaturated fatty acids
References
H. Fernández, C. Pérez, R. Sánchez-Tamés, Plant Growth Regul. 30, 125 (2000)
FAO Cropping Database (2016), http://faostat3.fao.org/browse/Q/QC/E. Accessed June 2016
J.A. Pereira, I. Oliveira, A. Sousa, P. Valentão, P.B. Andrade, I.C.F.R. Ferreira, F. Ferreres, A. Bento, R. Seabra, L. Estevinho, Food Chem. Toxicol. 45, 2287 (2007)
I. Oliveira, A. Sousa, I.C. Ferreira, A. Bento, L. Estevinho, J.A. Pereira, Food Chem. Toxicol. 46, 2326 (2008)
C. Sánchez-González, V. Noé, M. Izquierdo-Pulido, Food Funct. 5, 2922 (2014)
C.W.C. Kendall, A. Esfahani, A.R. Josse, L.S.A Augustin, E. Vidgen, D.J.A. Jenkins, Nutr. Metab. Cardiovasc. Dis. 21, 34 (2011)
G.P. Savage, P.C. Dutta, D.L. McNeil, J. Am. Oil Chem. Soc. 76, 1059 (1999)
J.S. Amaral, S. Cunha, M.R. Alves, J.A. Pereira, R.M. Seabra, M.B.P.P. Oliveira, J. Agric. Food Chem. 52, 7664 (2004)
L.P. Vanhanen, G.P. Savage, Food Chem. 99, 64 (2006)
T. Fukuda, H. Ito, T. Yoshida, Phytochemistry 63, 795 (2003)
S. Anjum, A. Gani, M. Ahmad, A. Shah, F.A. Masoodi, Y. Shah, A. Gani, J. Food Process Preserv. 41, n/a, e12756. doi:10.1111/jfpp.12756 (2017)
Z. Zhang, L. Liao, J. Moore, T. Wu, Z. Wang, Food Chem. 113, 160 (2009)
J.M. Landete, Food Res. Int. 44, 1150 (2011)
A. Laajimi, B. Thabet, Economics of nuts in the Mediterranean Basin (CIHEAM). (Zaragoza, Spain, 1999)
I.B. Abdallah, I. Bouali, E. Martínez-Force, A. Albouchi, M.C. Perez-Camino, S. Boukhchina, Nat. Prod. Res. 28, 1826 (2014)
I.B. Abdallah, N. Tlili, E. Martinez-Force, A.G.P. Rubio, M.C. Perez-Camino, A. Albouchi, S. Boukhchina, Food Chem. 173, 972 (2015)
J.S. Amaral, S. Casal, J. Pereira, R. Seabra, B. Oliveira, J. Agric. Food Chem. 51, 7698 (2003)
A. De Leonardis, F. Cuomo, V. Macciola, F. Lopez, RSC Adv. 6, 101098 (2016)
J.A. John, F. Shahidi, J. Funct. Foods 2, 196 (2010)
A. De Leonardis, V. Macciola, F. Cuomo, F. Lopez, Food Chem. 175, 568 (2015)
J.A. Pereira, I. Oliveira, A. Sousa, I.C. Ferreira, A. Bento, L. Estevinho, Food Chem. Toxicol. 46, 2103 (2008)
S. Ruggeri, L. Cappelloni, S. Gambelli, E. Carnovale, Ital. J. Food Sci. 10, 243 (1998)
G.P. Savage, Plant Foods Hum. Nutr. 56, 75 (2001)
M.M. Özcan, Iran, J. Chem. Chem. Eng. 28, 57 (2009)
A. De Leonardis, L. Pizzella, V. Macciola, Eur. J. Lipid Sci. Technol. 110, 941 (2008)
A. De Leonardis, V. Macciola, A. Nag, Acta Alim. 38, 77 (2009)
G.A. Agbor, J.E. Oben, J.Y. Ngogang, C. Xinxing, J.A. Vinson, J. Agric. Food Chem. 53, 6819 (2005)
D. Bera, D. Lahiri, A. De Leonardis, K.B. De, A. Nag, J. Food Eng. 81, 688 (2007)
Acknowledgements
This study was supported by the Ministry of Higher Education and Scientific Research of Tunisia. The authors express their sincere gratitude to the University of Molise, Italy for supporting this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdallah, I.B., Macciola, V., Boukhchina, S. et al. The negligible role of ellagic acid in preventing fat oxidation of Tunisian walnuts (Juglans regia L.). Food Measure 11, 1406–1411 (2017). https://doi.org/10.1007/s11694-017-9519-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9519-0