Abstract
Andrographis paniculata Nees (A. paniculata) has been used as herbal medicine for thousands of years in China. In this work, a quick extraction method with microwave-assisted treatment was studied for a complete extraction of active compounds from A. paniculata. Furthermore, the proposed fingerprint method, enhanced fingerprint by HPLC-DAD, has the advantage of efficiency and accuracy. In comparison with common fingerprint at fixed wavelength, enhanced fingerprint compiled additional spectral data and was hence more informative. It could efficiently identify, distinguish and assess A. paniculata. So it could be used to conduct the quality control of this traditional Chinese medicine comprehensively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Andrographis paniculata Nees (A. paniculata), one of the main traditional Chinese medicines (TCM), commonly known as “King of Bitters”, belongs to the Acanthaceae family. It grows widely in the tropical area of South East Asia, India and China [1]. This herb has been used traditionally for several applications such as against fever, dysentery, inflammation sore throat and snakebite [2, 3], antimicrobial [4], antimalarial [5, 6]. Furthermore, it is a promising new way for the treatment of several diseases caused by immune disorders such as HIV and AIDS [7].
During the last decade, as the use of TCM becomes popular in the world, identification and the quality control of it has attracted more and more attention. But the traditional quality control technique of TCM encounters more and more challenges because one or two markers or pharmacological active compounds in the herbal medicine employed for evaluating the quality and authenticity of herbal medicine cannot give a complete picture of herbal product. Chromatographic fingerprint, a more significant formulation for controlling the quality of herbal medicines and their products, has been accepted by many countries and organizations [8, 9]. This technique emphasizes the systemic characterization of compositions of samples and focuses on the identification and assessment of the stability of components [8, 10].
Conventional fingerprint contains only chromatographic information. However, in some cases, the limited information provided by conventional fingerprint might not be enough to reveal the quality characteristics of some extremely complex TCM products comprehensively. Therefore, it is necessary to develop a more effective fingerprint. The proposed fingerprint, enhanced fingerprint, seems promising to fulfill the purpose. Compared with conventional fingerprint, enhanced fingerprint with additional spectral information contained, has many more advantages. Firstly, it contains more peaks which can not be detected at a fixed wavelength but have UV responses at other wavelengths; Secondly, in quality assessment, conventional fingerprint might give different chromatographic patterns at different wavelengths, whereas enhanced fingerprint technique is more objective and credible [11].
By using the fingerprint analysis, a particular herb can be identified and distinguished from some closely related species. Due to the variation of the collecting time, sites, processing methods and so on, there are some differences in the content of active compounds in the same plant. Therefore, it is necessary to establish fingerprint of A. paniculata for its quality control.
Conventionally, the sample preparation of herbal medicines in fingerprint studies was accomplished by heating, boiling, refluxing or ultrasonic extraction [12–14]. But all of them are time-consuming, laborious, involving laborious operation and bulk amount of organic solvents [15]. The microwave-assisted extraction [16–21] has been extensively used for herbal medicine analysis; however, its application in the study on the fingerprint of A. paniculata has been rarely reported.
Although there were different approaches to the analysis of active compounds in A. paniculata [22–26], to our knowledge, few fingerprint analysis methodologies have been reported. Zhang et al. [27] performed the fingerprint analysis of A. paniculata. He used refluxing to extract active compounds and HPLC-UV at a fixed wavelength to establish fingerprint. However, as a traditional extraction method, refluxing could not extract active compounds completely and effectively. The objective of our study is to establish a simple and timesaving extraction method: microwave-assisted extraction and a more effective fingerprint which contains additional spectral information: enhanced fingerprint.
In the present study, different sample preparation procedures were compared, including microwave-assisted and marinated extraction, reflux and ultrasonic extraction. Microwave-assisted extraction was utilized with HPLC-DAD for identification and fingerprint establishment of A. paniculata. At the same time principal component analysis, similarity analysis and hierarchical cluster analysis were used to study the factor affecting the quality of A. paniculata.
Experimental
Instrumentation and reagents
HPLC analysis was carried out on a Agilent Series 1100 liquid chromatograph, equipped with a vacuum degasser, a quaternary pump, an auto sampler and a diode array detection (DAD) system, connected to a reversed-phase column (Diamonsil C18 5 μm 150 mm × 4.6 mm i.d., Agilent, USA). Data collection was performed using Chem. Station software (Agilent). The water used for all the solutions and dilutions was prepared with a Millipore ZMQS50F01 water purification system (USA). Pressure Self-Control Microwave Decomposition System (Shanghai, P.R. China) and Auto Science AS-2060B Ultrasonic Cleaner (Tianjin, P.R. China) were used for extraction. Ethanol (95%) is of AR grade and methanol (99.8%) is of HPLC grade (Ludu, P.R. China).
Materials
Raw herbs of A. paniculata were collected directly from various production sites in China. They are presented in Table 1. Commercial drugs, purchased in different drugstores, are presented in Table 2. Andrographolide (98%) and dehydroandrographolide (98%) were purchased from Chinese Medicine Research Institute (Nanjing, P.R. China).
Sample preparation
Before extraction, A. paniculata was ground into powder with particle diameter of 0.2–0.9 mm by plant grinder. Samples were prepared by four procedures as follows:
Marinated extraction
A 1 g aliquot of sample was added with 30 mL 85% ethanol and then placed into the sealed vessel, followed by the extraction for 30 h. The extracts were filtered and concentrated to dryness under vacuum. The dried extracts were dissolved in 25 mL of methanol, filtered through 0.45-μm millipore membrane and an aliquot of 10 μL of the filtrate was used for HPLC analysis.
Reflux extraction
A 1 g aliquot of sample was added with 30 mL 85% ethanol and then placed into the reflux device, followed by the extraction for two times, 2 h each. The extracts were collected together, filtered and concentrated to dryness under vacuum. Then the remaining steps were done as described in “Marinated extraction”.
Ultrasonic extraction
A 1 g aliquot of sample was added with 30 mL 85% ethanol and then placed into the sealed vessel, followed by ultrasonication for two times, 0.5 h each. The extracts were collected together, filtered and concentrated to dryness under vacuum. Then the remaining steps were done as described in “Marinated extraction”.
Microwave-assisted extraction
A 1 g aliquot of sample was added with 30 mL 70% ethanol and then placed into the pressure self-control microwave decomposition system. The irradiation time was kept for 4 min, then the extraction solution was cooled down to ambient temperature. After that, the resulted solution was centrifugal separated, filtered and concentrated to dryness under vacuum. Then the remaining steps were done as described in “Marinated extraction”.
Preparation of reference substance solutions
Andrographolide and dehydroandrographolide are the two typical medicinal components of A. paniculata, so both of them were considered as the reference substances in fingerprint. Stock solutions of andrographolide and dehydroandrographolide were prepared by dissolving suitable amount of pure andrographolide and dehydroandrographolide, respectively, in methanol. Reference substance solution was obtained by mixing andrographolide and dehydroandrographolide stock solutions together. After that, the reference substance solution was directly injected into the HPLC.
HPLC analysis
A reversed-phase C18 column (150 mm × 4.6 nm, i.d. 5 μm) from Agilent was used with the mobile phase consisting of methanol (A) and water (B). Gradient elution was performed using the following linear gradient: 0 min—30% A; 5 min—45% A; 35 min—70% A; 40 min—80% A; 45 min—80% A. The column compartment was kept at the temperature of 25 °C. When the wavelengths were below 230 nm or above 300 nm, most components have weak or even no responses, so the DAD detection was performed in the range of 230–300 nm at 2 nm/step. The sample volume injected was 20 μL.
Enhanced fingerprint generating
The information of fingerprint extracted from DAD data is two-dimensional matrices. Principal Component Analysis (PCA), a chemometrics method, was used for decomposition of two-dimensional matrices. According to the PCA,matrix X (n × m) was set to store the data. The retention time of fingerprint is set as rows (n) and the wavelength as columns (m). X can be described as m conventional chromatographic fingerprints at various wavelengths, and each fingerprint is measured at n regular time intervals. It can be described as follows:
By PCA, X can be decomposed into two matrices: the score matrix T and the loading matrix V. According to Eq. 1, the score matrix T can be described as:
Commonly, an authentic sample (AUS) is required for reference. It is arranged that X0 is the DAD matrix of AUS and t 0 i is the ith principal component (PC) of the score matrix. T 0 i can be described as follows:
Therefore the first principal component (PC1) can be described as follows:
Considering that PC1 represents the maximal variation of the data matrix, we simply used PC1 to describe the systematic information as follows:
In Eq. 5, x 01 –x 0 m is chromatographic fingerprint detected at various wavelengths and the loading vector v 11–v m1 is the weight of each fingerprint. Obviously, t 01 transforms the two dimensional information into one dimensional linear relationship and it contains both the chromatogram and spectrum information.
In PCA, singular value decomposition of original matrix, an algebra method, was applied to calculate the Covariance Matrix as follows:
λ1–λn is the variance of every PC. With the assistance of MATLAB 7.0, the variance percentage of PC1 of A. paniculata is calculated. The value is 98.87%, which means PC1 of A. paniculata can accounts for 98.87% variance in the original data and the most information of DAD data is successfully extracted. Thus using only PC1 to explain the original matrix is sufficient.
Because of the quality of PC1 and AUS, v 01 derived from AUS describes the common spectral characteristics for samples on the same species. Therefore, according to Eq. 5, the fingerprint of one sample is as follows
With the assistance of MATLAB 7.0 and professional similarity evaluation system named “Similarity Evaluation System for Chromatographic Fingerprint of TCM”, the enhanced fingerprint of A. paniculata was established which is shown in Fig. 1.
Data analysis
Data analysis was performed using the professional software named “Similarity Evaluation System for Chromatographic Fingerprint of TCM”. It is mainly applied to the similarity analysis of chromatographic patterns. The software about the calculation of similarity was mainly based on the peak area and retention time. Firstly, the chromatogram data should be imported into the software. Then the marker peaks were chosen by the user and all peaks would be matched. Subsequently, the mean chromatogram was produced, and the similarity value of all introduced chromatograms relative to that of mean chromatogram would be calculated [15]. The correlation coefficient is expressed by the following formula:
where r is the correlation coefficient between fingerprint x and the reference fingerprint x 0. \( x_{i} {\text{ and }}x_{i}^{0} \) represent the ith elements of the ith peak of each fingerprint, which can be the response value of signal, area or height of the peak, etc. The value r of the correlation coefficient is in the range 0 < r ≤ 1. The larger the value of r is, the higher the quality of target samples is. It is considered as the best, the better or the worst drug with a corresponding correlation coefficient above 0.9, between 0.8 and 0.9, or below 0.8, respectively. When r equals 1, it is identical.
Results and discussion
Selection of extraction method
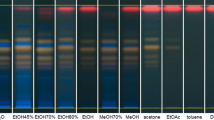
Different extraction methods were compared (shown in Fig. 2) to select an optimum sample preparation. From Fig. 2, we can see the HPLC fingerprint marked as “D” has more peaks than other fingerprints, which implies microwave-assisted extraction is a more effective method. At the same time it is timesaving. So it is more suitable for complete extraction of main components.
Preliminary test of microwave-assisted extraction conditions
In microwave-assisted extraction, there are many factors affecting the extraction yield, such as the extraction time, solid–liquid ratio and the concentration of extraction solvent. An orthogonal test design in the extraction mode was used to optimize the extraction conditions. The levels and factors of the orthogonal test are shown in Table 3 and the results are presented in Table 4. From Table 4, we can see that the maximum extraction yield of the extract was 1.53%. However, we cannot select the best extraction conditions only based on these outcomes in Table 4. Therefore a further orthogonal analysis was warranted. Thus, the K and R values were calculated and listed in Table 4. K value is the average extracting efficiency of every factor at each level. R value is the range of K value. As seen in Table 4, according to the R values, we can find that the influence to the mean extraction yields of the compounds decreases in the order: factor B > factor C > factor A. We also can see that K2j has the highest K value in factor A, which means that compared with other levels of factor A, level two has the highest extracting efficiency, and is the most suitable level for extraction. Based on the same principle, level four of factor B and level two of factor C were chosen for extraction. In other words, the maximum yield of the active compound was obtained at the following conditions: 30 mL solvent per gram of sample, 70% alcohol solution, 4 min of irradiation time, respectively.
Fingerprints of A. paniculata
The establishment of the reference fingerprint
In order to identify the main active components of A. paniculata, the HPLC profile of the standard compounds is shown in Fig. 3. Andrographolide and dehydroandrographolide could be identified by comparison of their retention time in Fig. 3 and the HPLC chromatography of the sample. Their structures are as follows: (a): Andrographolide, (b): Dehydroandrographolide.

The sample, from Huazhou, P.R. China (No. 4), extracted by microwave-assisted extraction, was detected by HPLC-DAD with the wavelength in the range of 230–300 nm. It is worthy to demonstrate that sample No. 4 was the raw material like others and is reliable to show the common character of all samples. Therefore it is feasible to pick No. 4 to show the HPLC profile of samples. The chromatography is presented in Fig. 4.
For the establishment of the reference fingerprint, all the raw materials of A. paniculata, presented in Table 1, were analyzed by HPLC. Then the HPLC profiles were inputted into the similarity evaluation system software. The average fingerprint, that was computed by this software, was considered as the reference fingerprint of samples which is shown in Fig. 5. Based on the retention time, seven common peaks were determined and peaks 4, 5 were identified as andrographolide and dehydroandrographolide, respectively, by comparing their retention time in Fig. 3.
The reference fingerprint is in practice a chromatographic pattern of some common kinds of pharmacologically active and chemically characteristic components in A. paniculata. This chromatographic profile should feature the fundamental attributions of “integrity”, in other words, “sameness”. It could demonstrate the “sameness” between various samples successfully. If the HPLC profile of one sample has the common peaks in the reference fingerprint and the retention time of the peaks are consistent with the ones in the reference fingerprint, the sample could be identified as A. paniculata. Meanwhile, in similarity analysis, the reference fingerprint is considered as the standard. Because the reference fingerprint can show the common character of A. paniculata, the sample could be considered as an authentic one if the similarity value of it is high.
The fingerprints of A. paniculata from different sources
In order to indicate the differences from various treatments, five batches of samples which were listed in Tables 1 and 2 were analyzed statistically. RSD values of seven common peaks (shown in Fig. 5) in HPLC chromatograms among five batches of samples were shown in Table 5. From Table 5, we can see RSD values were all less than 1.15% for retention time and less than 4.18% for peak area, which means the common peaks were in good correspondence in five batches of every sample. Therefore the average HPLC chromatogram of five batches which was generated by MATLAB 7.0 can show the sameness of every sample. Meanwhile average HPLC chromatograms from different samples can show the differences among various treatments.
On the basis of the demonstrations above, we use the average HPLC chromatogram among five batches of every sample to do the HPLC fingerprints and the clustering analyses in the following study.
All the samples (showed in Table 1) were evaluated by their similarities, which came from the calculation on the correlation coefficient or cosine value of vectorial angle of original data. The results are shown in Fig. 6 and Table 6. The bigger the similarity value is, the better the approach degree between the sample and reference. Peaks (A)–(G) in Fig. 6 are the seven peaks (marked as peaks 1–7) showed in Fig. 5. And the similarity values in Table 5 are all above 0.8. So they could be identified as A. paniculata.
Enhanced HPLC fingerprints of A. paniculata (Nos. 1–12). Peaks (A)–(G) are the seven common peaks showed in Fig. 5 (peaks 1–7)
The fingerprints of A. paniculata collected in different time
Samples (Nos. 1–4 showed in Table 1) were collected in July, August, September and October at the same location (Huazhou, P.R. China). The fingerprints are presented in Fig. 7. The results show that absorption intensity of some peaks is different, especially peak (A) (andrographolide) and peak (B) (dehydroandrographolide). Obviously, peaks of Nos. 2 and 3 have greater absorption intensity which implies that the concentration of principal components in Nos. 2 and 3 is higher than that in other samples. So it is favorable to collect A. paniculata in August and September.
The fingerprints of different medicinal parts of A. paniculata
It has been well known that the different medicinal parts of raw herbs usually are used for different curative purpose. It is therefore important to learn about any difference among various medicinal parts of A. paniculata. Samples (Nos. 10–12 showed in Table 1) with different effective parts of the plant were analyzed with the software and are presented in Fig. 8. Although the chromatograms of the Sample Nos. 10–12 have the similar peaks, peaks of No. 11 have greater absorption intensity which means that the concentration of principal effective compounds in No. 11 is higher than that in other samples. Therefore the leaf was better for the medicinal part of A. paniculata than others.
The fingerprints of A. paniculata collected in different locations
Samples from various locations (No. 3 and Nos. 5–10, showed in Table 1) were compared with the software and are presented in Fig. 6 and Table 5. Based on the similarity values, it is interesting to note that all the samples fell into two groups: Group A and Group B. The similarities of Groups A, B are 0.947–0.984 and 0.868–0.879, respectively. Group A consisted of No. 3 and Nos. 5–7 was collected from south of China. Group B consisted of Nos. 8–10 was collected from north of China. The important finding is that the similarities of herbs are very relative to their collecting locations (Tables 1, 5). Moreover, the effects brought from collecting locations are more visible than those from collecting time and medicinal parts of A. paniculata. So in order to control the stability of A. paniculata, the collecting location should be fixed.
As a result, in order to control the quality of A. paniculata, attentions should be paid to collecting time, location and the medicinal parts. Especially, location is the main factor affecting the quality of A. paniculata.
The clustering analyses of samples from different sources
Hierarchical cluster analysis based on peak characteristics from the average fingerprint profiles among five batches of every sample presented in Table 1 was applied, and the Euclidean distance, which is a pattern similarity measure, was selected as measurement for hierarchical cluster analysis. The cluster analysis was operated with the assistance of MATLAB 7.0 and the result is shown in Fig. 9. In cluster analysis, samples can be classified as one category because of the short Euclidean distance, which means they are more similar to each other than to others. In Fig. 9, comparing sample Nos. 1–4, we can see that Nos. 2 and 3 can be classified as one category for the higher similarity. According to Table 1, Nos. 2 and 3 were collected in August and September, therefore it is favorable to collect A. paniculata in August and September.
The clustering analyses of chromatograms of samples from different sources (the samples are presented in Table 1)
Meanwhile, using this method, we are able to classify all the samples into two broad categories, samples 1–7 collected from south of China and samples 8–12 collected from north of China. The result proves that in order to control the stability of A. paniculata, the collecting location should be fixed.
The fingerprints of A. paniculata purchased in different commercial drugs
Commercial drugs (Nos. 13–16, showed in Table 2) purchased from different drugstores were analyzed. The results are presented in Fig. 10 and Table 7. The results in Fig. 10 indicated that they all have the seven common peaks showed in the reference fingerprint in Fig. 5 (peaks (A)–(G) in Fig. 10 corresponding to peaks 1–7 in Fig. 5). So they could be identified as A. paniculata. However, the similarities of Nos. 15 and 16 in Table 7 are not very high, they are both below 0.80. If 0.80 is set as an appropriate threshold, it is easy to find that sample Nos. 15 and 16 are unacceptable. Meanwhile, hierarchical cluster analysis was applied. The result is presented in Fig. 11. Seen from Fig. 11, Nos. 13 and 14 are clustered together with the reference fingerprint firstly, which suggests that the quality of Nos. 13 and 14 are more similar to the reference. Sample Nos. 15 and 16 are different from other samples and the reference, which indicates they are unacceptable samples for quality control. As a result, the conclusion of similarity analysis and hierarchical cluster analysis are consistent.
Enhanced HPLC fingerprints of commercial drugs (Nos. 13–16). Peaks (A)–(G) are the seven common peaks showed in Fig. 5 (peaks 1–7)
The clustering analyses of chromatograms of different commercial drugs (the samples are presented in Table 2). Re the reference fingerprint
Comparison of enhanced fingerprint and conventional chromatographic fingerprint
The conventional chromatographic fingerprints of A. paniculata were obtained at wavelengths of 235 or 250 nm (shown in Fig. 12) which were the characteristic UV absorption wavelengths of the two typical medicinal components respectively (i.e. andrographolide and dehydroandrographolide). From Fig. 12, we can see that compared with conventional fingerprints, enhanced fingerprint contains more peaks, especially in the retention time of 15–25. Moreover, the signals in enhanced fingerprint are much stronger than those in conventional fingerprints.
Meanwhile, the similarities of conventional fingerprint (250 nm) and enhanced fingerprint are compared in Table 8. From Table 8, it can be easily seen that using enhanced fingerprint, all the samples are classified into two groups with different quality grades. Numbers 1–7 are classified into Group A with the similarities of 0.9171–0.9749, and Group B is consists of Nos. 8–12, whose similarity values are in the range of 0.8680–0.8759. Whereas all the samples from different sources could not be distinguished by conventional fingerprint. Thus, compared with enhanced fingerprint, the classification ability and discrimination power of conventional chromatographic fingerprint is limited to perform further quality control.
Methodology study
Precision test of the apparatus
Apparatus precision was evaluated by the analysis of five successive injections of the same sample solution. The correlation coefficients between the reference HPLC fingerprint and the sample HPLC fingerprint were: 0.9973, 0.9959, 0.9984, 0.9982 and 0.9963, respectively. These data showed that the precision of the apparatus was satisfactory.
Stability test of the solution
Stability was evaluated by the analysis of five injections of the same sample solution every 4 h. The correlation coefficients were 0.9987, 0.9991, 0.9972, 0.9978 and 0.9983, respectively. The data indicated that the stability of the solution was very nice.
Repeatability of analytical method
Repeatability was evaluated by the analysis of five injections of five sample solutions prepared by the same method. The correlation coefficients were 0.9979, 0.9992, 0.9995, 0.9987 and 0.9988, respectively, showing that the repeatability of the analytical method is very suitable.
Discussion
In the present study, an impersonal, valid and rapid extraction method and an efficiency fingerprint analysis method was developed and applied. Compared with traditional extraction, microwave-assisted extraction was suitable for the sample preparation of A. paniculata. It has the characters of speediness and no-pollution. Meanwhile, samples from different sources were analyzed. According to their similarities and hierarchical cluster analysis, those herbs were assorted into two groups. The taxonomy based on similarities had a fair consistency with the reference fingerprint in Fig. 5. In a word, our results have demonstrated that the enhanced fingerprint with the similarity analysis may be accepted in general quality standards of herbal medicines in the recent future. The important finding is that the most relevant factor on the quality of A. paniculata was the collecting location then the harvesting time and the medicinal parts. In order to get the consistent raw materials of A. paniculata, the collecting location should be fixed and then the harvest time. At last, commercial drugs purchased from different drugstores were analyzed to show the feasibility and applicability of the extraction and analysis method. As a result, two commercial drugs were identified as unqualified ones for their poor similarity.
Conclusion
In summary, the microwave-assisted extraction and enhanced fingerprint can be established for the quality control of A. paniculata. This method is very simple with high precision, stability and repeatability. The HPLC enhanced fingerprint, with additional spectral information compiled, can comprehensively and properly reveal the quality characteristics of TCM, and will become a very useful complementary technique for Chinese herbal quality control and a powerful support for the progress of Chinese herbal medical prescription.
References
A.C. Kumoro, M. Hasan, Supercritical carbon dioxide extraction of Andrographolide from Andrographis paniculata: effect of the solvent flow rate, pressure and temperature. Chin. J. Chem. Eng 15, 877–883 (2007)
R. Wongkittipong, L. Prat, S. Damronglerd, C. Gourdon, Solid–liquid extraction of andrographolide from plants—experimental study, kinetic reaction and model. Sep. Purif. Technol. 40, 147–154 (2004)
W. Martz, Plants with a reputation with snakebite. Toxicon 30, 1131–1142 (1992)
P.K. Singha, S. Roy, S. Dey, Antimicrobial activity of Andrographis paniculata. Fitoterapia 74, 692–694 (2003)
N.N.N.A. Rahman, T. Furuta, S. Kojima, K. Takane, M.A. Mohd, Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 64, 249–254 (1999)
V.K. Dua, V.P. Ojha, R. Roy, B.C. Joshi, N. Valecha, C.U. Devi, M.C. Bhatnagar, V.P. Sharma, S.K. Subbarao, Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J. Ethnopharmacol. 95, 247–251 (2004)
R.A. Kumar, K. Sridevi, N.V. Kumar, S. Nanduri, S. Rajagopal, Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 92, 291–295 (2004)
S. Wang, H.-Q. Ma, Y.-J. Sun, C.-D. Qiao, S.-J. Shao, S.-X. Jiang, Fingerprint quality control of Angelica sinensis (Oliv.) Diels by high-performance liquid chromatography coupled with discriminant analysis. Talanta 72, 434–436 (2007)
W. Li, Y. Deng, R. Dai, Y. Yu, M.K. Saeed, L. Li, W. Meng, X. Zhang, Chromatographic fingerprint analysis of Cephalotaxus sinensis from various sources by high-performance liquid chromatography—diodearray detection—electrospray ionization-tandem mass spectrometry. J. Pharm. Biomed. Anal. 45, 38–46 (2007)
P. Drašar, J. Moravcova, Recent advances in analysis of Chinese medical plants and traditional medicines. J. Chromatogr. B 812, 3–21 (2004)
S.-K. Yan, W.-F. Xin, G.-A. Luo, Y.-M. Wang, Y.-Y. Cheng, An approach to develop two-dimensional fingerprint for the quality control of Qingkailing injection by high-performance liquid chromatography with diode array detection. J. Chromatogr. A 1090, 90–97 (2005)
M. Palma, C.G. Barroso, Ultrasound-assisted extraction and determination of tartaric and malic acids from grapes and winemaking by-products. Anal. Chim. Acta 458, 119–130 (2002)
L. Tong, Y. Wang, J. Xiong, Y. Cui, Y. Zhou, L. Yi, Selection and fingerprints of the control substances for plant drug Eucommia ulmodies Oliver by HPLC and LC–MS. Talanta 76, 80–84 (2008)
B. Zhang, R. Yang, C.-Z. Liu, Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep. Purif. Technol. 62, 480–483 (2008)
J. Kang, L. Zhou, J. Sun, J. Han, D.-A. Guo, Chromatographic fingerprint analysis and characterization of furocoumarins in the roots of Angelica dahurica by HPLC/DAD/ESI-MSn technique. J. Pharm. Biomed. Anal. 47, 778–785 (2008)
A.V. Filgueiras, J.L. Capelo, I. Lavilla, C. Bindicho, Talanta 53, 433–436 (2000)
Z.K. Guo, Q.H. Jin, G.Q. Fan, Y.P. Duan, C. Qin, M.J. Wen, Anal. Chim. Acta 436, 41–46 (2001)
E.D. Conte, C.Y. Shen, P.W. Perschbacher, D.W. Miller, J. Agric. Food. Chem. 44, 829–834 (1996)
B. Kaufmann, P. Christen, J.L. Venuthey, Phytochem. Anal. 12, 327–330 (2001)
N. Hong, V.A. Yaylayan, G.S. Raghavan, J.R. Park, J.M. Blanger, Nat. Prod. LETT 15, 197–200 (2001)
W.H. Ho, S.J. Hsieh, Anal. Chim. Acta 428, 111–116 (2001)
L. Chen, A. Yu, X. Zhuang, K. Zhang, X. Wang, L. Ding, H. Zhang, Determination of andrographolide and dehydroandrographolide in rabbit plasma by on-line solid phase extraction of high-performance liquid chromatography. Talanta 74, 146–152 (2007)
L. Chen, H. Jin, L. Ding, H. Zhang et al., On-line coupling of dynamic microwave-assisted extraction with high-performance liquid chromatography for determination of andrographolide and dehydroandrographolide in Andrographis paniculata Nees. J. Chromatogr. A 1140, 71–77 (2007)
Q. Dua, G. Jerz, P. Winterhalter, Separation of andrographolide and neoandrographolide from the leaves of Andrographis paniculata using high-speed counter-current chromatography. J. Chromatogr. A 984, 147–151 (2003)
M.K. Bhan, A.K. Dhar, S. Khan, S.K. Lattoo, K.K. Gupta, D.K. Choudhary, Screening and optimization of Andrographis paniculata (Burm.f.) Nees for total andrographolide content, yield and its components. Sci. Hortic. 107, 386–391 (2006)
K. Muntha Reddy, V.B. Mopuru Reddy, D. Gunasekar, M. Madugula Murthy, C. Caux, B. Bodo, A flavone and an unusual 23-carbon terpenoid from Andrographis paniculata. Phytochemistry 62, 1271–1275 (2003)
Z. Zhang, H. Dong, J. Yu, The fingerprints of Andrographis paniculata by HPLC/UV/MS. Chin. J. Nat. Med 3, 373–377 (2005). (In Chinese)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 20875025, J0830415), the Hunan Provincial Natural Science Foundation (No. 07JJ3018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Y., Wang, Y., Ouyang, X. et al. Fingerprint profile of active components for Andrographis paniculata Nees by HPLC-DAD. Sens. & Instrumen. Food Qual. 3, 165–179 (2009). https://doi.org/10.1007/s11694-009-9082-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-009-9082-4