Abstract
Leaf-eating monkeys (colobines) are a highly diversified subfamily with 61 species in ten genera, in which patterns and constraints of morphological evolution are still poorly resolved. In the present study, we measured the skulls of 452 specimens collected from different museums worldwide. Using one of the most extensive samples ever employed, and geometric morphometric techniques, we aimed to elucidate the evolutionary processes that have led to the craniofacial diversification of colobines. Our comprehensive analyses of the colobine cranium demonstrated that phylogeny is the first order signal to emerge, with clear interspecific patterns of differentiation. Allometric trend constrains shape variation for most colobine taxa, but to a lesser degree than phylogeny. We also confirmed that diet is significantly associated with the variation in cranial shape among colobines. In particular, the mechanical advantage of the masseter for biting at the anterior dentition is linked to seed intake. We postulate that such ecomorphological patterns explain, in part, the non-phylogenetic and non-allometric variations in the colobine skull, and indicate the importance of diet in interspecific resource partitioning, allowing for species coexistence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colobinae represents a highly diversified subfamily of Old World monkeys, a sister group of the Cercopithecinae, from which they diverged ~ 19 million years ago (Liedigk et al. 2012). Colobines, also known as “leaf-eating monkeys,” are a group that is currently facing extinction (Kamilar and Paciulli 2008), largely owing to the strong anthropogenic pressures exerted upon them (e.g., deforestation and/or hunting). Cercopithecines are considered a model of choice for human evolution, given their complex social structures, and have been extensively studied in the past; colobines, on the contrary, have been largely neglected.
Extant colobines cover a wide geographical range and are distributed from western Africa to eastern Asia (Yan-Zhang et al. 1993; Ting 2008). In the past 12 million years, since the estimated date of their divergence (Liedigk et al. 2012), colobines have experienced a rapid diversification, and the last intergeneric divergence, between Simias and Nasalis, occurred as recently as ~ 1.5 million years ago. Such evolution is intimately related to the geographical and environmental changes that occurred throughout the Tertiary and Quaternary periods. As an example, Southeast Asia, with its high diversity of biotas, witnessed the radiation of the odd-nosed monkeys (namely members of Pygathrix, Rhinopithecus, Nasalis, and Simias), which occupy a remarkable range of habitats, from mangrove swamps (Bennett and Sebastian 1988) to high altitude and temperate forests (Li et al. 2002). This variety of habitats highlights the diversity of colobines in terms of behavior, feeding ecology, and social organization (Kirkpatrick and Grueter 2010).
Species in the subfamily Colobinae were long thought to be predominantly folivorous primates, in contrast to cercopithecines (Hylander 1979a; Chivers and Hladik 1980; Bouvier 1986; Ravosa 1990; Kamilar and Paciulli 2008). Morphological traits of colobines, such as relatively sharp molar crests, thin tooth enamel, long molar rows, small incisors, robust mandibles, and reduced stiffness of the alveolar bone, have been considered adaptations for masticating leaves (Hylander 1979b; Teaford 1983; Ravosa 1996; Ravosa et al. 2000; Daegling et al. 2011). However, many studies have revealed an unexpectedly high dietary diversity, which has attracted a renewed interest in the study of their morphology (Koyabu and Endo 2009, 2010; McGraw et al. 2016). For example, species-specific diet preferences have been observed in the field, with the intake frequencies of food items such as mature leaves, young leaves, fruits, flowers, lichens, and seeds varying considerably between species (e.g. Fimbel et al. 2001; Chapman et al. 2002, 2004; Teichroeb et al. 2003; Zhou et al. 2006; Anderson et al. 2007; Harris and Chapman 2007; Hanya and Bernard 2012). Overall, most colobines prefer young leaves (Bennett and Davies 1994; Oates 1994) as they are often highly nutritious and contain relatively few secondary compounds (Matsuda et al. 2013). They generally require low masticatory forces compared to more resistant foods such as mature leaves and seeds (Lucas et al. 2012). However, long-term field observations have demonstrated that other food items mostly fill the role of fallback resources during seasons with low food availability (Li et al. 2010; Vandercone et al. 2012; Ehlers Smith et al. 2013; Kibaja 2014). In particular, it is now clear that seed eating is a major aspect of the colobine diet in both Africa (Maisels et al. 1994; Oates 1994; Davies et al. 1999) and Asia (Davies et al. 1988, 1999; Davies 1991; Bennett and Davies 1994; Hanya and Bernard 2015).
Morphologically, colobines show considerable craniodental variation (e.g. Bergmann 1848; Verheyen 1962; Hull 1979; Daegling and McGraw 2001; Nowak et al. 2008; Cardini and Elton 2009), but the evolutionary factors driving this variation are not well understood (Daegling and McGraw 2001; Pan 2006; Wright and Willis 2012). Comparative studies on the craniodental morphology of selected colobines have proposed that the greater mechanical advantage of the masticatory apparatus (the length of the masseter lever arm relative to the load arm of the bite point) found in a handful of Asian (Koyabu and Endo 2010) and African colobines (Koyabu and Endo 2009) is related to seed eating. Among African colobines, it was found that species that are reported to feed frequently on seeds (Colobus angolensis and C. polykomos) show greater mechanical advantage of the masseter for chewing than do species that feed rarely on seeds (C. guereza, Pi. badius, and Pro. verus) (Koyabu and Endo 2009). Similarly, among Asian species, seed eaters such as P. rubicunda and T. phayrei were found to have a greater mechanical advantage for mastication than P. comata, S. vetulus, and T. obscurus, which rarely exploit seeds (Koyabu and Endo 2010). However, despite this work on a still understudied group, Koyabu and Endo (2009, 2010) were limited to only a few colobines (five species for both studies) and were not able to investigate the relationship between seed-eating and skull form for most of the colobines. In addition, the effect of phylogeny was not addressed in these studies owing to limited taxon sampling. Therefore, in a phylogenetic context, whether cranial variation among the whole clade of colobines actually reflects differences in seed eating remains to be tested.
Although dietary variation is an important driver of craniodental evolution owing to the variation in mechanical constraints occurring during mastication (Wright et al. 2008; Yamashita et al. 2016), non-dietary factors such as allometry should also be considered. Allometry consists of a correlated and proportional variation in shape changes relative to size (e.g., Steudel 1982; Rilling and Seligman 2002; Mitteroecker et al. 2004; Bruner 2007; Zollikofer and Ponce de León 2010), and the mammalian cranium is known to be subject to allometry (Emerson and Bramble 1993; Marroig et al. 2009). In primates, it has been reported that small alterations in size can produce major changes in the shape of the skull (Singleton 2005). For example, allometry is associated with major differences in skull shape between howler monkeys and capuchins (Meloro et al. 2014). It is very likely that skull variation in colobines reflects allometric effects, but to what extent allometric effects have shaped the morphology of colobine skulls is still largely unknown.
The aim of the present study is to test the hypothesis proposed by Koyabu and Endo (2009, 2010) that seed eating explains most of the overall variation in the colobine skull. Covering, for the first time, all ten recognized genera, and 44 of 61 recognized species (Groves 2005), we provide the most extensive analysis of the patterns of morphological differentiation among colobines using geometric morphometric techniques. Furthermore, we examine the patterns of differentiation in relation to non-dietary factors such as phylogeny and allometry. Hence, size and shape variations are discussed in light of both phylogenetic history and local ecology, with a particular focus on seed consumption.
Materials and Methods
Sampling
In this study, 452 colobine specimens from different museums worldwide were analyzed (Table 1), representing 44 species and ten genera from Africa and Asia. The specimens are housed at the Natural History Museum (BMNH), Primate Research Institute of Kyoto University (KUPRI), Smithsonian National Museum of Natural History (USNM), and the Zoological Reference Collection of Lee Kong Chian Natural History Museum at the National University of Singapore (ZRC) (Online Resource 1). Captive individuals were not included, and all specimens were limited to wild-caught individuals. As a consequence of the sampling process in the wild (males were predominantly shot), too few females were available in the collections. The developmental age of the specimens was unknown; therefore, we were unable to test ontogeny-related variation. To avoid any major effects from this factor, patterns of differentiation were investigated at the interspecific and intergeneric level, and only specimens with fully erupted dentition were considered. Specimens showing any signs of pathology, or supernumerary teeth, were excluded.
Phylogenetic Relationships and Their Projection in the Morphospace
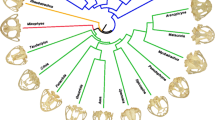
In this study, we compiled a composite tree of the 44 species (Fig. 1) presenting the commonly accepted relationships found in the literature between taxa, based on molecular analyses (Md-Zain et al. 2002; Meijaard and Groves 2004; Karanth et al. 2008; Osterholz et al. 2008; Roos et al. 2008; Ting 2008; Ting et al. 2008; Chatterjee et al. 2009; Meyer et al. 2011; Liedigk et al. 2012; Wang et al. 2015). These studies used different genetic markers, such as mitochondrial DNA alone (e.g. Ting 2008) or in association with nuclear markers (e.g. Karanth et al. 2008; Ting et al. 2008). Divergence dates were given as an indication (Fig. 1) and were obtained from Liedigk et al. (2012) for the Asian clade and Ting (2008) for the African clade. When no clear consensus was reached in the literature (e.g., Trachypithecus and Semnopithecus), we represented them as unresolved multiple branches. We also included T. pileatus in this phylogeny, but the topology here is tentative as this species is considered to be the result of hybridization between Semnopithecus and Trachypithecus (Wang et al. 2015).
Owing to the composite nature of this tree, and unresolved divergence dates, all terminal nodes were set as equal distances from the root, treating the tree as ultrametric. This allowed us to test for a statistically significant phylogenetic signal in our dataset (using lambda and Blomberg’s K estimates), and to project the phylogenetic relationships between taxa in morphological space using the phylomorphospace function from the ‘phytools’ R package (Revell 2012).
Diet
Following Davies et al. (1999), the annual feeding frequencies on five distinct food categories (mature leaves, young leaves, flowers, seeds, and fruits) were considered in this study. Although we used dietary information from long-term observations (available for 26 species, Table 1), diet may vary among localities and observers. Thus, dietary data were averaged across studies when possible, following Koyabu and Endo (2009). Reports of animal matter in the colobine diet are extremely rare, therefore faunivory was assumed to be negligible. Furthermore, because taxonomic names have been confused for some species (Pr. femoralis, Pr. melalophos, and Pi. badius, S. entellus, S. priam), some previous studies assigned incorrect dietary information to species (e.g. Wright and Willis 2012). Therefore, the localities of field observations and species identity were carefully checked prior to their use in this study.
Mechanical Advantage Analyses and Comparisons with Diet
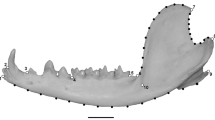
Coordinates of the muscle attachment positions, bite points, and temporomandibular joint (TMJ) were recorded using a Microscribe 3DX digitizer (Immersion Corp., San Jose, CA) to estimate the mechanical advantage for biting, based on Koyabu and Endo (2010). Landmark positions adopted in this study are illustrated in Fig. 2 and definitions provided in Online Resource 2. The mechanical advantage of the masticatory apparatus was calculated as the ratio between the length of the jaw muscle’s moment arm and the length of the moment arm of the corresponding bite point in the occlusal plane. The mechanical advantage of canine biting in Trachypithecus poliocephalus was not calculated because the original canine of the only specimen available was lost. Bite-point moment arm lengths were measured from the center of the articular eminence to the center of the trigon basin of M1 (molar biting) and P3 (premolar biting), to the tip of the canine (canine biting), and to the point between the central incisors (incisor biting). Moment arm lengths of the masseter, temporalis and medial pterygoid muscles were measured in this study. The moment arm length of the masseter muscle was measured as the distance from the center of the articular eminence to the inferior edge of the malar at the most anterior point of attachment of the superficial masseter muscle. The moment arm length of the temporalis muscle was defined as the distance from the center of the articular eminence to frontotemporale. The moment arm length of the medial pterygoid muscle position was estimated as the distance from the center of the articular eminence to the pterygopalatine suture at the posterior edge of the palatine. In order to adjust for the vertical difference, the measurements described above were taken as projections onto the occlusal plane, defined by the point between the central incisors and the right and left centers of the trigon basin of M1 (Spencer and Demes 1993; Wright 2005). We evaluated the correlation between the mean mechanical advantage of each species and the dietary information of each species, derived from long-term observations using phylogenetically-independent contrasts (Felsenstein 1985), using the PDAP module of Mesquite (Maddison and Maddison 2011). As noted earlier, because branch lengths were not available for all species, we used an ultrametric tree in this study, i.e. all terminal nodes were considered as equally distant from the root. The alpha level of significance was adjusted using Bonferroni corrections (Sokal and Rohlf 2012).
Morphometric Data Collection and Statistics
For each specimen, three-dimensional Cartesian coordinates of 26 anatomical landmarks were digitized on the left side of the skull (Fig. 2, Online Resource 3) by a single observer (DK) using a Microscribe 3DX digitizer.
Differences in specimen positioning during data acquisition, as well as isometric variations in size, were corrected using a generalized Procrustes analysis (GPA) (Rohlf and Slice 1990). This GPA resulted in a set of standardized Procrustes coordinates, which were then considered for later statistical analyses. The centroid size (i.e., the square root of the sum of squared distances of each landmark from the centroid of the configuration) was also calculated and used as a size estimator. At a large taxonomic scale, in studies focusing on the skull, this parameter is considered to be a reliable proxy for body size (Damuth and MacFadden 1990). Even though we considered a more restricted taxonomic scale (i.e., Colobinae), the results obtained provide a good overview of the size trends existing in this group. However, a one-to-one relationship between centroid size and body mass remains to be tested and we therefore limit our interpretations to the centroid size, referred to hereafter as “size.” Centroid size differences between genera and between species were investigated using Kruskal–Wallis tests for pairwise multiple comparisons (as the normality assumption was rejected using a Shapiro–Wilk’s test), a nonparametric test for univariate data. All statistics and visualizations were performed in R (R-Core-Team 2016), using the packages PMCMR (Pohlert 2016), ade4 (Dray and Dufour 2007), and Morpho (Schlager 2013).
The presence of allometry was first investigated using a linear regression between original Procrustes coordinates and centroid size. Allometric patterns of shape variation were then further studied by calculating the regression score and plotting it against the centroid size following Drake and Klingenberg (2008). The regression score corresponds to the projection of the data points in shape space onto an axis in the direction of the regression vector. It is the shape variable that has the maximal covariation with centroid size. Associated shape changes were visualized using color maps along the regression score by calculating hypothetical configurations at the 0.05 and 0.95 quantiles. The dependence of shape on size was then tested using the ‘ffmanova’ R package (Langsrud and Mevik 2012), with aligned shape coordinates as the response variable. The residuals of the regression between the aligned coordinates and centroid size were extracted and considered as size-adjusted variables in further analyses.
Patterns of size-adjusted shape differentiation were investigated using these residuals as shape variables, and were visualized using a between-group principal component analysis (bgPCA) with species as the grouping variable (Culhane et al. 2002; Renaud et al. 2015). Among-group differences were tested using permutation tests (Procrustes ANOVA; 9999 permutations) performed on the first 12 axes summarizing 90% of the total variance. Shape differences were visualized using the mean of deformation maps (extrapolated from fixed landmarks) between group means, or along shape axes by calculating hypothetical configurations at known coordinates. For each morphospace the phylogenetic relationships were projected on the bgPC axes using the ‘phylomorphospace’ function from the ‘phytools’ package.
Multivariate Regressions
Multivariate multiple regression (MMR) was used to assess the influence of size, phylogeny, and diet on the cranial shape variation. Centroid size was considered as size variable. Phylogeny variable was included as the first four meaningful axes of a principal coordinate analysis performed on the distance matrix of the composite tree using the R ‘ape’ package (Paradis et al. 2004). The feeding frequencies of five food items were included as diet variable. These three groups of variables were considered as independent variables to explain the dependent variable composed of the first five meaningful principal component axes calculated on the original Procrustes coordinates. The relationship between cranial geometry and diet was also assessed by two-block partial least-squares (2B-PLS) (Rohlf and Corti 2000), using MorphoJ. In 2B-PLS models there is no predictor or predicted variables, and both blocks of variables (here, cranial shape as Block 1 and diet as Block 2) are equally weighted. Then, vectors of maximum covariation between the blocks were extracted.
Results
Mechanical Advantage Analyses and Comparison with Diet
All values of measured moment arms and calculated mechanical advantages of the masseter, temporalis, and medial pterygoid muscles are shown in Online Resource 4. Our results show a strong overall consistency between bite points (incisor, canine, third premolar and first molar). Regarding the mechanical advantage of the masseter, Pr. rubicunda (incisor 0.64 and canine 0.67), T. francoisi (incisor 0.61 and canine 0.63), Py. nigripes (incisor 0.59 and canine 0.62), and R. avunculus (incisor 0.61 and canine 0.63) showed high values for anterior dentition. This indicates these species are more mechanically advantageous than other species when biting at the incisor and canine. Pr. rubicunda (premolar 0.78 and molar 0.99), T. francoisi (premolar 0.77 and molar 0.95), and T. poliocephalus (premolar 0.76 and molar 0.97) showed high values for the posterior dentition. These species appear to be more mechanically advantageous than other species when biting at the premolar and molar with the masseter. Semnopithecus entellus and members of Piliocolobus, such as Pi. pennantii, Pi. tephrosceles, and Pi. tholloni, exhibited a rather low mechanical advantage of the masseter at all bite points. Pr. rubicunda and Pr. chrysomelas showed high mechanical advantage of the temporalis at all bite points. On the contrary, Nasalis, Simias and R. avunculus showed low temporalis mechanical advantage at all bite points. Regarding the mechanical advantage of the medial pterygoid, Pr. chrysomelas, Pr. robinsoni, T. phayrei, and Se. priam showed high values, whereas Pi. pennantii, T. francoisi, and R. roxellana presented low values at all bite points.
The results of the correlation analyses between dietary items and mechanical advantages are given in Table 2. Among all food items, seeds showed the strongest level of correlation to mechanical advantages of the masticatory apparatus. Seeds showed a significant strong correlation with the mechanical advantage of the masseter for incisor biting (Fig. 3a, r = 0.64, P = 0.0004) and canine biting (Fig. 3b, r = 0.61, P = 0.0009). P3 biting (r = 0.56, P = 0.0026) and M1 biting (r = 0.55, P = 0.0035) showed moderate correlations with seed intake, but these were not statistically significant after Bonferroni corrections. Fruits exhibited a weak correlation to the mechanical advantages of the medial pterygoid at all bite points, although not significant under the Bonferroni criterion. The mechanical advantages of the temporalis presented no significant correlation with any of the food items. In addition, all mechanical advantage values were negatively correlated with centroid size (Online Resource 5).
Size Differences
Overall, size was relatively homogeneous within each genus. Some species included in our analysis were rare, and in order to reach a minimum number of specimens for better statistical resolution, we tested for size differences (using Kruskal–Wallis tests) at the genus level (Table 3). However, as inter-generic tests could mask the underlying variation of size between species we chose to plot size differences between species (Fig. 4) and we also provide inter-specific Kruskal–Wallis tests (Online Resource 6). As no significant difference in size was evidenced between species within each genus, we considered that investigating size differences at the genus level was representative.
Among African clades, Colobus and Piliocolobus presented no difference in size (P = 0.436), while Procolobus was significantly smaller than the other two (P < 0.001).
Among Asian taxa, Presbytis was the smallest, being significantly smaller than most of the other genera (P < 0.001), with the exception of Simias (P = 0.624). Its average size is similar to the African Procolobus (P = 0.903). Trachypithecus also displayed a small size on average, with an important intrageneric variation. This variation can be mostly explained by the larger size of T. pileatus which lay outside of the range of other Trachypithecus species. Because of a high degree of intrageneric variation and low sample size, Semnopithecus appeared as significantly different in size only compared to Presbytis (P < 0.001) and Procolobus (p < 0.001).
Within odd-nosed monkeys, the two recently diverged sister genera, Simias and Nasalis, were morphometrically different. Only two Simias specimens were available for this study, but given our observations on thirteen additional specimens, which were not included in this study because of partial damage, we assume that our Simias specimens were not unusually large or small for this genus (Online Resource 7). Our results are in accordance with previous descriptions of those taxa: Nasalis is considered a large species, while Simias is considered an average-size species (Jablonski 1998). This contrasts with their close phylogenetic relationship and recent date of divergence. On the contrary Pygathrix and Rhinopithecus, which are closely related to each other, are similar in size (P = 0.999). Overall, with the exception of Simias, the odd-nosed monkeys include the largest taxa within the colobines.
Allometric Patterns
Using a multivariate regression of size on the original Procrustes coordinates, we observed a moderate influence of allometry on our dataset (P < 0.001, R2 = 0.18). Plotting the regression score (i.e., shape variable) as a function of the centroid size (Fig. 5a, Online Resource 8), we observed that while most of the genera were well-aligned along the regression line (P < 0.001, R2 = 0.72), some taxa such as Procolobus (n = 18), Colobus (n = 68), and Simias (n = 2) were slightly shifted above the regression line. Looking at the reconstructions performed along the regression and calculated at the 0.05 and 0.95 size quantiles (Fig. 5b), we saw that the larger the specimen, the less protrusive the orbits and flatter the cranium (evidenced by a downward shift of landmark 4, the bregma). The top posterior part of the parietal was, on the contrary, slightly more rounded. The zygomatic arch was shifted medially in larger taxa. Those changes were associated with an elongation of the anterior region of the maxilla.
a The regression score (Drake and Klingenberg 2008) is plotted as a function of centroid size. The black line represents the regression line and the two dashed lines the 95% confident interval. Each grey dot represents a single specimen and colored dots of different symbols represent the mean per species. Abbreviated names of the species are given at proximity of their respective mean. b Distance map showing shape differences associated to the allometric effect. The original landmarks used to build the distance map are shown as red dots. The color scale ranges from blue (i.e. compression) toward yellow (i.e. expansion). Deformations localized at each landmark, without interpolation, can be found in Online Resource 8. (Color figure online)
On the contrary, Rhinopithecus displayed a larger size than expected from their morphology and were clearly shifted below the regression line. A similar pattern was observed within Semnopithecus, S. entellus being similar in size to Nasalis, while its morphology was less extreme along the regression score. We also noted that the extended variation of Trachypithecus toward larger sizes was mostly driven by T. pileatus, which is an unusually large species among Trachipithecus.
Phylogenetic Patterns
Patterns of morphological variation between taxa were further investigated based on a between-group analysis performed on size-adjusted variables, with species as the grouping variable. All genera taken together were significantly different (P < 0.001, R2 = 0.783), and clear interspecific differences emerged when plotting the first axes of the bgPCA (Fig. 6). This was confirmed using pairwise comparisons between genera with Procrustes ANOVAs performed on the first 12 axes of the bgPCA (summarizing > 90% of the total variance) and displaying highly significant probabilities (Table 4). In addition, the existence of a phylogenetic signal was confirmed by Blomberg’s K tests (bgPC1: K = 1.43, P = 0.001; bgPC2: K = 1.07, P = 0.001; bgPC3: K = 0.78, P = 0.001) and Lambda tests (bgPC1: Lambda = 0.79, P < 0.001; bgPC2: Lambda = 0.99, P < 0.001; bgPC3: Lambda = 0.99, P < 0.001).
a–b Plots of the first three axes of a between-group principal component analysis (bgPCA) performed on size-adjusted variables with the species as grouping variable. Each dot represents a mean by species, and all species belonging to a single genus are grouped together using manual outlining. c Distance map showing shape differences associated along the first three axes of the bgPCA from negative to positive values (bgPC1, bgPC2 and bgPC3). The original landmarks used to build the distance map are shown as red dots. The color scale ranges from blue (i.e. compression) toward yellow (i.e. expansion). Deformations localized at each landmark, without interpolation, can be found in Online Resource 8. (Color figure online)
When considering the first three axes of the bgPCA, the African groups were clearly differentiated according to phylogeny: Colobus along bgPC1, and Procolobus and Piliocolobus along bgPC3 (Fig. 6a–b). Morphologically, for Colobus this differentiation involved a more prominent maxilla associated with a smaller neurocranium overall (Fig. 6c). The posterior region of the zygomatic was wider. The orbits were also slightly shifted posteriorly. Concerning Piliocolobus and Procolobus, the differentiation along bgPC3 corresponded to anteriorly shifted orbital and nasal regions. Similar to Colobus, the bregma region was flatter.
Within the Asian group, Presbytis and Trachypithecus were clearly differentiated along bgPC2 (Fig. 6a–b), moving from, respectively, a more elongated and flattened cranium to a less broad cranium displaying a shorter face and rounder superior part (Fig. 6c, Online Resource 8). Semnopithecus appeared to be intermediate between them. Concerning the odd-nosed monkeys, Rhinopithecus and Pygathrix were opposite to the African genera along bgPC1 (short face, small neurocranium), and were differentiated from one another on the bgPC2-bgPC3 plane. Finally, Simias and Nasalis genera clearly diverged along bgPC1 despite relatively recent speciation (Fig. 1), and they strongly segregated from other genera in the bgPC2-bgPC3 plane, at the opposite end from other odd-nosed monkeys.
Multivariate Regressions
MMR was built to determine the proportion of cranial shape variation explained by phylogeny, size, and diet. As a result, the model indicated that phylogeny is the first order signal, explaining 28.8% of total variation, while the second and third order signals were respectively diet (4.9%) and size (3.3%). All three variables were determined to be contributing significantly to cranial shape variation (P < 0.001).
A 2B-PLS was performed between the Procrustes coordinates and the matrix composed of the feeding frequencies for each food item. Only specimens for which diet information was available were considered (i.e. 325 specimens representing 26 species). Basic statistics of 2B-PLS are summarized in Table 5. Covariance explained by PLS1 was 53.6%, PLS 2 was 28.3%, PLS 3 was 14.6%, PLS 4 was 2.6%, cumulatively explaining 99.1% of total variance. The correlation coefficient between Block 1 (morphology variable) and Block 2 (diet variable) was r = 0.50 (p = 0.0101) in PLS1, r = 0.63 (p = 0.0005) in PLS2, r = 0.53 (p = 0.0053) in PLS3, and r = 0.50 (p = 0.0001) in PLS4 (Table 5; Fig. 7). Among the first two major PLS, Block 2 of PLS1 was loaded particularly by mature leaves (0.65) and fruits (− 0.64), and Block 2 of PLS2 was loaded particularly by seeds (− 0.73) and young leaves (0.53) (Table 5).
Plots of the first two pairs of 2B-PLS for morphology and diet (x-axis is morphology block, y axis is diet block) and distance maps showing shape differences associated along the first two axes of 2B-PLS. a Plots and distance map for PLS1. b Plots and distance map for PLS2. The original landmarks used to build the distance map are shown as red dots. The color scale ranges from blue (i.e. compression) toward yellow (i.e. expansion). Deformations localized at each landmark, without interpolation, can be found in Online Resource 8. (Color figure online)
Discussion
Size Differences Contribute to Interspecific Coexistence Between Closely Related Species
Being univariate and straightforward in its analysis and visualization, size is a parameter routinely investigated in biogeographic studies (Bergmann 1848, see; Meiri and Dayan 2003 for a review concerning birds and mammals). In our study, although intraspecific patterns of size (estimated by the centroid size of the cranium) could not be investigated owing to a lack of information about the precise origins of the specimens, we still observed marked differences at the intergeneric level depending on their geographical origin and local environment.
African colobines are often found in sympatry, which is the case, for example, of Colobus polykomos, Pi. badius and Pro. verus at Taï (Davies et al. 1999; Teichroeb et al. 2003), C. angolensis and Pi. badius at Salonga (Maisels et al. 1994), C. guereza and Pi. tephrosceles at Kibale (Oates 1994), or C. polykomos, and Pi. temminckii at Cantanhez (Minhós et al. 2015). For sympatric species, several mechanisms aimed at reducing the negative effects of competition have been described, including, for example, behavioral changes (Snaith and Chapman 2008), or morphological modification of the feeding apparatus allowing a differential exploitation of resources (Dayan and Simberloff 1998; Simberloff et al. 2000; Ledevin et al. 2012).
Our results showed a major size difference between Procolobus and the two other African genera (Piliocolobus and Colobus). Phylogenetically, Colobus are the most basal African genus, and the Procolobus-Piliocolobus divergence occurred ~ 1.1 million years after their differentiation (Ting 2008, Fig. 1). The geographic distribution of Pro. verus is similar to that of Colobus vellerosus, and their diet is similar, relying on mature and young leaves (Davies et al. 1999; Teichroeb et al. 2003); therefore, the small body size of Pro. verus may have facilitated the sympatric coexistence of the two species. Oates (1988) previously pointed out a possible link between the small body size of Pro. verus and its high selectivity on young leaves. From 21 months of observations at Tiwai forest in Sierra Leone, Oates (1988) found that the diet of Pro. verus is particularly dominated by young leaves, comprising up to 59% of its feeding. It was pointed out that Pro. verus (approximately 4 kg body weight at Tiwai) may have evolved in competition with its close, but much larger, relative Pi. badius (approximately 8 kg at Tiwai), with both living broadly in sympatry in West Africa. Size differentiation, and consequently niche separation, may have occurred in the two species. Generally, in mammals, larger body size allows for relatively reduced energy requirements, longer passage rates, and thorough digestion, therefore facilitating the use of lower quality forage (Parra 1978). The larger size of Pi. badius should therefore facilitate digestion of mature leaves, which generally requires more time to digest and include less protein (Huang et al. 2010; Lucas et al. 2001; Milton 1979). This food item is more frequently observed in its diet than in the smaller Pro. verus (Table 1). Members of the small-sized Asian Presbytis occupy a similar size range to Procolobus. Similar to Pro. verus, the annual diet of Presbytis appears to include only a small amount of mature leaves, ranging from 1% (Pr. rubicunda) to 11% (Pr. femoralis) and they feed more frequently on other food items such as young leaves, fruits, or seeds. We postulate that the small size of Presbytis constrains it to be highly selective in its choice of food, as with Pro. verus.
Insularity can also be responsible for unexpected size differences because of peculiar and local evolutionary processes (Raia and Meiri 2006). Known as the “Island rule” (Van Valen 1973), this effect has been interpreted in different ways, with a combination of many factors governing body size evolution in an insular context. Among these factors, a reduced predation pressure allowing large species to attain smaller sizes (Sondaar 1977; Sinclair et al. 2003), or resource shortage on small islands leading to smaller body sizes (Lomolino 1985; Burness et al. 2001) have been proposed. Piliocolobus kirkii, endemic to Unguja Island in Tanzania, is known to present remarkably small size compared to other closely related species, possibly resulting from insularity (Nowak et al. 2008). Although our study included only two Pi. kirkii specimens, our results are in agreement with this previous observation. Our study also evidenced a case of a relatively recent, but drastic, diminution in size within the odd-nosed monkeys (i.e. members of Pygathrix, Rhinopithecus, Nasalis, and Simias). Southeast Asia presents a wide variety of environments, including small insular ecosystems that were colonized by colobines. Our results show that Si. concolor presented a surprisingly small size compared to other odd-nosed monkeys, particularly in comparison to its sister-species N. larvatus (divergence ~ 1.5 Ma) (Liedigk et al. 2012). Skulls of Si. concolor are extremely rare in collections and have rarely been studied in a comparative context. We explain the notable difference found between Simias and other odd-nosed taxa by the size of their home islands: the first one living in Borneo (area: 743,330 km2) (Matsuda et al. 2009), while Simias is found on the much smaller islands of the Mentawai Archipelago (area of largest island ~ 4000 km2) (Watanabe 1981). Nasalis is known as a foregut fermenter in which regurgitation and remastication (i.e. rumination) were observed in the wild (Matsuda et al. 2014). Nasalis appears to have one of the largest skulls among Asian colobines (Fig. 4), and its large body mass is arguably related to its diet and “ruminant-like strategy” (Matsuda et al. 2014). Thus, both insularity in Simias and dietary adaptation in Nasalis may have resulted in the contrasting size difference between the closely related Simias and Nasalis.
Allometric Patterns
MMR indicated that size explains 3.3% of whole shape variation. The reconstructions we made to visualize the allometric effect in our dataset showed a relative elongation of the face with increasing cranial size, associated with an overall flattening of the cranium (Fig. 5). This pattern of facial elongation with increasing size has been established for cercopithecines and more generally for cercopithecoids (e.g. Singleton 2002). Although allometric constraints are evident among colobines, it appears that some taxa depart from this trend. For example, two genera (Rhinopithecus and Simias) deviated notably from the 95% CI of the regression line. Other genera appeared shifted above (e.g., Procolobus), or below (Presbytis), the regression line describing the allometric constraints. We speculate that such deviation is partly associated with dietary specialization, as discussed later. Strikingly, N. larvatus, which is known for their long face (Benefit and McCrossin 1991), does not deviate significantly from the allometric trend line, suggesting that their long face is mostly a result of allometric constraints.
When the allometric pattern of shape variation is interpreted in the light of the results obtained on the mechanical advantage analysis, we observed a negative correlation between size and mechanical advantage for all measurements, suggesting the presence of allometric constraints on mechanical advantage. For instance, large-bodied N. larvatus and C. guereza showed very low mechanical advantages for biting (Fig. 3, Online Resource 4). These results support the proposition made on cercopithecines that a decrease in mechanical advantage occurs as the face becomes proportionally longer by allometric constraints (Singleton 2005).
Shape Changes Primarily Reflect Phylogeny
Overall, the phylogenetic signal in colobine cranial shape variation was clearly supported by Blomberg’s K tests and Lambda tests. Phylogeny was indicated to explain 28.8% of whole shape variation by MMR, being much more influential than diet and size. From their initial radiation ~ 12 million years ago (Liedigk et al. 2012), colobines became a highly diversified subfamily comprising 61 species in ten genera (Groves 2005). The most recent species diverged ~ 1.5 Ma in Southeast Asia and our results showed that even on this timescale, strong morphological differences have emerged. Asian colobines display a much greater craniodental variation compared to African monkeys. Some genera are strongly diverged and stand in the extreme range of phenotypic variation (e.g., Rhinopithecus and Pygathrix along bgPC1, or Nasalis and Simias along bgPC1 and bgPC2). This is not surprising as Asian colobines are found in a wider range of habitats, with highly variable environmental conditions such as swamps, tropical or temperate forests, high or low altitude landscapes, mainland or islands (Kirkpatrick and Grueter 2010; Ehlers Smith et al. 2013). Contrary to what one would expect, the first order signal (bgPC1) was not a dichotomy between African and Asian clades. It reflected mostly the within-Asia variation, differentiating Presbytis, Trachypithecus, Pygathrix, and Nasalis. Although not an Asian clade, the African Colobus is also clearly separated from other clades along this axis. Procolobus and Piliocolobus are not well differentiated from the Asian clade along this axis. bgPC2 separated Colobus from other African clades, and bgPC3 also reflected the Colobus-Piliocolobus-Procolobus differentiation.
The phylogenetic position of T. pileatus has been a focus of dispute in the studies of colobine evolutionary history (Roos et al. 2008; Lavrenchenko 2014; Wang et al. 2015). Recently, a surprising case of intergeneric hybridization between Semnopithecus (dispersing in the Indian subcontinent) and Trachypithecus (found from mainland southeast Asia to the Sundaland) have been evidenced (Roos et al. 2008; Wang et al. 2015). In particular, it has been proposed that T. pileatus originated from a unidirectional introgression hybridization process that occurred between Semnopithecus and Trachypithecus (Lavrenchenko 2014; Wang et al. 2015). The overlap in their range of geographic distribution in Bhutan, Bangladesh, and northeast India, and an analysis of full-length nuclear mitochondrial DNA segments, estimated this hybridization to be the result of a short-term event that occurred relatively recently ~ 3.5 million years ago (Wang et al. 2015). Semnopithecus entellus represents one of the largest species among Asian colobines, and the unusually large size of T. pileatus is argued to be a result of genetic inheritance to Semnopithecus (Wang et al. 2015). Our results are congruent with this proposition, considering both size and shape parameters. The larger size of T. pileatus relative to other Trachypithecus species has already been described in previous studies (Delson et al. 2000; Wang et al. 2015); however, our study is the first to reveal the clear divergence of T. pileatus from other Trachypithecus and its morphometric resemblance to Semnopithecus. In the morphological space defined by the bgPC1-bgPC2 axes, this species is more similar to S. entellus than to any of the other Trachypithecus species. This is in agreement with genetic studies that suggest S. entellus played a role in the introgression event (Roos et al. 2008; Wang et al. 2015), highlighting the importance of considering a combination of morphometric and genetic approaches when focusing on hybridization processes.
Diet and Cranial Morphology
MMR indicated that diet explains 4.9% of whole shape variation. Comparing the craniodental morphologies of five species of African and five species of Asian colobines, Koyabu and Endo (2009, 2010) proposed that craniodental configurations in colobines, and more particularly the mechanical advantage of the masseter for biting, are linked to seed eating. We found that species with a high proportion of seeds in their diet exhibited greater mechanical advantage of the masseter muscle for all studied bite points. Biting at the anterior dentition showed a strong and significant correlation with seed eating (r = 0.62 for the incisor and r = 0.63 for the canine; Fig. 3). On the other hand, postcanine bite points showed weaker and nonsignificant correlations with seed eating (r = 0.57 for P3, r = 0.55 for M1; Table 2). However, our 2B-PLS analysis supported the hypothesis that cranial shape is significantly associated with all food items (Table 5; Fig. 7, Online Resources 8). Block 2 of PLS1, which showed a significant correlation to Block 1 of PLS1 (r = 0.50, p = 0.0101), was loaded mainly by the frequencies of mature leaves (0.65) and fruits (− 0.64), suggesting that PLS1 indicates the contrast of leaf eaters vs. fruit eaters. Block 2 of PLS2, which showed a significant correlation to Block 1 of PLS2 (r = 0.63, p = 0.0005), was loaded mainly by the frequencies of seeds (0.73) and young leaves (− 0.53), suggesting that PLS2 reflects the degree of seed eating. Correlation between Block1 (morphology) and Block 2 (diet) was the highest in this PLS2. These results suggest that the overall craniodental shape variation clearly reflects the degree of seed eating but also that correspondence to other food items is also evident. Such ecomorphological patterns for food items possibly explain, in part, the non-phylogenetic and non-allometric variation in the colobine skull. PLS1, which strongly reflected the frugivory vs. folivory ditochomy, indicated that more folivorous species are characterized by a taller dental crown and anteroposteriorly longer postcanine dentition while more frugivorous species tend to exhibit wider face and more anteriorly positioned malar (Fig. 7a, Online Resources 8). PLS2, which strongly reflect seed eating, showed that seed eaters exhibit a more anteriorly protruded alveolar region, wider lower facial region, and more posteriorly shifted dentition relative to the temporomandibular joints (Fig. 7b, Online Resources 8). Specifically, morphological configurations of more posteriorly shifted dentition relative to the temporomandibular joints contribute to enhance the mechanical advantage of the masticatory apparatus and possibly facilitate seed predation (Koyabu and Endo 2009, 2010).
Ecological studies have suggested that seeds are selectively predated by colobines because they are rich sources of nutritive elements such as lipids, digestible carbohydrates, and protein (McKey 1978; Maisels et al. 1994; Waterman and Kool 1994; Auta and Anwa 2007; Hanya and Bernard 2012, 2015).Thus, seeds can be a desirable food item for animals as long as the defense of the seeds (the pod or pericarp) can be overcome. Seeds can be mechanically resistant whereas young leaves are generally less hard and less tough, therefore requiring less masticatory force to be processed (Lucas 2004; Williams et al. 2005). Although the frequency of seed eating can capture only one aspect of the complex animal diet—and further studies incorporating the mechanical properties of seeds predated by colobines are needed—at least some species, such as C. polykomos and Pr. Rubicunda, include mechanically challenging seeds in their diet that require relatively stronger masticatory forces to break down (Lucas and Teaford 1994; Lucas et al. 2000; Scott et al. 2012).
Among the Asian species, Pr. rubicunda, T. francoisi, Py. nigripes, and R. avunculus showed a greater mechanical advantage of the masseter for biting at the anterior dentition. Dietary information on the critically endangered R. avunculus and T. francoisi is largely lacking, but observations on Pr. rubicunda and Py. nigripes confirm that these two species frequently predate seeds (Davies 1991; Rawson 2009). Davies (1991) reported that the diet of Pr. rubicunda at Sepilok in Northern Borneo can comprise 80% seeds, depending on the season. Similarly, in the Danum Valley, seeds account for more than 80% of its diet in September, confirming the status of seed predator for Pr. rubicunda (Hanya and Bernard 2012). Seeds predated by Pr. rubicunda are pliant and tough (Lucas and Teaford 1994; Scott et al. 2012), and most seeds are “bitten, chewed, swallowed and digested, leaving no chance for survival” (Davies 1991). Davies (1991) also reported that the resistant arils of fruits such as Xerospermum internedium, Wallucharia wallichii, and Knema laterica, are chiseled off and removed to access the seeds. While most colobines prefer young leaves over other food items (Bennett and Davies 1994; Oates 1994), Rawson (2009) pointed out that young leaves eaten by Py. nigripes were, on the contrary, fallback resources; seeds such as Sindora siamensis, Peltophorum cf. dasyrrhachis, Terminalia spp., and Dracontomelon appeared to be the most preferred food items, which may even determine group movements and, more particularly, fission–fusion behavior (Rawson 2009). Duc et al. (2009) observed that Py. nigripes in southern Vietnam fed more frequently on seeds from unripe fruits than ripe ones, using their canines to gouge out seeds so they could eat those alone. While Pr. rubicunda has been a classic model for morphological studies examining the influence of seed eating (Lucas and Teaford 1994; Koyabu and Endo 2010; Scott et al. 2012), the significance of seed eating for Py. nigripes’ morphology has been overlooked. Its high mechanical advantage for biting (Fig. 3 and Online Resource 4) and the consistent dietary observations (Rawson 2009) place this species as another potential model for seed predation. Rhinopithecus avunculus is one of the most endangered mammalian species today, but its diet is largely unknown; a preliminary investigation reported that it predates on tougher food items than other colobines in Vietnam (R. avunculus 1393 J/m2; Trachypithecus laotum and Trachypithecus delacouri 1156 J/m2; Py. nemaeus and cinerea 1238 J/m2) (Quyet et al. 2007). In line with these observations, R. avunculus presents one of the greatest mechanical advantages of the masseter for biting at the anterior dentition among all species considered in our study. Long-term observations of feeding frequencies in R. avunculus, and how its dentition is recruited during the feeding process, are of high interest as they could provide useful information on its feeding habits and preferred environments, thereby informing conservation efforts to sustain its populations.
Among African colobines, C. satanas is known as a selective seed eater (McKey 1978; McKey et al. 1981; Oates 1994), exhibiting relatively flat molars compared to the more folivorous C. guereza and Pi. badius (Kay 1975; Ungar 1998). In agreement with this, C. satanas showed greater mechanical advantage of the masseter than C. guereza and P. badius for all bite points, and showed the greatest mechanical advantage of the masseter for most bite points among African colobines. Among Colobus, this study further revealed that C. polykomos and C. angolensis exhibited greater mechanical advantage of the masseter than C. guereza and C. vellerosus. “Molarized” premolars, a character that is often linked to seed eating in primates (Fleagle and McGraw 1999; Daegling and McGraw 2001), have also been observed in C. satanas and C. polykomos (Swindler 1976). In Tiwai Forest, Sierra Leone, C. polykomos has been observed to laboriously gnaw through the full-sized, thick woody pods of Pentaclethra macrophylla and eat the seeds, whereas the sympatric P. badius avoids the heavily lignified pods (Maisels et al. 1994). A study on the processing behaviors of C. polykomos and P. badius, and the material properties of P. macrophylla seeds and pods in Tai Forest, has shown that the anterior dentition (incisors and/or canines) is more frequently employed in C. polykomos than in P. badius, and is particularly recruited to broach the exceptionally tough pods of P. macrophylla (mean of 10 603.32 ± 5148.85 J/m2) for accessing the seeds within (McGraw et al. 2016). Consistent with this observation, C. polykomos exhibited greater mechanical advantage of the masseter for biting at the anterior dentition than P. badius.
As exemplified by the cases of Pr. rubicunda (Davies 1991), Py. nigripes (Duc et al. 2009), and C. polykomos (McGraw et al. 2016), biting with the anterior dentition (incisors and canines) appears to be highly important for seed eating. Similarly, the Neotropic primate Chiropotes satanas is observed to use its anterior dentition to remove the puncture-resistant husks of unripe fruits and gain access to the seeds, a behavior referred as “sclerocarpic harvesting” (Kinzey 1992). It also presents greater mechanical advantage of the masseter for biting at the anterior dentition compared to other closely related primates, which do not conduct this behavior (Wright 2005). The alveolar region of the maxilla is oriented more anteriorly, resulting in procumbent incisors that function as an efficient nipping or cropping device (Kinzey 1992). As exemplified by the morphometric analysis, the oral tip of seed-eating colobines is similarly tilted more anteriorly. Thus, we postulate that the incisor/canine position and masseter insertion are particularly linked for seed eating in some colobines (namely Pr. rubicunda, Py. nigripes, C. angolensis, C. polykomos, and C. satanas). Although the mechanical advantage of biting is an essential parameter affecting the amount of force production, muscle mass (i.e., physiological cross-sectional area) is another important contributor to the bite forces an animal can generate (Raadsheer et al. 1999). As was previously done for other taxa (e.g., Taylor and Vinyard 2009; Koyabu et al. 2012; Furuuchi et al. 2013; Ito and Endo 2016), muscle mass should be further explored in future studies in order to better characterize the relationship between bite force and seed eating.
In conclusion, our expectation, following Koyabu and Endo (2009, 2010), that seed-eating would be the dominant signal to emerge from variation in the colobine skull, was not fully supported. Phylogeny explains 28.8%, diet explains 4.9%, and allometry explains 3.3% of the overall shape variation, all of which contributing significantly. The mechanical advantage of the masseter for biting at the anterior dentition exhibited correlation to frequencies of seed intake and our multivariate analyses of the colobine cranium demonstrated that cranial variation does reflect dietary variation in seed eating, but seed eating cannot, by itself, explain the overall variation of the skull and other food items also significantly explain the variation. Concerning phylogeny, clear interspecific patterns of differentiation and a robust phylogenetic signal was confirmed. For further studies, integrated phylogenetic analyses including all described taxa of colobines could be of high value in order to test these issues more precisely (including, for example, a correspondence for each specimen between the genetic and morphological data). However, in this study we considered specimens from most of the major collections of colobines worldwide, and the scarcity of additional specimens could be an impediment to further analyses. Size evolution, which is arguably driven by diet, biogeographic effects, and niche displacement, have a strong influence on shape variation for most colobine taxa, as shown by the allometric pattern we described. Size displacement and the diversity of food items consumed by colobines may have facilitated the sympatric coexistence of closely related species following colobine radiations.
Colobines for which diet have been studied in detail and phylogeny is resolved overall, provided us with a unique opportunity to explore patterns of craniodental variation, which aids in further understanding the cranial variation found among mammals. Due to the paucity of female specimens, this study was conducted only on males, but a dedicated study should be performed on females to determine if similar results are observed. Our results also highlight the potential of colobines as a model for future studies testing the relationship between morphology and ecological factors. In the past few decades, more and more studies have revealed the peculiarity of their feeding habits and highly specialized ecological niches, which hopefully will assist with building conservation plans for species such as R. avunculus that are currently facing extinction.
References
Anderson, J., Cowlishaw, G., & Rowcliffe, J. M. (2007). Effects of forest fragmentation on the abundance of Colobus angolensis palliatus in Kenya’s Coastal Forests. International Journal of Primatology, 28, 637–655.
Auta, J., & Anwa, E. P. (2007). Preliminary studies on Albizzia lebbeck seeds: Proximate analysis and phytochemical screening. Research Journal of Biological Sciences, 2, 33–35.
Benefit, B. R., & McCrossin, M. L. (1991) Ancestral facial morphology of Old World higher primates. Proceedings of the National Academy of Sciences United States of America, 88, 5267–5271.
Bennett, E. L. (1983). The banded langur: Ecology of a colobine in West Malaysian rain-forest. Ph.D. thesis, University of Cambridge, Cambridge.
Bennett, E. L., & Davies, A. G. (1994). The ecology of Asian colobines. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 129–171). Cambridge: Cambridge University Press.
Bennett, E. L., & Sebastian, A. C. (1988). Social organization and ecology of proboscis monkeys (Nasalis larvatus) in mixed coastal forest in Sarawak. International Journal of Primatology, 9, 233–255.
Bergmann, C. (1848). Über die Verhältnisse der Wärme-ökonomie der Thiere zu ihrer Grösse. Göttinger Studien, 3, 595–708.
Bouvier, M. (1986). Biomechanical scaling of mandibular dimensions in New World monkeys. International Journal of Primatology, 7, 551–567.
Brugiere, D., Gautier, J. P., Moungazi, A., & Gautier-Hion, A. (2002). Primate diet and biomass in relation to vegetation composition and fruiting phenology in a rain forest in Gabon. International Journal of Primatology, 23, 999–1024.
Bruner, E. (2007). Cranial shape and size variation in human evolution: Structural and functional perspectives. Child’s nervous system, 23, 1357–1365.
Burness, G. P., Diamond, J., & Flannery, T. (2001). Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proceedings of the National Academy of Sciences of the United States of America, 98, 14518–14523.
Cardini, A., & Elton, S. (2009). The radiation of red colobus monkeys (Primates, Colobinae): Morphological evolution in a clade of endangered African primates. Zoological Journal of the Linnean Society, 157, 197–224.
Chapman, C. A., Chapman, L. J., Cords, M., Gathua, J. M., Gautier-Hion, A., Lambert, J. E., Rode, K., Tutin, C. E. G., & White, L. J. T. (2004). Variation in the diets of Cercopithecus species: Differences within forests, among forests, and across species. In M. E. Glenn & M. Cords (Eds.), The guenons: Diversity and adaptation in African monkeys (pp. 325–350). New York: Kluwer Academic.
Chapman, C. A., Chapman, L. J., & Gillespie, T. R. (2002). Scale issues in the study of primate foraging: Red colobus of Kibale National Park. American Journal of Physical Anthropology, 117, 349–363.
Chatterjee, H. J., Ho, S. Y. W., Barnes, I., & Groves, C. (2009). Estimating the phylogeny and divergence times of primates using a supermatrix approach. BMC Evolutionary Biology, 9, 259.
Chivers, D. J., & Hladik, C. M. (1980). Morphology of the gastrointestinal tract in primates: Comparisons with other mammals in relation to diet. Journal of Morphology, 166, 337–386.
Clutton-Brock, T. H. (1975). Feeding behaviour of red colobus and black and white colobus in East Africa. Folia Primatologica, 23, 165–207.
Culhane, A. C., Perrière, G., Considine, E. C., Cotter, T. G., & Higgins, D. G. (2002). Between-group analysis of microarray data. Bioinformatics, 18, 1600–1608.
Curtin, S. H. (1976). Niche separation in sympatric Malaysian leaf-monkeys (Presbytis obscura and Presbytis melalophos). Yearbook of Physical Anthropology, 20, 421–439.
Curtin, S. H. (1980). Dusky and banded leaf monkeys. In D. J. Chivers (Ed.), Malayan forest primates: Ten years' study in tropical rain forest (pp. 105–145). New York: Plenum Press.
Daegling, D. J., Granatosky, M. C., McGraw, W. S., & Rapoff, A. J. (2011). Reduced stiffness of alveolar bone in the colobine mandible. American Journal of Physical Anthropology, 144, 421–431.
Daegling, D. J., & McGraw, W. S. (2001). Feeding, diet, and jaw form in West African Colobus and Procolobus. International Journal of Primatology, 22, 1033–1055.
Damuth, J., & MacFadden, B. J. (1990). Body size in mammalian paleobiology: Estimation and biological implications. Cambridge: Cambridge University Press.
Davies, A. G. (1991). Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo. International Journal of Primatology, 12, 119–144.
Davies, A. G., Bennett, E. L., & Waterman, P. G. (1988). Food selection by two South-east Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biological Journal of the Linnean Society, 34, 33–56.
Davies, A. G., Oates, J. F., & Dasilva, G. L. (1999). Patterns of frugivory in three West African colobine monkeys. International Journal of Primatology, 20, 327–357.
Dayan, T., & Simberloff, D. (1998). Size patterns among competitors: Ecological character displacement and character release in mammals,with special reference to island populations. Mammal Review, 28, 99–124.
Dela, J. D. S. (2007). Seasonal food use strategies of Semnopithecus vetulus nestor, at Panadura and Piliyandala, Sri Lanka. International Journal of Primatology, 28(3), 607–626.
Delson, E., Terranova, C. J., Jungers, W. L., Sargis, E. J., Jablonski, N. G., & Dechow, P. C. (2000). Body mass in Cercopithecidae (Primates, Mammalia): Estimation and scaling in extinct and extant taxa. Anthropological Papers of the American Museum of Natural History, 83, 1–159.
Drake, A., & Klingenberg, C. (2008) The pace of morphological change: Historical transformation of skull shape in St Bernard dogs. Proceedings of the Royal Society B: Biological Sciences, 275, 71–76.
Dray, S., & Dufour, A.-B. (2007). The ade4 package: Implementing the duality diagram for ecologists. Journal of Statistical Software, 22, 1–20.
Duc, H. M., Baxter, G. S., & Page, M. J. (2009). Diet of Pygathrix nigripes in southern Vietnam. International Journal of Primatology, 30, 15–28.
Ehlers Smith, D. A., Husson, S. J., Ehlers Smith, Y. C., & Harrison, M. E. (2013). Feeding ecology of red langurs in sabangau tropical peat-swamp forest, Indonesian Borneo: Extreme granivory in a non-masting forest. American Journal of Primatology, 75, 848–859.
Emerson, S. B., & Bramble, D. M. (1993). Scaling, allometry and skull design. In J. Hanken & B. K. Hall (Eds.), The skull (Vol. 3, pp. 384–421). Chicago: University of Chicago Press.
Felsenstein, J. (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1–15.
Fimbel, C., Vedder, A., Dierenfeld, E., & Mulindahabi, F. (2001). An ecological basis for large group size in Colobus angolensis in the Nyungwe Forest, Rwanda. African Journal of Ecology, 39, 83–92.
Fleagle, J. G., & McGraw, W. S. (1999). Skeletal and dental morphology supports diphyletic origin of baboons and mandrills. Proceedings of the National Academy of Sciences of the United States of America, 96, 1157–1161.
Furuuchi, K., Koyabu, D., Mori, K., & Endo, H. (2013). Physiological cross-sectional area of the masticatory muscles in the giraffe (Giraffa camelopardalis). Mammal Study, 38, 67–71.
Groves, C. P. (2005). Subfamily Colobinae. In D. E. Wilson & D. M. Reeder (Eds.), Mammal species of the world (3rd ed., pp. 167–177). Baltimore: Johns Hopkins University Press.
Gupta, A. K., & Kumar, A. (1994). Feeding ecology and conservation of the Phayre’s leaf monkey Presbytis phayrei in northeast India. Biolological Conservation, 69, 301–306.
Hanya, G., & Bernard, H. (2012). Fallback foods of red leaf monkeys (Presbytis rubicunda) in Danum Valley, Borneo. International Journal of Primatology, 33, 322–337.
Hanya, G., & Bernard, H. (2015). Different roles of seeds and young leaves in the diet of red leaf monkeys (Presbytis rubicunda): Comparisons of availability, nutritional properties, and associated feeding behavior. International Journal of Primatology, 36, 177–193.
Harris, T. R., & Chapman, C. A. (2007). Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates, 48, 208–221.
Huang, Z. P., Huo, S., Yang, S. G., Cui, L. W., & Xiao, W. (2010). Leaf choice in black-and-white snub-nosed monkeys Rhinopithecus bieti is related to the physical and chemical properties of leaves. Current Zoology, 56, 643–649.
Hull, D. B. (1979). A craniometric study of the black and white Colobus Illiger 1811 (Primates: Cercopithecoidea). American Journal of Physical Anthropology, 51, 163–181.
Hylander, W. L. (1979a). Mandibular function in Galago crassicaudatus and Macaca fascicularis: An in vivo approach to stress analysis of the mandible. Journal of Morphology, 159, 253–296.
Hylander, W. L. (1979b). The functional significance of primate mandibular form. Journal of Morphology, 160, 223–239.
Ito, K., & Endo, H. (2016). Comparative study of physiological cross-sectional area of masticatory muscles among species of Carnivora. Mammal Study, 41, 181–190.
Jablonski, N. G. (1998). The evolution of doucs and snub-nosed monkeys and the question of the phyletic unity of the odd-nosed colobines. In N. G. Jablonski (Eds.), The Natural History of the Doucs and Snub-Nosed Monkeys (pp. 13–52). Singapore: World Scientific.
Kamilar, J. M., & Paciulli, L. M. (2008). Examining the extinction risk of specialized folivores: A comparative study of colobine monkeys. American Journal of Primatology, 70, 816–827.
Karanth, K. P., Singh, L., Collura, R. V., & Stewart, C. B. (2008). Molecular phylogeny and biogeography of langurs and leaf monkeys of South Asia (Primates: Colobinae). Molecular Phylogenetics and Evolution, 46, 683–694.
Kay, R. F. (1975). The functional adaptations of primate molar teeth. American Journal of Physical Anthropology, 43, 195–215.
Kibaja, M. (2014). Diet of the ashy red colobus (Piliocolobus tephrosceles) and crop-raiding in a forest-farm mosaic, Mbuzi, Rukwa Region, Tanzania. Primate Conservation, 28, 109–116.
Kinzey, W. G. (1992). Dietary and dental adaptations in the Pitheciinae. American Journal of Physical Anthropology, 88, 499–514.
Kirkpatrick, R. C., & Grueter, C. C. (2010). Snub-nosed monkeys: Multilevel societies across varied environments. Evolutionary Anthropology, 19, 98–113.
Kool, K. M. (1993). The diet and feeding behavior of the silver leaf monkey (Trachypithecus auratus sondaicus) in Indonesia. International Journal of Primatology, 14, 667–700.
Koyabu, D., Oshida, T., Nguyen, S. T., Dang, C. N., Nguyen, N. X., Nguyen, D. X., Motokawa, M., Kimura, J., Sasaki, M., & Endo, H. (2012). Comparison of jaw muscle morphology in two sympatic callosciurine squirrels (Callosciurus erythraeus and Dremomys rufigenis) in Vietnam. Mammal Study, 37, 237–242.
Koyabu, D. B., & Endo, H. (2009). Craniofacial variation and dietary adaptations of African colobines. Journal of Human Evolution, 56, 525–536.
Koyabu, D. B., & Endo, H. (2010). Craniodental mechanics and diet in asian colobines: Morphological evidence of mature seed predation and sclerocarpy. American Journal of Physical Anthropology, 148, 137–148.
Langsrud, Ø., & Mevik, B. (2012). ffmanova: Fifty-fifty MANOVA. R package version 0.2-2. Retrieved from https://CRAN.R-project.org/package=ffmanova.
Lavrenchenko, L. a. (2014). Hybrid speciation in mammals: Illusion or reality? Biology Bulletin Reviews, 4, 198–209.
Ledevin, R., Quéré, J.-P., Michaux, J. R., & Renaud, S. (2012). Can tooth differentiation help to understand species coexistence? The case of wood mice in China. Journal of Zoological Systematics and Evolutionary Research, 50, 315–327.
Li, B., Pan, R., & Oxnard, C. E. (2002). Extinction of snub-nosed monkeys in China during the past 400 years. International Journal of Primatology, 23, 1227–1244.
Li, Y., Jiang, Z., Li, C., & Grueter, C. C. (2010). Effects of seasonal folivory and frugivory on ranging patterns in Rhinopithecus roxellana. International Journal of Primatology, 31, 609–626.
Liedigk, R., Yang, M., Jablonski, N. G., Momberg, F., Geissmann, T., Lwin, N., Hla, T. H., Liu, Z., Wong, B., Ming, L., Yongcheng, L., Zhang, Y. P., Nadler, T., Zinner, D., & Roos, C. (2012) Evolutionary history of the odd-nosed monkeys and the phylogenetic position of the newly described myanmar snub-nosed monkey Rhinopithecus strykeri. PLoS ONE, 7, e37418.
Liu, X., Stanford, C. B., Yang, J., Yao, H., & Li, Y. (2013). Foods eaten by the sichuan snub-nosed monkey (Rhinopithecus roxellana) in Shennongjia National Nature Reserve, China, in relation to nutritional chemistry. American Journal of Primatology, 75, 860–871.
Lomolino, M. V. (1985). Body size of mammals on islands: The island rule reexamined. The American Naturalist, 125, 310–316.
Lucas, P. W. (2004) Dental functional morphology: How teeth work (pp. 1–355). Cambridge: Cambridge University Press.
Lucas, P. W., Beta, T., Darvell, B. W., Dominy, N. J., Essackjee, H. C., Lee, P. K. D., & Yuen, T. D. B. (2001). Field kit to characterize physical, chemical and spatial aspects of potential primate foods. Folia Primatologica, 72, 11–25.
Lucas, P. W., Copes, L., Constantino, P. J., Vogel, E. R., Chalk, J., Talebi, M., Landis, M., & Wagner, M. (2012). Measuring the toughness of primate foods and its ecological value. International Journal of Primatology, 33, 598–610.
Lucas, P. W., & Teaford, M. F. (1994). Functional morphology of colobine teeth. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 173–203). Cambridge: Cambridge University Press.
Lucas, P. W., Turner, I. M., Dominy, N. J., & Yamashita, N. (2000). Mechanical defences to herbivory. Annals of Botany, 86, 913–920.
Maddison, W. P., & Maddison, D. R. (2011) Mesquite: A modular system for evolutionary analysis. Version 2.75. Retrieved from http://mesquiteproject.org
Maisels, F., Gautier-Hion, A., & Gautier, J.-P. (1994). Diets of two sympatric colobines in Zaire: More evidence on seed-eating in forests on poor soils. International Journal of Primatology, 15, 681–701.
Marroig, G., Shirai, L. T., Porto, A., de Oliveira, F. B., & De Conto, V. (2009). The evolution of modularity in the mammalian skull II: Evolutionary consequences. Evolutionary Biology, 36, 136–148.
Marsh, C. W. (1981). Diet choice among Red Colobus (Colobus badius rufomitratus) on the Tana River, Kenya. Folia Primatologica, 35(2–3), 147–178.
Matsuda, I., Tuuga, A., Bernard, H., Sugau, J., & Hanya, G. (2013). Leaf selection by two Bornean colobine monkeys in relation to plant chemistry and abundance. Scientific Reports, 3, 1873.
Matsuda, I., Tuuga, A., Hashimoto, C., Bernard, H., Yamagiwa, J., Fritz, J., Tsubokawa, K., Yayota, M., Murai, T., Iwata, Y., & Clauss, M. (2014). Faecal particle size in free-ranging primates supports a ‘rumination’strategy in the proboscis monkey (Nasalis larvatus). Oecologia, 174, 1127–1137.
Matsuda, I., Tuuga, A., & Higashi, S. (2009). The feeding ecology and activity budget of proboscis monkeys. American Journal of Primatology, 71, 478–492.
McGraw, W. S., van Casteren, A., Kane, E., Geissler, E., Burrows, B., & Daegling, D. J. (2016). Feeding and oral processing behaviors of two colobine monkeys in Tai Forest, Ivory Coast. Journal of Human Evolution, 98, 90–102.
McKey, D. B. (1978). Soils, vegetation, and seed-eating by black colobus monkeys. In G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 423–437). Washington D.C: Smithsonian Institution Press.
McKey, D. B., Gartlan, J. S., Waterman, P. G., & Choo, G. M. (1981). Food selection by black colobus monkeys (Colobus satanas) in relation to plant chemistry. Biological Journal of the Linnean Society, 16, 115–146.
Md-Zain, B. M., Hasan, M. H., & Melnick, D. J. (2002) Defining evolutionary significant units for Presbytis melalophos conservation using mitochondrial DNA sequences. In Proceedings of the regional symposium on environment and natural resources, (Vol. 1, pp. 279–286).
Meijaard, E., & Groves, C. P. (2004) The biogeographical evolution and phylogeny of the genus Presbytis. Primate Report, 68, 71–90.
Meiri, S., & Dayan, T. (2003). On the validity of Bergmann’s rule. Journal of Biogeography, 30, 331–351.
Meloro, C., Cáceres, N., Carotenuto, F., Sponchiado, J., Melo, G. L., Passaro, F., & Raia, P. (2014). In and out the Amazonia: Evolutionary ecomorphology in howler and capuchin monkeys. Evolutionary Biology, 41, 38–51.
Meyer, D., Rinaldi, I. D., Ramlee, H., Perwitasari-Farajallah, D., Hodges, J. K., & Roos, C. (2011). Mitochondrial phylogeny of leaf monkeys (genus Presbytis, Eschscholtz, 1821) with implications for taxonomy and conservation. Molecular Phylogenetics and Evolution, 59, 311–319.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: A test of some hypotheses of food selection by generalist herbivores. The American Naturalist, 114, 362–378.
Minhós, T., Sousa, C., Vicente, L. M., & Bruford, M. W. (2015). Kinship and intragroup social dynamics in two sympatric African colobus species. International Journal of Primatology, 36, 871–886.
Mitteroecker, P., Gunz, P., Bernhard, M., Schaefer, K., & Bookstein, F. L. (2004). Comparison of cranial ontogenetic trajectories among great apes and humans. Journal of Human Evolution, 46, 679–698.
Mturi, F. A. (1993). Ecology of the Zanzibar red colobus monkey, Colobus badius kirkii (Gray, 1968), in comparison with other red colobines. In J. C. Lovett & S. K. Wasser (Eds.), Biogeography and ecology of the rain forest of eastern Africa (pp. 243–266). Cambridge: Cambridge University Press.
Nowak, K., Cardini, A., & Elton, S. (2008). Evolutionary acceleration and divergence in Procolobus kirkii. International Journal of Primatology, 29, 1313.
Oates, J. F. (1988). The diet of the olive colobus monkey, Procolobus verus, in Sierra Leone. International Journal of Primatology, 9, 457–478.
Oates, J. F. (1994). The natural history of African colobines. Colobine monkeys: Their ecology, behaviour and evolution. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 75–128). Cambridge: Cambridge University Press.
Osterholz, M., Walter, L., & Roos, C. (2008). Phylogenetic position of the langur genera Semnopithecus and Trachypithecus among Asian colobines, and genus affiliations of their species groups. BMC Evolutionary Biology, 8, 58.
Pan, R. (2006). Dental morphometric variation between African and Asian colobines, with special reference to the other Old World monkeys. Journal of Morphology, 267, 1087–1098.
Paradis, E., Claude, J., & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290.
Parra, R. (1978). Comparison of foregut and hindgut fermentation in herbivores. In G. Montgomery (Ed.), The ecology of arboreal folivores (pp. 205–230). Washington D.C.: Smithsonian Institution Press.
Pohlert, T. (2016). The pairwise multiple comparison of mean ranks package (PMCMR). Resource document. Retrieved July 26, 2017, from https://cran.r-project.org/web/packages/PMCMR/vignettes/PMCMR.pdf.
Quyet, L. K., Duc, N. A., Tai, V. A., Wright, B. W., & Covert, H. H. (2007). Diet of the Tonkin snub-nosed monkey (Rhinopithecus avunculus) in the Khau Ca area, Ha Giang Province, northeastern Vietnam. Vietnamese Journal of Primatology, 1, 75–83.
Raadsheer, M. C., Van Eijden, T., Van Ginkel, F. C., & Prahl-Andersen, B. (1999). Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. Journal of Dental Research, 78, 31–42.
Raia, P., & Meiri, S. (2006). The island rule in large mammals: Paleontology meets ecology. Evolution, 60, 1731–1742.
Ravosa, M. J. (1990). Functional assessment of subfamily variation in maxillomandibular morphology among Old World monkeys. American Journal of Physical Anthropology, 82, 199–212.
Ravosa, M. J. (1996). Jaw morphology and function in living and fossil Old World monkeys. International Journal of Primatology, 17, 909–932.
Ravosa, M. J., Noble, V. E., Hylander, W. L., Johnson, K. R., & Kowalski, E. M. (2000). Masticatory stress, orbital orientation and the evolution of the primate postorbital bar. Journal of Human Evolution, 38, 667–693.
Rawson, B. (2009). The socio-ecology of the black-shanked douc (Pygathrix nigripes) in Mondulkiri Province. Ph.D. thesis, The Australian National University.
R-Core-Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Resource document. Retrieved July 26, 2017, from http://www.R-project.org/.
Renaud, S., Dufour, A., Hardouin, E. A., & Ledevin, R. (2015). Once upon multivariate analyses: When they tell several stories about biological evolution. PLoS ONE, 10, 1–18.
Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x.
Rilling, J. K., & a Seligman, R. (2002). A quantitative morphometric comparative analysis of the primate temporal lobe. Journal of Human Evolution, 42, 505–533.
Rode, K. D., Chapman, C. A., Chapman, L. J., & McDowell, L. R. (2003). Mineral resource availability and consumption by colobus in Kibale national park, Uganda. International Journal of Primatology, 24, 541–573.
Rohlf, F. J., & Corti, M. (2000). Use of two-block partial least-squares to study covariation in shape. Systematic Biology, 49, 740–753.
Rohlf, F. J., & Slice, D. (1990). Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59.
Roos, C., Nadler, T., & Walter, L. (2008). Mitochondrial phylogeny, taxonomy and biogeography of the silvered langur species group (Trachypithecus cristatus). Molecular Phylogenetics and Evolution, 47, 629–636.
Ruhiyat, Y. (1983). Socio-ecological study of Presbytis aygula in West Java. Primates, 24, 344–359.
Schlager, S. (2013). Package ‘Morpho’. Resource document. Retrieved July 26, 2017, from https://cran.r-project.org/web/packages/Morpho/Morpho.pdf.
Scott, R. S., Teaford, M. F., & Ungar, P. S. (2012). Dental microwear texture and anthropoid diets. American Journal of Physical Anthropology, 147, 551–579.
Simberloff, D., Dayan, T., Jones, C., & Ogura, G. (2000). Character displacement and release in the small Indian mongoose, Herpestes javanicus. Ecology, 81, 2086–2099.
Sinclair, A. R. E., Mduma, S., & Brashares, J. S. (2003). Patterns of predation in a diverse predator–prey system. Nature, 425, 288–290.
Singleton, M. (2002). Patterns of cranial shape variation in the Papionini (Primates: Cercopithecinae). Journal of Human Evolution, 42, 547–578.
Singleton, M. (2005). Functional shape variation in the cercopithecine masticatory complex. In D. E. Slice (Ed.), Modern morphometrics in physical anthropology (pp. 319–348). New York: Kluwer Academic/Plenum Publishers.
Snaith, T. V., & Chapman, C. A. (2008). Red colobus monkeys display alternative behavioral responses to the costs of scramble competition. Behavioral Ecology, 19, 1289–1296.
Sokal, R. R., & Rohlf, F. J. (2012) Biometry (4th ed.). New York: WH Freeman and Company.
Sondaar, P. Y. (1977). Insularity and its effect on mammal evolution. In M. K. Hecht, P. C. Goody & B. M. Hecht (Eds.), Major patterns in vertebrate evolution (pp. 671–707). New York: Plenum.
Spencer, M. A., & Demes, B. (1993). Biomechanical analysis of masticatory system configuration in Neandertals and Inuits. American Journal of Physical Anthropology, 91, 1–20.
Stanford, C. B. (1991). The diet of the capped langur (Presbytis pileata) in a moist deciduous forest in Bangladesh. International Journal of Primatology, 12, 199–216.
Steudel, K. (1982). Patterns of intraspecific and interspecific allometry in Old World primates. American Journal of Physical Anthropology, 59, 419–430.
Starin, E. D. (1991). Socioecology of the red colobus monkey in the Gambia with partial reference to female-male differences and transfer patterns. Ph.D. thesis, City University of New York, New York.
Struhsaker, T. T. (1975). The red colobus monkey. Chicago: University of Chicago Press.
Sunderraj, S. F. W. (2001). Ecology and conservation of Nilgiri langur (Trachypithecus johnii). Envis Bulletin: Wildlife and Protected Areas, 1, 49–59.
Swindler, D. R. (1976). Dentition of living primates. London: Academic Press.
Taylor, A. B., & Vinyard, C. J. (2009). Jaw-muscle fiber architecture in tufted capuchins favors generating relatively large muscle forces without compromising jaw gape. Journal of Human Evolution, 57, 710–720.
Teaford, M. F. (1983). The morphology and wear of the lingual notch in macaques and langurs. American Journal of Physical Anthropology, 60, 7–14.
Teichroeb, J. A., Saj, T. L., Paterson, J. D., & Sicotte, P. (2003). Effect of group size on activity budgets of Colobus vellerosus in Ghana. International Journal of Primatology, 24, 743–758.
Ting, N. (2008). Mitochondrial relationships and divergence dates of the African colobines: Evidence of Miocene origins for the living colobus monkeys. Journal of Human Evolution, 55, 312–325.
Ting, N., Tosi, A. J., Li, Y., Zhang, Y.-P., & Disotell, T. R. (2008). Phylogenetic incongruence between nuclear and mitochondrial markers in the Asian colobines and the evolution of the langurs and leaf monkeys. Molecular phylogenetics and evolution, 46, 466–474.
Ungar, P. (1998). Dental allometry, morphology, and wear as evidence for diet in fossil primates. Evolutionary Anthropology, 6, 205–217.
Vandercone, R. P., Dinadh, C., Wijethunga, G., Ranawana, K., & Rasmussen, D. T. (2012). Dietary diversity and food selection in Hanuman langurs (Semnopithecus entellus) and purple-faced langurs (Trachypithecus vetulus) in the Kaludiyapokuna Forest Reserve in the dry zone of Sri Lanka. International Journal of Primatology, 33, 1382–1405.
Van Valen, L. (1973). Pattern and the balance of nature. Evolutionary Theory, 1, 31–49.
Verheyen, W. N. (1962) Contribution à la craniologie comparée des primates: les genres Colobus Illiger 1811 et Cercopithecus Linné 1758. Musée royal de l’Afrique centrale.
Wang, B., Zhou, X., Shi, F., Liu, Z., Roos, C., Garber, P. A., Li, M., & Pan, H. (2015). Full-length Numt analysis provides evidence for hybridization between the Asian colobine genera Trachypithecus and Semnopithecus. American Journal of Primatology, 77, 901–910.
Watanabe, K. (1981). Variations in group composition and population density of the two sympatric Mentawaian leaf-monkeys. Primates, 22, 145–160.
Waterman, P. G., & Kool, K. M. (1994). Colobine food selection and plant chemistry. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys: Their ecology, behaviour and evolution (pp. 251–284). Cambridge: Cambridge University Press.
Williams, S. H., Wright, B. W., Truong, V., Daubert, C. R., & Vinyard, C. J. (2005). Mechanical properties of foods used in experimental studies of primate masticatory function. American Journal of Primatology, 67, 329–346.
Wright, B. W. (2005). Craniodental biomechanics and dietary toughness in the genus Cebus. Journal of Human Evolution, 48, 473–492.
Wright, B. W., Ulibarri, L., O’brien, J., Sadler, B., Prodhan, R., Covert, H. H., & Nadler, T. (2008). It’s tough out there: Variation in the toughness of ingested leaves and feeding behavior among four Colobinae in Vietnam. International Journal of Primatology, 29, 1455–1466.
Wright, B. W., & Willis, M. S. (2012). Relationships between the diet and dentition of Asian leaf monkeys. American Journal of Physical Anthropology, 148, 262–275.
Yamashita, N., Cuozzo, F. P., Sauther, M. L., Fitzgerald, E., Riemenschneider, A., & Ungar, P. S. (2016). Mechanical food properties and dental topography differentiate three populations of Lemur catta in southwest Madagascar. Journal of Human Evolution, 98, 66–75.
Yan-Zhang, P., Ru-Liang, P., & Jablonski, N. G. (1993). Classification and evolution of Asian colobines. Folia Primatologica, 60, 106–117.
Zhou, Q., Wei, F., Li, M., Huang, C., & Luo, B. (2006). Diet and food choice of Trachypithecus francoisi in the Nonggang Nature Reserve, China. International Journal of Primatology, 27, 1441–1460.
Zollikofer, C. P. E., & Ponce de León, M. S. (2010). The evolution of hominin ontogenies. Seminars in Cell & Developmental Biology, 21, 441–452.
Acknowledgements
Authors thank the museum staffs for access to specimens and Goro Hanya, Takeshi Nishimura, Gen Suwa, Daisuke Shimizu, and Masanaru Takai for discussions. This study was supported by KAKENHI (Grant Nos. 26711023, 18H02492, 18H04816, and 18K19359), by the Cooperation Research Program of Primate Research Institute, Kyoto University (Grant No. 2010-A-3) and by the LabEx Sciences Archéologiques de Bordeaux (Grant No. ANR-10-LABX-52).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11692_2019_9469_MOESM1_ESM.xlsx
Online Resource 1 List of the specimens analyzed in this study, with accession ID and institutes housing them: Natural History Museum (BMNH), Primate Research Institute of Kyoto University (KUPRI), Smithsonian National Museum of Natural History (USNM), and Zoological Reference Collection of Lee Kong Chian Natural History Museum at the National University of Singapore (ZRC)—Supplementary material 1 (XLSX 32 KB)
11692_2019_9469_MOESM3_ESM.xlsx
Online Resource 3 Landmark definitions employed in the geometric morphometric analyses—Supplementary material 3 (XLSX 9 KB)
11692_2019_9469_MOESM4_ESM.xlsx
Online Resource 4 Mean values of measured moment arms and calculated mechanical advantages of the masseter, temporalis, and medial pterygoid muscles for each species. Canine position measurement is not available for T. policephalus due to missing canine in the studied specimen—Supplementary material 4 (XLSX 23 KB)
11692_2019_9469_MOESM5_ESM.xlsx
Online Resource 5 Pearson’s correlation coefficients for comparsions between mechanical advantage and centroid size—Supplementary material 5 (XLSX 9 KB)
11692_2019_9469_MOESM6_ESM.xls
Online Resource 6 Inter-specific comparison of size using Kruskal-Wallis tests. Significant probabilities are either in bold (P < 0.001) or in italic (P < 0.05). Species for which the number of specimens available if lower than five are written in red—Supplementary material 6 (XLS 51 KB)
11692_2019_9469_MOESM7_ESM.xlsx
Online Resource 7 Basic measurements (n = 15) and centroid size (n = 2) for Simias concolor—Supplementary material 7 (XLSX 9 KB)
11692_2019_9469_MOESM8_ESM.pdf
Online Resource 8 Alternative representation of shape deformations associated to: 1) shape axes bgPC1, bgPC2 and bgPC3, 2) allometry, and 3) 2B-PLS. Here the red dots correspond to the reference configuration compared to the target configuration (in green). The line between each red dot and its homologous green dot represents the deformation—Supplementary material 8 (PDF 1894 KB)
Rights and permissions
About this article
Cite this article
Ledevin, R., Koyabu, D. Patterns and Constraints of Craniofacial Variation in Colobine Monkeys: Disentangling the Effects of Phylogeny, Allometry and Diet. Evol Biol 46, 14–34 (2019). https://doi.org/10.1007/s11692-019-09469-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-019-09469-7