Abstract
Cognitive impairment is now recognized in a subset of patients with amyotrophic lateral sclerosis (ALS). The objective of the study was to identify group differences and neuroanatomical correlates of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) in participants ALS. Fifty-three ALS patients and 43 healthy controls recruited as a part of our multicentre study (CALSNIC) were administered the ECAS and underwent an MRI scan. Voxel-based morphometry and tract based spatial statistics (TBSS) was performed to identify structural changes and associations with impaired ECAS scores. Lower performance in the ECAS verbal fluency and executive domains were noted in ALS patients as compared to controls (p < 0.01). Extensive white matter degeneration was noted in the corticospinal tract in all ALS patients, while ALS patients with impaired verbal fluency or executive domains (ALS-exi, n = 22), displayed additional degeneration in the corpus callosum, cingulum and superior longitudinal fasciculus as compared to controls (p < 0.05, TFCE corrected). Mild grey matter changes and associations with ECAS verbal fluency or executive performance were noted at lenient statistical thresholds (p < 0.001, uncorrected). Executive impairment was detected using the ECAS in our multicentre sample of Canadian ALS patients. White matter degeneration in motor regions was revealed in ALS patients with extensive spread to frontal regions in the ALS-exi sub-group. Mild associations between ECAS verbal fluency, executive function scores and MRI metrics suggest that reduced performance may be associated with widespread structural integrity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clinical signs of both upper and lower motor neuron (UMN and LMN respectively) degeneration is required for a diagnosis of amyotrophic lateral sclerosis (ALS). This terminal neurodegenerative condition is characterised by increased muscle tone and hyperreflexia (UMN signs) in combination with muscle atrophy, weakness and fasciculations (LMN signs). Survival is estimated at 2–3 years after diagnosis and LMN driven respiratory failure accounts for death in most patients (Brooks et al. 2000; Kiernan et al. 2011). Additional features include cognitive and behavioural changes due to frontotemporal lobar degeneration (FTLD). The most prevalent presentation of FTLD in ALS is mild to moderate executive dysfunction 25%–50% patients and apathy in 25–30% patients (Phukan et al. 2012; Witgert et al. 2010). A smaller proportion 10%–15% of ALS patients may meet criteria for frontotemporal dementia (FTD) (Phukan et al. 2012).
Reduced verbal fluency is the hallmark of executive dysfunction in ALS with patients performing worse than controls in tests of letter-based fluency (Kew et al. 1993; Abrahams et al. 1997; Abe et al. 1997; Abrahams et al. 2000; Phukan et al. 2012). Cognitive impairments have also been implied in planning and decision making (executive functions), language and, more recently, social cognition in ALS (Goldstein and Abrahams 2013). Memory deficits have been reported in smaller ALS sub-groups (Abdulla et al. 2014; Machts et al. 2014) with a suggestion that this may be driven by executive dysfunction in ALS (Burke et al. 2017).
Most of these studies include extensive neuropsychometric testing with administration time ranging from 1 to 3 h (Goldstein and Abrahams 2013). Administration of such extensive tests may be challenging in a clinical setting given the time constraints of a busy clinic. The Edinburgh Cognitive and Behavioural ALS Screen (ECAS) was developed to address the above needs and is emerging as a useful screening tool in ALS (Abrahams et al. 2014). It tests multiple cognitive domains (language, verbal fluency, executive, memory and visuospatial abilities) within an administration time of 20–30 min. It includes a behavioural screen which can be completed by the caregivers. The behavioural screen assesses apathy, disinhibition, loss of empathy, stereotypical or perseverative behaviours and changes in dietary habits. The ECAS has been validated against standard neuropsychological assessment (Niven et al. 2015; Pinto-Grau et al. 2017) and adapted to multiple languages (Lulé et al. 2015; Loose et al. 2016; Ye et al. 2016; Poletti et al. 2016; Siciliano et al. 2017).
However, few studies have investigated neuroimaging correlates of the ECAS (Keller et al. 2017; Lulé et al. 2018; Trojsi et al. 2019). There is evidence for cortical function changes such as reduced activation in the left inferior frontal gyrus in ALS patients with impaired ECAS verbal fluency (n = 3) as compared to patients with higher verbal fluency performance (n = 50), while no such differences were noted for alteration (ECAS executive sub-test) (Keller et al. 2017). The study included small sub-groups with cognitive impairment and did not report structural brain changes in association with ECAS performance. Lulé et al. (2018) associated performance on specific domains of the ECAS (executive, memory and disinhibited behaviour) with stages of disease progression identified using pathology (Brettschneider et al. 2013) and diffusion tensor imaging (DTI) (Müller et al. 2016). ALS patients with no cognitive impairments were classified as stage 1 (structures involved: corticospinal tract, somatomotor neurons and brainstem nuclei). Patients with ECAS executive deficits were classified as stage 2 with 100% sensitivity and specificity (structures involved: corticopontine tract, rubral tract and middle frontal gyrus, in addition to stage 1). Patients with disinhibited behaviour were classified as stage 3 or higher with 100% sensitivity and 95% specificity (structures involved: corticostriatal and prefrontal neocortex, in addition to stages 1 and 2). Patients with memory impairments were classified as stage 4 with only 55% sensitivity and 52% specificity (structures involved: perforant path, hippocampus and anteriomedial temporal lobe). While this provides some evidence for extra-motor involvement in cognitive dysfunction identified using the ECAS, Lulé et al. (2018) did not report grey matter changes, thus limiting measures of cortical regions such as middle frontal gyrus, prefrontal cortex and the hippocampus.
Trojsi et al. (2019) report microstructural white matter associations between ECAS verbal fluency, inferior longitudinal fasciculus and inferior frontal occipital fasciculus in ALS patients (n = 36). ECAS memory scores were associated with changes in the thalamus and corpus callosum (genu, anterior body and splenium). The authors propose that alterations in verbal fluency may be related to frontotemporal connectivity while memory impairments may be associated with damage to the thalamocortical circuits. The study did not investigate differences in ALS sub-groups with or without cognitive impairment.
Observing the involvement of different brain regions at specific stages of the disease course, there is an emerging emphasis on sub-groups that may be present in ALS patients (Braak et al. 2013; Brettschneider et al. 2013; Müller et al. 2016). Identifying these sub-groups of patients with cognitive impairment may be crucial for clinical trials and biomarker research. Each sub-group may reflect characteristic features that would enable researchers to better understand underlying disease pathology or spread. If the ECAS is able to identify sub-groups, it is crucial to verify that these sub-groups correspond with global or local brain changes that have been reported in ALS.
Neuroimaging evidence in ALS primarily indicates grey matter degeneration predominantly in the precentral gyrus and evidence of atrophy in frontal and temporal lobes (Chang et al. 2005; Turner et al. 2011; Agosta et al. 2016). Previous studies reported grey matter degeneration in the postcentral gyrus, anterior cingulate cortex (ACC), orbitofrontal gyrus, caudate, putamen, amygdala and hippocampus in cognitively impaired ALS patients when compared to controls (Schuster et al. 2014; Menke et al. 2014; Agosta et al. 2016; Christidi et al. 2018). Lower white matter integrity as identified by decreased fractional anisotropy (FA) was reported in the corticospinal tract (CST) in ALS patients (Kaufmann et al. 2004; Mitsumoto et al. 2007; Senda et al. 2009; Agosta et al. 2009; Agosta et al. 2010; Y. Zhang et al. 2011). In cognitively impaired ALS patients, widespread white matter degeneration was reported in the corpus callosum (CC), cingulum, superior longitudinal fasciculus (SLF) and uncinate fasciculus (UF) (Kasper et al. 2014; Agosta et al. 2016; Christidi et al. 2018).
Considering the evidence of cognitive impairments and frontotemporal changes in ALS patients, the present study aimed to investigate structural brain differences in cognitive sub-groups using the ECAS and to identify the neuroanatomical associations of the cognitive domains of the ECAS. It was hypothesised that: (1) ALS participants impaired on the ECAS have lower grey matter density and reduced white matter integrity in frontotemporal regions, and (2) there is an association between the ECAS and neuroimaging metrics of grey matter density and white matter integrity of frontotemporal regions in ALS patients.
Methods
Participants
The participants for the current study were recruited from ALS clinics in Calgary, Edmonton, Montreal and Toronto (referred to as sites 1–4 respectively) as a part of the Canadian ALS Neuroimaging Consortium (CALSNIC). Inclusion criteria included a diagnosis of ALS according to the revised El Escorial criteria (Ludolph et al., 2015). Healthy controls were age-matched to a patient. Exclusion criteria for both patients and controls were a history of neurological or psychiatric illness, English as second language, and age greater than 80 years. Ethics approval was obtained at each site and all participants signed a written informed consent prior to participating in the study.
Fifty-three ALS patients were included for the study. Demographic details of the patients are outlined in Table 1. Forty-three patients presented with limb-onset, eight with bulbar onset, and two with both bulbar and limb onset (Table 2). Disability was measured by the ALS Functional Rating Scale – Revised (ALSFRS-R) (Cedarbaum et al. 1999). Respiratory status was assessed as forced vital capacity (FVC, percent reference). Disease progression rate was computed as: (48 – ALSFRS-R) / symptom duration (Kimura et al. 2006). In addition to this rate, a scale of UMN burden was derived from the neurological evaluations. The scale includes two lateralized sub-scores for the right and left side of the body, and a sub-score for jaw related UMN symptoms. These scores account for the presence of spasticity and hyperreflexia in respective limbs, Babinski’s sign or clonus in the lower extremity, and the presence of a brisk reflex or clonus in the jaw. Clinical characteristics of all patients and at each site are outlined in Table 2.
Forty-three healthy controls met the inclusion criteria for the study. Beck’s Depression Inventory - II (BDI-II) (Beck et al. 1996) was used to assess mood in all participants. The demographics of the overall and per site control group are described in Table 1.
ECAS and cognitive sub-groups
The ECAS is a screening tool with an average administration time of 20 to 30 min (Abrahams et al. 2014). It can be administered in a written or spoken version and has accommodations for motor impairments in ALS patients. A North American adaptation of the ECAS was used for the current study. Research assistants and a graduate student were trained in administering the ECAS on participants. The ECAS includes assessment of the cognitive domains of language (naming, spelling and comprehension, max score = 28), verbal fluency (letter S, four-letter words beginning with T, max score = 24), executive (digit span backward, alternation, sentence inhibition, social cognition, max score = 48), memory (immediate and delayed recall, recognition, max score = 24) and visuospatial abilities (dot counting, cube counting, dot position, max score = 12). These five domains are summed to obtain the ECAS total score (max score = 136). The individual scores of the domains and the ECAS total score were considered for analysis.
Based on the revised Strong criteria (Strong et al. 2017), the current study considered impairments in either verbal fluency or executive domain scores in a single group. Patients were regarded as impaired on executive function (ALS-exi) if they obtained scores below 2 standard deviations (SD) from the mean scores of the healthy control group for either ECAS verbal fluency or executive scores. Patients who scored above the cut-off scores were classified as normal on EF (ALS-n). Five of six participants who were impaired on language were also impaired in executive functions and hence were classified as ALS-exi. Considering the small sample size for only language (n = 1) or memory (n = 1) impairments, these sub-group analyses were not pursued. The behavioural screen of the ECAS was completed by caregivers in a subset of only 15 patients. Two patients displayed behavioural impairments in all six domains and one displayed impairment in 5 domains.
MR image acquisition
A harmonised MR imaging protocol was implemented across the CALSNIC sites of which three-dimensional T1 sequence (3D-T1) and diffusion tensor imaging (DTI) were examined for the current study (Table 3). All sites acquired MR images on scanners operating at 3 T. The scanner at Sites 1 and 4 was GE Discovery MR750 scanner while sites 2 and 3 used Siemens Prisma and Tim Trio models, respectively.
MR preprocessing
Voxel based morphometry
Voxel-wise analysis of 3D-T1 data was performed using the computational anatomy toolbox (CAT-12 in SPM-12; http://dbm.neuro.uni-jena.de/cat12/). All T1 images were aligned to the anterior commissure – posterior commissure (AC-PC) line using mango (Research Imaging Institute, UTHSCA; http://ric.uthscsa.edu/mango/). Next, corrections for field intensity inhomogeneities were completed and the images were normalised to the International Consortium for Brain Mapping (ICBM) template using both linear and non-linear transformations. These images were segmented into grey matter, white matter and cerebrospinal fluid (CSF) tissue maps. The grey matter tissue maps were smoothed using a Gaussian kernel (8 mm isotropic full-width at half maximum, FWHM) for further analysis. Group comparisons for patients and controls as well as with patient sub-groups (ALS-exi and ALS-n) were performed using the full-factorial model in SPM-12 with diagnosis and site as factors, and age, gender and total intracranial volume (TIV) as covariates. Frontal and temporal masks from the pickatlas toolbox (Maldjian et al. 2003) were employed for identifying region of interest (ROI) based correlations between grey matter densities and ECAS scores.
Diffusion tensor imaging (DTI)
The DTI images of each participant were preprocessed to correct for temporal signal drifts, Gibbs ringing artifacts, motion, Eddy currents and echo planar imaging (EPI) distortions (using T1 as reference) in Explore DTI (Leemans et al. 2009). To facilitate optimal registration of white matter, the preprocessed DTI images were processed with DTI Toolkit (DTI-TK) (H. Zhang et al. 2007a) and were registered to a study-specific template (H. Zhang et al. 2007b). Registration involved affine and deformable alignments with template refinement to allow for alteration of global size and shape of DTI images to match the study-specific template as well as minimize differences of size and shape in local structures respectively. Individual DTI volumes were warped to the final template using a single interpolation operation that combined affine and deformable alignments. The registered DTI volumes were then subjected to a custom implementation of post-registration step to generate required inputs for voxel-wise tract based spatial statistics (TBSS) (Bach et al. 2014). Site and diagnosis were used as a factor and age was a covariate in the GLM models. Fractional anisotropy (FA) which is a measure of diffusion directionality was used for the primary analysis. It is an index of white matter integrity with values ranging from 0 (isotropy) to 1 (anisotropy). To further understand biological changes in the white matter, axial diffusivity (AD, indicating diffusion rate along the main axis), radial diffusivity (RD, indicating diffusion rate in the transverse direction) and mean diffusivity (MD, molecular diffusion rate) were also investigated.
Statistical analysis
Normality of demographic variables (age and education), mood (BDI-II scores) and ECAS was tested using Shapiro-Wilk test. Since these were not possible to rectify using transformation, Mann-Whitney U-tests compared demographic (age, years of education), mood (BDI-II scores), and ECAS performance between patients and controls. Data across sites were compared using Kruskal-Wallis tests, including clinical characteristics of the patients. Statistical models for imaging included site and diagnosis as factors, and age was included as covariate. Correction for multiple comparisons was done using false discovery rate (FDR) for ECAS analysis and threshold free cluster enhancement (TFCE) for imaging analyses at p < 0.05. Supplementary material also includes findings that failed multiple comparisons correction (p < 0.001, uncorrected).
Results
Demographic and clinical characteristics
Demographic and clinical characteristics of participants are outlined in Table 1. The ALS and control participants were similar for gender (χ2 = 1, p = 0.26) and age (U = 1237, p = 0.36). Median education was higher in control participants (16 vs. 15 years, U = 845, p = 0.03). Total BDI-II score was significantly higher in patients (15 vs 3, U = 1652, p < 0.01). Within healthy control participants (HC) and ALS patients, there were no significant differences across sites for age (p-HC = 0.10, p-ALS = 0.18), education (p-HC = 0.53, p-ALS = 0.45) and BDI-II (p-HC = 0.87, p-ALS = 0.52). Education and BDI-II did not show significant associations with ECAS performance and were not used as covariates in group comparisons.
Symptom duration was different between sites (χ2 [3] = 16, p < 0.01; Table 2) and post-hoc analysis revealed longer symptom duration for Sites 1 and 4 (Supplementary Fig. 1A). Similarly, ALSFRS-R scores also differed between sites (χ2 [3] = 10, p = 0.02), with post-hoc tests revealing lower ALSFRS-R score for Site 1 (Supplementary Fig. 1B). Both symptom duration and ALSFRS-R were not associated with performance on the ECAS and hence were not considered as covariates in group comparisons. There were no significant differences in the disease progression rates, respiratory capacity (FVC) and UMN burden between sites. Only the disease progression rate was associated with ECAS language score (rs = −0.3, p = 0.03) while the other clinical variables showed no significant associations with ECAS performance.
Lower ECAS performance in ALS
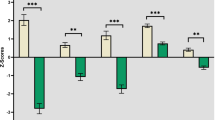
ALS patients displayed significantly lower performance on the ECAS total score (median-ALS = 109, range: 73–121) as compared to controls (median-HC = 115, range: 94–128), U = 645, p < 0.001. Specifically, ALS patients performed poorly on verbal fluency (U = 748, p < 0.01) and executive domains of the ECAS (U = 704, p < 0.01; Fig. 1). Post-hoc comparisons revealed that within verbal fluency, patients performed poorly for both letter S (U = 795, p < 0.01) and letter T fluency (U = 767, p < 0.01), and within the executive domain, patients performed poorly for reverse digit span (U = 821, p = 0.02), alternation (U = 931, p = 0.04) and sentence completion (U = 774, p < 0.01), but not social cognition (see Fig. 2). Performance of patients and controls within each site is displayed in Supplementary Fig. 3.
Twenty-two patients were classified as ALS-exi and 31 patients were classified as ALS-n. These classifications were used to explore and identify neuroanatomical differences between the sub-groups. Impairments on individual domains of the ECAS are displayed in Supplementary Table 2.
ALS patients performed lower than controls on both verbal fluency subtests (letters S and T). Decreased performance was also noted for executive domain sub-tests (digit span, alternation and sentence completion). No significant difference was noted on social cognition sub-test. **p < 0.01, *p < 0.05.
Neuroimaging findings
Mild grey matter degeneration was noted in ALS patients and cognitive sub-groups at lenient thresholds (p < 0.001) and these results did not survive multiple comparisons corrections (Supplementary Fig. 4).
Lower white matter integrity as indicated by reduced FA was noted in the upper segment of the CST and body of the corpus callosum (CC) in ALS patients when compared to healthy controls (p < 0.05, TFCE corrected; Fig. 3A). At more lenient thresholds, reduced FA was noted in the internal capsule of the CST and rostral body of the CC (p < 0.001, uncorrected; Fig. 3A). Regarding patient sub-groups, ALS-n patients displayed reduced FA in the CST and corpus callosum when compared to healthy controls (p < 0.001, uncorrected; Fig. 3B). Similar changes were noted for ALS-exi as compared to healthy controls with additional involvement of the rostral corpus callosum (p < 0.001, uncorrected; Fig. 3C). ALS-exi patients had reduced FA in the corpus callosum and the body of the cingulum compared to ALS-n patients (p < 0.001, uncorrected; Fig. 3D). There was no difference in FA within the CST of ALS-exi patients compared to ALS-n patients.
Reductions in FA were primarily accompanied by increased RD and MD (Fig. 4, Table 4). Group comparisons revealed increased RD and MD in the CST, CC and SLF in ALS patients compared to healthy controls (p < 0.05, TFCE corrected; Fig. 4A) and in the ALS-exi sub-group compared to healthy controls (p < 0.05, TFCE corrected; Fig. 4C), while no significant differences were noted in AD. At lenient thresholds, reduced AD, and increased RD and MD were found predominantly in the CST of the ALS-n sub-group when compared to controls (p < 0.001, uncorrected; Fig. 4B), while minor alterations were noted in the frontal regions. Cognitive sub-group comparisons revealed increased RD and MD in anterior regions of the CC in the ALS-exi sub-group (Fig. 4D, Table 4), while no significant differences were noted in the CST. AD was also non-significant between ALS-exi and the ALS-n groups.
Alterations in AD, RD and MD in (a) ALS patients compared to healthy controls (HC; RD and MD in ALS > HC), (b) ALS patients with normal EF compared to HC (AD in ALS-n < HC; RD and MD in ALS-n > HC), (c) ALS patients with impaired EF (ALS-exi) compared to HC (RD and MD in ALS-exi > HC) and (d) ALS-exi compared to ALS-n patients (RD and MD in ALS-exi > ALS-n). Note: Green color represents p < 0.05 (TFCE corrected) clusters, while red color represents p < 0.001 (uncorrected) clusters. Images are displayed in neurological convention (left is left)
Voxel-wise correlations revealed mild associations between decreased performance on the ECAS (verbal fluency and executive scores) and reduced grey matter density and FA (p < 0.001, uncorrected; Supplementary Fig. 5). No associations were found for other ECAS scores (language, memory and visuospatial abilities). These results did not survive multiple comparisons correction.
Tract Based Spatial Statistics (TBSS) revealed reduced fractional anisotropy (FA): (a) in all patients as compared to controls, (b-c) in ALS cognitive sub-groups compared to controls, and (d) between ALS cognitive sub-groups. Colour bars represent T-values at p < 0.001 (uncorrected). Clusters corrected for multiple comparisons using threshold free cluster enhancement (TFCE) are represented in green colour. Note: ALS-n = ALS patients with normal EF, ALS-exi = ALS patients with impaired EF. Images are displayed in neurological convention (left is left).
Discussion
The current study aimed at evaluating the neuroanatomical associations of the ECAS in ALS patients. Reduced performance was noted in ALS patients for ECAS verbal fluency and executive domains indicating executive dysfunction in the ALS group. Twenty-two (42%) patients were classified as impaired (ALS-exi) on either ECAS verbal fluency or executive domain with the remaining 31 classified as normal (ALS-n). Extensive white matter degeneration in the CST was noted in ALS patients while the ALS-exi sub-group also had prominent changes in anterior regions of the CC and SLF. Mild grey matter changes were present in ALS and mild associations were noted between ECAS verbal fluency and executive performance and neuroimaging (grey matter density and FA); however, these findings did not survive correction for multiple comparisons.
Executive dysfunction detected by ECAS
To our best knowledge, this is the first report on the clinical use of the North American adaptation of the ECAS. The observed cognitive profile in this study reflected executive dysfunction as commonly reported in ALS cohorts. Verbal fluency and executive domains were found to be impaired in ALS patients, as noted in previous studies (Lulé et al. 2015; Ye et al. 2016). Prior reports of impaired language and memory performance as detected by the ECAS were not present in the current cohort (Lulé et al. 2015; Ye et al. 2016). A small proportion of patients (n = 6, 11%) did score below 2SDs for ECAS language; among these, five were classified as ALS-exi, and one had borderline performance (1.5-2SD) in the executive domain. This proportion of patients with impaired language is lower than in previous reports, which could be due to the relatively simple language tests included in the ECAS compared to more extensive neuropsychological assessments (Taylor et al. 2013). There were no differences observed in the visuospatial domain, although post-hoc analysis indicated impairments in a very small proportion of patients (Supplementary Table 2).
Predominant white matter degeneration in ALS
Degeneration of the CST was evident by the finding of reduced FA and increased RD and MD in ALS patients. In addition to the CST, the ALS-exi group revealed reduced FA and increased RD and MD in the CC, the cingulum and the SLF indicating greater spread of the disease as previously reported (Kasper et al. 2014; Christidi et al. 2018).
No differences were noted in the CST of the ALS-exi group when compared to the ALS-n group, supporting previous findings (Kasper et al. 2014). One previous study indicated similar but less extensive changes in a smaller sub-group of cognitively impaired (n = 9) and competent (n = 5) ALS patients (Dimond et al. 2017). Another study indicated extensive cortical thinning and white matter loss in MND patients with cognitive or behavioural impairments (MND-plus) when compared to unimpaired patients (MND-motor) and heathy controls, with higher severity in temporal lobes (Agosta et al. 2016). The authors reported a higher number of MND-plus patients, which could be to the inclusion of patients with ALS as well as both UMN and LMN phenotype variants (primary lateral sclerosis [PLS] and progressive muscular atrophy [PMA] respectively). Cognitive (39% PLS, 36% PMA) and behavioural (13% PLS) impairments were identified in these phenotype variants, with neuroimaging evidence of FTLD in the MND-plus group. However, the authors did not distinguish changes within these MND-plus phenotypes. Nevertheless, our findings are in line with previous literature suggesting a greater spatial extent of degeneration in ALS-exi patients as indicated by reduced FA and increased RD and MD in extra-motor regions.
Previous studies have indicated that reduced FA in ALS correspond with increased RD and MD values (Agosta et al. 2010; Kasper et al. 2014; Christidi et al. 2018). FA, an indicator of diffusion directionality, represents the integrity of white matter but is non-specific to its underlying biological alterations. RD represents the diffusion of water perpendicular to the axonal tract and is modulated by the biological factors such as distance between axonal membranes, diameter of the axon and degree of myelination (Beaulieu 2002). Increased RD suggests free diffusion across tracts and is representative of demyelination (Song et al. 2003). MD represents the mean diffusion across x, y and z directions and increased MD could be secondary to alterations in perpendicular diffusion (RD). The current findings are consistent with loss of white matter integrity corresponding to demyelination, likely due to Wallerian degeneration of the axons (Song et al. 2003).
At lenient statistical threshold, the ALS-n sub-group also revealed mild degeneration in the genu of CC highlighting the existence of the pathology beyond motor regions. The ALS-n and ALS-exi groups did not differ on demographic or clinical variables, therefore it is unlikely that clinical factors contribute to the extra-motor changes noted in ALS-n patients. One possibility is that extra-motor changes may be present in ALS-n patients and may precede cognitive decline, similar to pre-symptomatic brain changes in ALS reported previously (Sgobio et al. 2008; Menke et al. 2016). Another possibility is that it may indicate the limitation of neuropsychometric testing in the detection of mild cognitive changes (Christidi et al. 2018). This latter hypothesis may better explain findings of extra-motor changes in ALS-n patients. It is also possible that a sub-set of ALS-n patients may have behavioural changes that were not captured in the study. Furthermore, given the subtle nature of cognitive changes in ALS, impairments may develop over time and patients may show greater cognitive or behavioural impairment at a later stage. Considering the cross-sectional nature of the study, it is beyond the scope of the current study to investigate conversion rate of cognitive impairment in ALS-n patients.
In contrast to white matter degeneration, grey matter degeneration (see supplementary material) was focal with some evidence of frontotemporal spread at lenient statistical threshold. Loss of grey matter density was primarily noted in the bilateral precentral gyrus bilateral and mPFC in ALS patients as compared to controls. ALS-exi sub-group displayed reduced grey matter density in the right precentral gyrus, inferior frontal gyrus and insula when compared to healthy controls and in the precuneus when compared to the ALS-n sub-group. These regions have been implied in previous studies suggesting evidence of mild grey matter loss in some relevant regions of cognitively impaired ALS patients (Chang et al. 2005; Bede et al. 2013; Schuster et al. 2014; Menke et al. 2014; Agosta et al. 2016; Christidi et al. 2018).
The dominance of white matter over grey matter degeneration has been reported previously in cross-sectional studies (Menke et al. 2014; Agosta et al. 2016; Christidi et al. 2018). It is likely that this pattern captures UMN pathology and disease spread via specific white matter tracts in ALS (Brettschneider et al. 2013). Longitudinally ALS patients were reported to display widespread grey matter changes in the frontotemporal regions at later time points, while longitudinal white matter degeneration was limited (Menke et al. 2014); suggesting that grey matter loss may be pronounced at a later point in the disease course.
Neuroanatomical associations
The performance on ECAS verbal fluency and executive scores was associated with focal degeneration in the grey matter and white matter at lenient statistical threshold (see Supplementary material). Lower verbal fluency was associated with reduced grey matter density in the right precentral gyrus, left premotor and left superior frontal gyri. In healthy controls, a laterality in performance during verbal fluency tasks have been reported, such that left hemisphere, especially Broca’s area, is dominant during letter fluency and the right hemisphere is dominant during automatic speech (Birn et al. 2010). In the current study, no specific lateralization was noted. However, the association with left premotor cortex and right precentral gyri suggests involvement of articulatory processes to generate the words. The involvement of the superior frontal gyrus may reflect a broader network contributing to executive dysfunction. In white matter, verbal fluency was associated with overall structural integrity (FA) of tracts such as the SLF suggesting that executive dysfunction may indeed be associated with diffuse structural integrity, such as frontotemporal circuits as suggested by Trojsi et al. (2019) and not localised connectivity. The association of reduced grey matter density in posterior region, reduced FA in posterior SLF and reduced executive performance is unexpected. It is likely that the ECAS executive domain requires sustained attention, typically associated with the parietal lobe.
Overall, the current study identified extensive white matter degeneration in ALS patients and the ALS-exi sub-group as compared to controls and is in line with previous studies (Christidi et al. 2018). These regions of degeneration align with regions of phosphorylated TAR-DNA binding protein 43 (pTDP-43) inclusions (Braak et al. 2013; Brettschneider et al. 2013), which spread along specific white matter tracts; greater pTDP-43 spread indicating progression of the disease. It is likely that impaired ECAS verbal fluency and executive performance is associated with this pathological spread in the ALS-exi sub-group (Lulé et al. 2018). However further evidence of grey matter atrophy with sufficient statistical power is required to corroborate with spread of the disease in regions such as the middle frontal gyrus, prefrontal neocortex and hippocampus.
Strengths, limitations and future directions
The ECAS is an emerging standard test for assessing cognition in ALS. To our best knowledge, this the first study providing evidence of both grey and white matter associations with ECAS performance and using data derived from a multicentre standardised protocol. Previous studies providing neuroimaging evidence have employed general cognitive screening measures such as the Addenbrooke’s Cognitive Exam – Revised (ACE-R) (Mioshi et al. 2006; Mioshi et al. 2013; Menke et al. 2014) or have employed extensive neuropsychometric testing for identifying ALS sub-groups (Sarro et al. 2011; Agosta et al. 2016; Christidi et al. 2018). The cognitive sub-groups in the current study were matched for age, education and other clinical parameters such as ALSFRS-R, FVC, symptom duration and disease progression rate, thus suggesting that neuroanatomical substrates reflect disease-related pathology.
Limitations in this study arise from a relatively small sample size, leading to an underpowered subgroup analysis. Hence, the use of multiple-comparisons corrections revealed degeneration confined to the CST. Increasing the sample size would provide statistical power to deal with such issues and strengthen sub-group analysis. This study benefited from the use of a data acquisition protocol harmonized across the 4 sites; however, it is possible that site-dependent variances could be further mitigated by the use of specific post-processing methods (Fortin et al., 2017). In our ALS participants, FVC was available for 45 patients, of which 7 were below 80%. Only one participant in this range was on BiPAP. Though we did not find correlations between ECAS and FVC, future studies should consider the possible effect of respiratory impairment on cognition (Huynh et al., 2019). Incorporating longitudinal analysis would enable investigation of both spatial and temporal neuroanatomical correlates, and their predictive potential for tracking the progression of cognitive impairment. The current sample did not include sufficient number of patients with memory impairments (n = 1) and thereby restricting the exploration of this sub-group. Incorporating behavioural assessment would enable a comprehensive evaluation of frontal-clinical associations.
Conclusions
In summary, in a standardised multicentre clinical and imaging study, the ECAS detected executive dysfunction in patients with ALS. Extensive white matter degeneration was present in the CST of ALS patients while sub-group analysis revealed degeneration of the CC, the cingulum and the SLF in those with executive impairment (ALS-exi). Grey matter changes were noted in the motor cortex and frontal regions, but these were relatively mild in comparison to the white matter abnormalities. Mild associations between structural MRI metrics, verbal fluency or executive performance suggest that reduced performance may be associated with widespread white matter integrity.
References
Abdulla, S., Machts, J., Kaufmann, J., Patrick, K., Kollewe, K., Dengler, R., Heinze, H. J., Petri, S., Vielhaber, S., & Nestor, P. J. (2014). Hippocampal degeneration in patients with amyotrophic lateral sclerosis. Neurobiology of Aging, 35(11), 2639–2645. https://doi.org/10.1016/j.neurobiolaging.2014.05.035.
Abe, K., Fujimura, H., Toyooka, K., Sakoda, S., Yorifuji, S., & Yanagihara, T. (1997). Cognitive function in amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 148(1), 95–100.
Abrahams, S., Goldstein, L. H., Al-Chalabi, A., Pickering, A., Morris, R. G., Passingham, R. E., et al. (1997). Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. Journal of Neurol Neurosurg Psychiatry, 62(5), 464–472.
Abrahams, S., Leigh, P. N., Harvey, A., Vythelingum, G. N., Grise, D., & Goldstein, L. H. (2000). Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia, 38(6), 734–747.
Abrahams, S., Newton, J., Niven, E., Foley, J., & Bak, T. H. (2014). Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener, 15(1–2), 9–14. https://doi.org/10.3109/21678421.2013.805784.
Agosta, F., Rocca, M. A., Valsasina, P., Sala, S., Caputo, D., Perini, M., Salvi, F., Prelle, A., & Filippi, M. (2009). A longitudinal diffusion tensor MRI study of the cervical cord and brain in amyotrophic lateral sclerosis patients. Journal of Neurology, Neurosurgery, and Psychiatry, 80(1), 53–55. https://doi.org/10.1136/jnnp.2008.154252.
Agosta, F., Pagani, E., Petrolini, M., Caputo, D., Perini, M., Prelle, A., Salvi, F., & Filippi, M. (2010). Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: A diffusion tensor MR imaging tractography study. AJNR. American Journal of Neuroradiology, 31(8), 1457–1461. https://doi.org/10.3174/ajnr.A2105.
Agosta, F., Ferraro, P. M., Riva, N., Spinelli, E. G., Chio, A., Canu, E., et al. (2016). Structural brain correlates of cognitive and behavioral impairment in MND. Human Brain Mapping, 37(4), 1614–1626. https://doi.org/10.1002/hbm.23124.
Bach, M., Laun, F. B., Leemans, A., Tax, C. M., Biessels, G. J., Stieltjes, B., et al. (2014). Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage, 100, 358–369. https://doi.org/10.1016/j.neuroimage.2014.06.021.
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine, 15(7–8), 435–455. https://doi.org/10.1002/nbm.782.
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck depression inventory-II. San Antonio: Psychological Corporation.
Bede, P., Bokde, A., Elamin, M., Byrne, S., McLaughlin, R. L., Jordan, N., Hampel, H., Gallagher, L., Lynch, C., Fagan, A. J., Pender, N., & Hardiman, O. (2013). Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): A neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. Journal of Neurology, Neurosurgery, and Psychiatry, 84(7), 766–773. https://doi.org/10.1136/jnnp-2012-302674.
Birn, R. M., Kenworthy, L., Case, L., Caravella, R., Jones, T. B., Bandettini, P. A., & Martin, A. (2010). Neural systems supporting lexical search guided by letter and semantic category cues: A self-paced overt response fMRI study of verbal fluency. Neuroimage, 49(1), 1099–1107. https://doi.org/10.1016/j.neuroimage.2009.07.036.
Braak, H., Brettschneider, J., Ludolph, A. C., Lee, V. M., Trojanowski, J. Q., & Del Tredici, K. (2013). Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nature Reviews. Neurology, 9(12), 708–714. https://doi.org/10.1038/nrneurol.2013.221.
Brettschneider, J., Del Tredici, K., Toledo, J. B., Robinson, J. L., Irwin, D. J., Grossman, M., et al. (2013). Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of Neurology, 74(1), 20–38. https://doi.org/10.1002/ana.23937.
Brooks, B. R., Miller, R. G., Swash, M., & Munsat, T. L. (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1(5), 293–299.
Burke, T., Lonergan, K., Pinto-Grau, M., Elamin, M., Bede, P., Madden, C., Hardiman, O., & Pender, N. (2017). Visual encoding, consolidation, and retrieval in amyotrophic lateral sclerosis: Executive function as a mediator, and predictor of performance. Amyotroph Lateral Scler Frontotemporal Degener, 18(3–4), 193–201. https://doi.org/10.1080/21678421.2016.1272615.
Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B., & Nakanishi, A. (1999). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). Journal of the Neurological Sciences, 169(1–2), 13–21.
Chang, J. L., Lomen-Hoerth, C., Murphy, J., Henry, R. G., Kramer, J. H., Miller, B. L., & Gorno-Tempini, M. L. (2005). A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology, 65(1), 75–80. https://doi.org/10.1212/01.wnl.0000167602.38643.29.
Christidi, F., Karavasilis, E., Riederer, F., Zalonis, I., Ferentinos, P., Velonakis, G., Xirou, S., Rentzos, M., Argiropoulos, G., Zouvelou, V., Zambelis, T., Athanasakos, A., Toulas, P., Vadikolias, K., Efstathopoulos, E., Kollias, S., Karandreas, N., Kelekis, N., & Evdokimidis, I. (2018). Gray matter and white matter changes in non-demented amyotrophic lateral sclerosis patients with or without cognitive impairment: A combined voxel-based morphometry and tract-based spatial statistics whole-brain analysis. Brain Imaging and Behavior, 12(2), 547–563. https://doi.org/10.1007/s11682-017-9722-y.
Dimond, D., Ishaque, A., Chenji, S., Mah, D., Chen, Z., Seres, P., Beaulieu, C., & Kalra, S. (2017). White matter structural network abnormalities underlie executive dysfunction in amyotrophic lateral sclerosis. Human Brain Mapping, 38(3), 1249–1268. https://doi.org/10.1002/hbm.23452.
Goldstein, L. H., & Abrahams, S. (2013). Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurology, 12(4), 368–380. https://doi.org/10.1016/S1474-4422(13)70026-7.
Kasper, E., Schuster, C., Machts, J., Kaufmann, J., Bittner, D., Vielhaber, S., Benecke, R., Teipel, S., & Prudlo, J. (2014). Microstructural white matter changes underlying cognitive and behavioural impairment in ALS--an in vivo study using DTI. PLoS One, 9(12), e114543. https://doi.org/10.1371/journal.pone.0114543.
Kaufmann, P., Pullman, S. L., Shungu, D. C., Chan, S., Hays, A. P., Del Bene, M. L., et al. (2004). Objective tests for upper motor neuron involvement in amyotrophic lateral sclerosis (ALS). Neurology, 62(10), 1753–1757.
Keller, J., Bohm, S., Aho-Ozhan, H. E. A., Loose, M., Gorges, M., Kassubek, J., et al. (2017). Functional reorganization during cognitive function tasks in patients with amyotrophic lateral sclerosis. Brain Imaging and Behavior, 12, 771–784. https://doi.org/10.1007/s11682-017-9738-3.
Kew, J. J., Goldstein, L. H., Leigh, P. N., Abrahams, S., Cosgrave, N., Passingham, R. E., et al. (1993). The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain, 116(Pt 6), 1399–1423.
Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., Hardiman, O., Burrell, J. R., & Zoing, M. C. (2011). Amyotrophic lateral sclerosis. Lancet, 377(9769), 942–955. https://doi.org/10.1016/S0140-6736(10)61156-7.
Kimura, F., Fujimura, C., Ishida, S., Nakajima, H., Furutama, D., Uehara, H., Shinoda, K., Sugino, M., & Hanafusa, T. (2006). Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology, 66(2), 265–267. https://doi.org/10.1212/01.wnl.0000194316.91908.8a.
Leemans, A, Jeurissen, B, Sijbers, J, and Jones, DK (2009). ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Paper presented at the 17th annual meeting of Intl Soc mag Reson med, Hawaii, USA,
Loose, M, Burkhardt, C, Aho-Ozhan, H, Keller, J, Abdulla, S, Bohm, S, et al. (2016). Age and education-matched cut-off scores for the revised German/Swiss-German version of ECAS. Amyotroph Lateral Scler Frontotemporal Degener, 1-3, doi:https://doi.org/10.3109/21678421.2016.1162814.
Lulé, D., Burkhardt, C., Abdulla, S., Bohm, S., Kollewe, K., Uttner, I., et al. (2015). The Edinburgh cognitive and Behavioural amyotrophic lateral sclerosis screen: A cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Frontotemporal Degener, 16(1–2), 16–23. https://doi.org/10.3109/21678421.2014.959451.
Lulé, D., Bohm, S., Muller, H. P., Aho-Ozhan, H., Keller, J., Gorges, M., et al. (2018). Cognitive phenotypes of sequential staging in amyotrophic lateral sclerosis. Cortex, 101, 163–171. https://doi.org/10.1016/j.cortex.2018.01.004.
Machts, J., Bittner, V., Kasper, E., Schuster, C., Prudlo, J., Abdulla, S., Kollewe, K., Petri, S., Dengler, R., Heinze, H. J., Vielhaber, S., Schoenfeld, M. A., & Bittner, D. M. (2014). Memory deficits in amyotrophic lateral sclerosis are not exclusively caused by executive dysfunction: A comparative neuropsychological study of amnestic mild cognitive impairment. BMC Neuroscience, 15, 83.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239.
Menke, R. A., Korner, S., Filippini, N., Douaud, G., Knight, S., Talbot, K., et al. (2014). Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain, 137(Pt 9), 2546–2555.
Menke, R. A., Proudfoot, M., Wuu, J., Andersen, P. M., Talbot, K., Benatar, M., et al. (2016). Increased functional connectivity common to symptomatic amyotrophic lateral sclerosis and those at genetic risk. Journal of Neurology, Neurosurgery, and Psychiatry, 87(6), 580–588. https://doi.org/10.1136/jnnp-2015-311945.
Mioshi, E., Dawson, K., Mitchell, J., Arnold, R., & Hodges, J. R. (2006). The Addenbrooke's cognitive examination revised (ACE-R): A brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry, 21(11), 1078–1085. https://doi.org/10.1002/gps.1610.
Mioshi, E., Lillo, P., Yew, B., Hsieh, S., Savage, S., Hodges, J. R., Kiernan, M. C., & Hornberger, M. (2013). Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology, 80(12), 1117–1123.
Mitsumoto, H., Ulug, A. M., Pullman, S. L., Gooch, C. L., Chan, S., Tang, M. X., et al. (2007). Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology, 68(17), 1402–1410. https://doi.org/10.1212/01.wnl.0000260065.57832.87.
Müller, H. P., Turner, M. R., Grosskreutz, J., Abrahams, S., Bede, P., Govind, V., Prudlo, J., Ludolph, A. C., Filippi, M., & Kassubek, J. (2016). A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 87(6), 570–579. https://doi.org/10.1136/jnnp-2015-311952.
Niven, E., Newton, J., Foley, J., Colville, S., Swingler, R., Chandran, S., Bak, T. H., & Abrahams, S. (2015). Validation of the Edinburgh cognitive and Behavioural amyotrophic lateral sclerosis screen (ECAS): A cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener, 16(3–4), 172–179. https://doi.org/10.3109/21678421.2015.1030430.
Phukan, J., Elamin, M., Bede, P., Jordan, N., Gallagher, L., Byrne, S., Lynch, C., Pender, N., & Hardiman, O. (2012). The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. Journal of Neurology & Psychiatry, 83(1), 102–108.
Pinto-Grau, M., Burke, T., Lonergan, K., McHugh, C., Mays, I., Madden, C., Vajda, A., Heverin, M., Elamin, M., Hardiman, O., & Pender, N. (2017). Screening for cognitive dysfunction in ALS: Validation of the Edinburgh cognitive and Behavioural ALS screen (ECAS) using age and education adjusted normative data. Amyotroph Lateral Scler Frontotemporal Degener, 18(1–2), 99–106. https://doi.org/10.1080/21678421.2016.1249887.
Poletti, B., Solca, F., Carelli, L., Madotto, F., Lafronza, A., Faini, A., Monti, A., Zago, S., Calini, D., Tiloca, C., Doretti, A., Verde, F., Ratti, A., Ticozzi, N., Abrahams, S., & Silani, V. (2016). The validation of the Italian Edinburgh cognitive and Behavioural ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener, 17(7–8), 489–498. https://doi.org/10.1080/21678421.2016.1183679.
Sarro, L., Agosta, F., Canu, E., Riva, N., Prelle, A., Copetti, M., Riccitelli, G., Comi, G., & Filippi, M. (2011). Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: A diffusion tensor tractography study. Ajnr: American Journal of Neuroradiology, 32(10), 1866–1872.
Schuster, C., Kasper, E., Dyrba, M., Machts, J., Bittner, D., Kaufmann, J., Mitchell, A. J., Benecke, R., Teipel, S., Vielhaber, S., & Prudlo, J. (2014). Cortical thinning and its relation to cognition in amyotrophic lateral sclerosis. Neurobiology of Aging, 35(1), 240–246. https://doi.org/10.1016/j.neurobiolaging.2013.07.020.
Senda, J., Ito, M., Watanabe, H., Atsuta, N., Kawai, Y., Katsuno, M., Tanaka, F., Naganawa, S., Fukatsu, H., & Sobue, G. (2009). Correlation between pyramidal tract degeneration and widespread white matter involvement in amyotrophic lateral sclerosis: A study with tractography and diffusion-tensor imaging. Amyotrophic Lateral Sclerosis, 10(5–6), 288–294. https://doi.org/10.3109/17482960802651717.
Sgobio, C., Trabalza, A., Spalloni, A., Zona, C., Carunchio, I., Longone, P., & Ammassari-Teule, M. (2008). Abnormal medial prefrontal cortex connectivity and defective fear extinction in the presymptomatic G93A SOD1 mouse model of ALS. Genes, Brain, & Behavior, 7(4), 427–434.
Siciliano, M., Trojano, L., Trojsi, F., Greco, R., Santoro, M., Basile, G., Piscopo, F., D’Iorio, A., Patrone, M., Femiano, C., Monsurrò, M., Tedeschi, G., & Santangelo, G. (2017). Edinburgh cognitive and Behavioural ALS screen (ECAS)-Italian version: Regression based norms and equivalent scores. Neurological Sciences, 38(6), 1059–1068. https://doi.org/10.1007/s10072-017-2919-4.
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 20(3), 1714–1722.
Strong, M. J., Abrahams, S., Goldstein, L. H., Woolley, S., McLaughlin, P., Snowden, J., et al. (2017). Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener, 18(3–4), 153–174. https://doi.org/10.1080/21678421.2016.1267768.
Taylor, L. J., Brown, R. G., Tsermentseli, S., AlChalabi, A., Shaw, C. E., Ellis, C. M., et al. (2013). Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? Journal of Neurology & Psychiatry, 84(5), 494–498.
Trojsi, F., Caiazzo, G., Siciliano, M., Femiano, C., Passaniti, C., Russo, A., Bisecco, A., Monsurrò, M. R., Cirillo, M., Esposito, F., Tedeschi, G., & Santangelo, G. (2019). Microstructural correlates of Edinburgh cognitive and Behavioural ALS screen (ECAS) changes in amyotrophic lateral sclerosis. Psychiatry Research: Neuroimaging, 288, 67–75. https://doi.org/10.1016/j.pscychresns.2019.04.001.
Turner, M. R., Grosskreutz, J., Kassubek, J., Abrahams, S., Agosta, F., Benatar, M., Filippi, M., Goldstein, L. H., van den Heuvel, M., Kalra, S., Lulé, D., Mohammadi, B., & first Neuroimaging Symosium in ALS (NISALS). (2011). Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurology, 10(5), 400–403. https://doi.org/10.1016/S1474-4422(11)70049-7.
Witgert, M., Salamone, A. R., Strutt, A. M., Jawaid, A., Massman, P. J., Bradshaw, M., Mosnik, D., Appel, S. H., & Schulz, P. E. (2010). Frontal-lobe mediated behavioral dysfunction in amyotrophic lateral sclerosis. European Journal of Neurology, 17(1), 103–110.
Ye, S., Ji, Y., Li, C., He, J., Liu, X., & Fan, D. (2016). The Edinburgh cognitive and Behavioural ALS screen in a Chinese amyotrophic lateral sclerosis population. PLoS One, 11(5), e0155496. https://doi.org/10.1371/journal.pone.0155496.
Zhang, H., Avants, B. B., Yushkevich, P. A., Woo, J. H., Wang, S., McCluskey, L. F., et al. (2007a). High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Transactions on Medical Imaging, 26(11), 1585–1597. https://doi.org/10.1109/TMI.2007.906784.
Zhang, H., Yushkevich, P. A., Rueckert, D., & Gee, J. C. (2007b). Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv, 10(Pt 2), 211–218.
Zhang, Y., Schuff, N., Woolley, S. C., Chiang, G. C., Boreta, L., Laxamana, J., Katz, J. S., & Weiner, M. W. (2011). Progression of white matter degeneration in amyotrophic lateral sclerosis: A diffusion tensor imaging study. Amyotrophic Lateral Sclerosis, 12(6), 421–429. https://doi.org/10.3109/17482968.2011.593036.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
SK received funding from the Canadian Institutes of Health Research (CIHR) Operating grant (CIHR MOP 123534).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the local human research ethics board at each participating site for CALSNIC.
Informed consent
Written informed consent was obtained from all participants in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1125 kb)
Rights and permissions
About this article
Cite this article
Chenji, S., Ishaque, A., Mah, D. et al. Neuroanatomical associations of the Edinburgh cognitive and Behavioural ALS screen (ECAS). Brain Imaging and Behavior 15, 1641–1654 (2021). https://doi.org/10.1007/s11682-020-00359-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-020-00359-7