Abstract
Cognitive deficits, especially in the domains of social cognition and executive function including verbal fluency, are common in amyotrophic lateral sclerosis (ALS) patients. There is yet sparse understanding of pathogenesis of the underlying, possibly adaptive, cortical patterns. To address this issue, 65 patients with ALS and 33 age-, gender- and education-matched healthy controls were tested on cognitive and behavioral deficits with the Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Using functional magnetic resonance imaging (fMRI), cortical activity during social cognition and executive function tasks (theory of mind, verbal fluency, alternation) adapted from the ECAS was determined in a 3 Tesla scanner. Compared to healthy controls, ALS patients performed worse in the ECAS overall (p < 0.001) and in all of its subdomains (p < 0.02), except memory. Imaging revealed altered cortical activation during all tasks, with patients consistently showing a hyperactivation in relevant brain areas compared to healthy controls. Additionally, cognitively high performing ALS patients consistently exhibited more activation in frontal brain areas than low performing patients and behaviorally unimpaired patients presented with more neuronal activity in orbitofrontal areas than behaviorally impaired patients. In conclusion, hyperactivation in fMRI cognitive tasks seems to represent an early adaptive process to overcome neuronal cell loss in relevant brain areas. The hereby presented cortical pattern change might suggest that, once this loss passes a critical threshold and no cortical buffering is possible, clinical representation of cognitive and behavioral impairment evolves. Future studies might shed light on the pattern of cortical pattern change in the course of ALS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder characterized by progressive loss of upper and lower motor neurons (Kiernan et al. 2011). It evolves in distinct pathophysiological cortical patterns (Brettschneider et al. 2013) and shares genetic, clinical and pathological abnormalities with frontotemporal dementia (Neumann et al. 2006; Kiernan et al. 2011; Al-Chalabi et al. 2012). Cognitive deficits also play a major role in the course of the disease and are prevalent in around 30–50% of ALS patients (Beeldman et al. 2016; Böhm et al. 2016). They have been extensively studied and occur most commonly in the domains of language, social cognition and executive function including verbal fluency (Ringholz et al. 2005; Phukan et al. 2012; Goldstein and Abrahams 2013; van der Hulst et al. 2015).
The neural substrates underlying these changes have already been investigated using different neuroimaging techniques (Tsermentseli et al. 2012; Ayaz et al. 2014), yet, studies using functional magnetic resonance imaging (fMRI) to determine neuronal substrates of cognitive impairments in ALS are scarce (see Chio et al. 2014 for a review) and provide inconclusive results. One recent study reported decreased activity in frontal, parietal and temporal areas of ALS patients when compared to healthy controls during tasks of executive function (Jelsone-Swain et al. 2015), whereas others observed a decrease as well as an increase in neural activation in frontal and temporal areas during cognitive tasks (Abrahams et al. 2004; Goldstein et al. 2011; Witiuk et al. 2014).
As previous studies only used domain specific tasks in rather small patient cohorts, we decided to use three well-validated tasks of social cognition and executive function from an ALS-specific screening tool, i.e. the Theory of Mind (ToM) task, the verbal fluency task and the alternation task, and apply them to one comparably large sample of ALS patients. The term Theory of Mind describes someone’s ability to attribute and make sense of others mental states (Baron-Cohen 1995). Earlier studies have found ALS patients to be impaired in this task (Gibbons et al. 2007; van der Hulst et al. 2015) making it a valuable test in the detection of deficits in social cognition among these patients. Verbal fluency is one of the most prominent cognitive functions in which impairments have been reported for ALS patients (Phukan et al. 2007). Abrahams et al. (2004) already showed that despite their deficits in this task, the underlying neuronal activation is not only characterized by a decrease in bilateral prefrontal, left temporal and left parietal areas but also by an increase of neuronal activation in the right middle temporal gyrus, left superior and right inferior frontal gyrus. Also, the alternation task, a verbal adaption of the Trail Making Test-B (Bowie and Harvey 2006) and thus a reliable measure of cognitive flexibility and executive functioning, was chosen. Specifically, all three tasks were amended to not require a verbal or motor response as speech and/or hand movements may have a confounding impact on the fMRI signal. For further reliability, they were directly adapted from the Edinburgh Behavioural and Cognitive ALS Screen (ECAS), a standard and widely used screening tool for the assessment of cognitive deficits among ALS patients (Abrahams et al. 2014).
The aim of this study was to test the hypothesis that ALS patients demonstrate changed cortical patterns during tasks of social cognition and executive function. This indicates a process of early adaptation to potential neuronal loss which might compensate cognitive deficits to a certain degree. Only after this threshold is exceeded, cognitive and/or behavioral impairments manifest clinically. This hypothesis is not only supported by previous findings from fMRI studies using cognitive tasks (Abrahams et al. 2004; Goldstein et al. 2011; Witiuk et al. 2014; Jelsone-Swain et al. 2015) but also by several studies investigating the neural underpinnings of motor-related tasks in patients with ALS, which also implicate functional neuronal reorganization and compensation in the presence of loss of motor function (Konrad et al. 2002; Schoenfeld et al. 2005; Lulé et al. 2007; Poujois et al. 2013).
Materials and methods
Participants
In total, 65 patients diagnosed with ALS by a board-certified neurologist according to the revised El-Escorial criteria issued by the World Federation of Neurology research group on ALS/MND (Ludolph et al. 2015) were recruited from the in- and outpatient clinic of the Department of Neurology, University of Ulm, Germany. Additionally, 33 age-, gender- and education-matched healthy controls (HC) participated in the study (Table 1). The revised ALS Functional Rating Scale (ALS-FRS) (Cedarbaum et al. 1999) was used to get a measure of patients’ physical impairments, ranging from mild to moderate. For further neuropsychological characterization the modified diagnostic criteria from Strong et al. (2017) was used. None had severe problems breathing when lying supine in the scanner. Also, no participant showed any clinical signs of a psychiatric or neurological condition (other than ALS), fulfilled the clinical criteria for accompanying frontotemporal dementia (ALS-FTD) according to Rascovsky et al. 2011 or had impaired vision which might have altered task performance. For all subjects, vascular brain alterations as well as inflammatory or neoplastic brain processes were excluded via conventional MRI. The study was approved by the Ethics Committee of the University of Ulm (Statement No. 19/12) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave written informed consent to the study.
Experimental design and stimuli
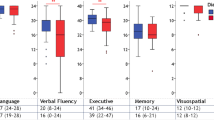
All participants completed the German version of the ECAS according to standard procedures (Lulé et al. 2015; Loose et al. 2016) to detect possible cognitive and behavioral impairments. Low performers were defined on the basis of their scores in the ECAS overall as well as in any of its five subdomains (memory, visuospatial, language, verbal fluency, executive function), as being more than two standard deviations below the mean of the control sample (Abrahams et al. 2014; Lulé et al. 2015). Unfortunately, not all ALS patients’ caregivers could be reached, therefore only caregivers of N = 41 patients filled out the caregiver questionnaire of the ECAS with regard to patients’ behavioral abnormalities. Participants underwent MRI scanning within three days after ECAS screening. Inside the scanner the theory of mind task was completed first, followed by the verbal fluency task and the alternation task, all three designed as block tasks. To ensure comparability, the tasks were directly adapted from the paper-and-pencil ECAS and adjusted to the MRI setting, even though this might create potential repetition effects. Yet, we hypothesize that these effects seem to be negligible, as the nature of the selected cognitive function tasks should reliably identify subjects with impairments in every trial. As the three tasks were adapted to not require any response during scanning, participants were firmly instructed to perform the tasks, which they all knew from the previous ECAS screening, mentally. All tasks were presented via video goggles (VisuaStim Digital, Resonance Technology Inc., Northridge, CA, USA) using Presentation® software (www.neurobs.com).
Theory of mind task
A digitized version of the judgment of preference task, as implemented in the ECAS, was used. It consisted of two conditions of six stimuli each. In the first, condition, each stimulus showed four different objects of which the participant was instructed to mentally, i.e. without talking or pointing, choose the one he/she liked best. This served as the control condition. The second, i.e. the actual ToM condition, consisted of the same six stimuli featuring an additional smiling face in the middle of the four objects with its gaze directed at one of them. Here, the subjects were instructed to internally, i.e. without talking or pointing, decide which object the face in the middle likes best. Each of the twelve stimuli was shown once for 8.1 s. The sequence of stimuli was the same for all participants and alternated between stimuli from each condition. Before each stimulus, an instruction (either: “Please choose the image you like best” or: “Please choose the image the face likes best”) was shown for 8.1 s (Fig. 1).
Verbal fluency task
Participants were required to either name as many animals as possible, or as many words with the initial letter “s” as possible. As before, the task had to be performed mentally, i.e. without talking or pointing. Each condition was shown three times in alternating order and consisted of an instruction that was displayed for 8.1 s, reading either: “Please name as many animals as possible” or: “Please name as many words with the initial “s” as possible”, followed by a black fixation cross in the middle of a white screen for 24.3 s during which the instructed task was executed (Fig. 1). For imaging analysis, brain activity of both conditions (animals and “s”-words) was pooled, as these two task conditions are too similar to allow for sufficient contrasting on a single subject level. Therefore, the neuronal activity measured during this task can be considered to represent both, semantic and phonetic verbal fluency.
Alternation task
In this task subjects had to either mentally count (starting from “one” to whichever number possible within the given time-frame), which is considered a very suitable minimum-cognitive demand control condition in fMRI experiments (Stark and Squire 2001), or to alternate between numbers and the letters of the alphabet in ascending order. Again, participants were told not to speak and make as little movements during the task as possible. Analogous to the verbal fluency task, they were presented the instruction for 8.1 s first, followed by a black fixation cross in the middle of a white screen for 24.3 s. Overall, subjects underwent both conditions three times in alternating order (Fig. 1).
MRI data acquisition
Images were acquired on a 3 Tesla head scanner (Allegra, Siemens Medical, Erlangen, Germany). Functional Images were collected using a gradient echo planar imaging sequence (EPI; TR = 1620 ms, TE = 35 ms, flip angle = 90°, interleaved slices = 25, slice thickness = 3 mm, voxel size = 3 mm3, axial slice orientation oriented along the anterior-posterior commissure, field of view = 192x192x93 mm) for blood-oxygen-level dependent (BOLD) imaging (Ogawa et al. 1990). To optimize data acquisition of sub-cortical areas image slice orientation was tilted by 30° (Deichmann and Turner 2002).
MRI data analysis
Data were analyzed using SPM12 (Functional Imaging Laboratory, Institute of Neurology, UCL, London, UK; Friston et al. 1995) running on MATLAB (Release 2011b, The MathWorks, Inc., Natick, Massachusetts, United States). The first five volumes were discarded from analysis to allow for steady field magnetization. Preprocessing encompassed slice time correction, motion correction by three-dimensional spatial realignment of the scans to the first scan of each run, normalization into standard stereotactic space (Montreal Neurological Institute, MNI) using a segmentation-based approach (Ashburner and Friston 2005) and smoothing with an 8 mm full width at half maximum isotropic Gaussian kernel. A mass-univariate approach based on General Linear Models was used. For the statistical model of cognitive tasks, a matrix including ToM, verbal fluency and alternation conditions was estimated using a hemodynamic response function to accommodate the lag time of the BOLD response including realignment parameters obtained from preprocessing as nuisance covariates to further control for potential movement artifacts (Johnstone et al. 2006). Contrasts on the subject-level were computed using a random effects analysis of variance by subtracting activation of the respective control condition from the ToM and alternation condition to create individual t-maps. Then, individual contrast images were subjected to between-group analyses and additional regression analyses were conducted to identify effects of behavioral performance scores on BOLD-activation. Inclusion masks of previously identified brain regions of interest (ROI; Table 2) for all three tasks were created using the WFU PickAtlas software tool (Version 3.0.5; Maldjian et al. 2003) to allow for more precise second-level analyses by reducing the severity of correction for multiple tests, i.e. restricting the analyses to brain areas previously implicated in these tasks (Poldrack 2007). A family-wise error threshold of p < 0.05 was chosen to identify significantly activated clusters of four or more voxels within these ROIs.
For each task, post-hoc analysis was performed to identify subjects with intolerable head movement during scanning and to identify those who failed to perform any of the tasks properly in the fMRI paradigm, as estimated by no activity in the respective contrast image at a threshold of puncorrected < 0.001. Either of this was the case for 12 ALS patients and one HC in the ToM task, 12 patients and 3 HC in the verbal fluency task as well as 5 patients in the alternation task. All of these participants were excluded from all imaging analyses of the respective task.
Statistical analysis
All analyses of demographic and ECAS data were performed using SPSS (IBM Corp. Version 21.0. IBM SPSS Statistics for Windows, Armonk, NY). A priori, data were checked for outliers using Grubbs’ test and analyzed for normal distribution using the Kolmogorov-Smirnov test. Accordingly, Mann-Whitney U tests or Pearson’s chi-squared tests were used where appropriate to detect group differences in performance accuracy between patients and HC. All analyses were two-sided and the significance level was set at p < 0.05.
Results
General cognitive and behavioral screening
Patients performed significantly worse in the ECAS overall (p < 0.001) and in the cognitive domains of language (p < 0.001), verbal fluency (p < 0.001), executive function (p < 0.001) and visuospatial perception (p = 0.015), but not in the memory domain (p = 0.201). With regards to specific cognitive functions used in the fMRI paradigm, ALS patients performed worse in the alternation task (p < 0.001) and the non-restricted verbal fluency task (verbal fluency “s”-words; p = 0.048) but not in the ToM task (p = 0.309) of the ECAS. For detailed results see Table 1.
There were no differences in cognitive performance between patients with familial compared to those with sporadic ALS and between those with bulbar and spinal disease onset.
Seven out of the 41 patients (≙ 17.1%) with behavioral information provided by their caregivers showed behavioral alterations but none fulfilled the criteria for ALS-FTD according to Rascovsky et al. (2011). Importantly, there were no statistically significant differences in any demographic variable or cognitive domain, as assessed by the ECAS, between behaviorally impaired and non-impaired patients apart from the memory subscore (p = 0.014) and family history of ALS (p = 0.018), with two out of three familial ALS cases exhibiting behavioral impairments.
fMRI analyses: Theory of mind task
Group comparison patients vs. HC: When contrasting brain activity as measured with BOLD during the ToM condition, the patient sample (n = 53) showed significantly higher activation than HC (n = 32) in the right posterior superior temporal sulcus (Table 3; Fig. 2a). No brain area was identified in which the control sample showed more activation than the patients.
Patient comparison performance: Brain activity of low performing ALS patients (n = 9) in the ToM task was significantly reduced in a cluster within the right precentral gyrus extending to the inferior frontal gyrus as well as in the left middle occipital gyrus, whereas they showed more activity in the right postcentral gyrus when compared to those who performed well (n = 44) (Table 3; Fig. 3).
Patient comparison behavioral impairment: Brain activity of five patients with behavioral impairments was reduced in the right rectal gyrus compared to the 32 behaviorally non-impaired ALS patients, whereas no area of increased activity could be identified (Table 3; Fig. 4a).
Theory of Mind task regression analyses: When performing a regression analysis of the Theory of Mind score on the corresponding activation in our patient sample (n = 53), a higher ToM score was associated with more fMRI activity in a cluster within the right precentral gyrus extending to the inferior frontal gyrus (Table 3).
fMRI Analyses: Verbal fluency task
Group comparison patients vs. HC
ALS patients (n = 53) showed more cortical activation than HC (n = 30) when performing the verbal fluency tasks in right superior frontal gyrus, right inferior parietal lobule, right thalamus, right pars triangularis and left caudate nucleus (Table 3; Fig. 2b). There were no areas in which HC showed more neuronal activation than ALS patients in this task.
Patient comparison performance
For the verbal fluency conditions this contrast revealed a hypoactivation in the left inferior frontal gyrus for the low performing patients (n = 3) when contrasted with high performing patients (n = 50), whereas they exhibited no area of increased activation (Table 3).
Patient comparison behavioral impairment
There were no statistically significant differences in neuronal activation during this task between behaviorally non-impaired and impaired ALS patients.
Verbal fluency task regression analysis
In our sample of ALS patients (n = 53), a higher score in the “s”-word condition of the verbal fluency task was associated with more activity bilaterally in various clusters of the frontal lobe, bilateral central sulcus, right postcentral gyrus extending to the inferior parietal lobule and bilateral insula (Table 3).
fMRI analyses: Alternation task
Group comparison patients vs. HC
The patient sample (n = 60) showed a higher neuronal activation in the right paracentral lobule when performing the alternation task as contrasted to simple counting (Table 3; Fig. 2c) and, yet again, HC (n = 33) failed to show more neuronal activation than ALS patients in any brain area.
Patient comparison performance
There were no statistically significant differences in neuronal activation of the alternation task between high (n = 46) and low performing ALS patients (n = 12).
Patient comparison behavioral impairment
Those ALS patients with behavioral impairments (n = 7) showed less brain activity in the right superior orbital gyrus (Table 3; Fig. 4B) than those without (n = 31).
Alternation task regression analyses
A regression analysis of patients’ (n = 60) scores in the alternation task on their corresponding neuronal activation identified an association of activity in the left calcarine and the left lingual gyrus with better performance (Table 3).
Discussion
The presence of cognitive impairment, especially in the domains of social cognition and executive function including verbal fluency, in ALS patients is a well described fact (Ringholz et al. 2005; Phukan et al. 2012; Goldstein and Abrahams 2013; Beeldman et al. 2016; Böhm et al. 2016). However, the neuronal processes underlying these impairments are still far from being well understood. The aim of this work therefore was to study these cognitive functions in a large sample of ALS patients, using three fMRI-adapted social cognition and executive function tasks.
Theory of mind
As in other recent studies which employed the ECAS, ALS patients performed significantly worse than controls in the overall score and in the domains of executive functioning, language and verbal fluency (Abrahams et al. 2014; Lulé et al. 2015). Additionally, there was a significant group difference in visuospatial performance between patients and HC (Keller et al. 2015; Poletti et al. 2016); however, none of the participants showed any limitation in his/her visuospatial abilities severe enough to be a potentially disturbing factor in execution of the fMRI tasks. Also, patients displayed a significantly lower alternation score, but did not perform significantly worse in the ToM task of the ECAS than HC. This is in contrast to some previous findings (Girardi et al. 2011; Cerami et al. 2014; van der Hulst et al. 2015) but in accordance with other studies, which also did not report significant group differences in ToM between ALS patients and HC (Jelsone-Swain et al. 2015; Cavallo et al. 2011). This might be due to the fact that only a small number of patients performed extremely poor in this task, apparently not grasping the concept behind it, while the rest made almost no mistakes. A recent study has come to similar conclusions with ALS patients without overt executive impairment performing not significantly different than HC in an identical task (Burke et al. 2016). Also, the ToM task used here is a measure of affective theory of mind aspects only (the belief about sb. else’s feelings) and thus does not account for potential deficits of other theory of mind tasks such as cognitive ToM (the belief about sb. else’s beliefs).
When performing the ToM task during scanning, our patient sample showed significantly more activation in the right posterior superior temporal sulcus, a crucial area for neural processing in theory of mind tasks (Saxe and Kanwisher 2003; Zaitchik et al. 2010; Abu-Akel and Shamay-Tsoory 2011). As there was no brain region in which HC showed more activation than ALS patients and considering the fact that there was no group difference in behavioral ToM performance, a compensatory over-recruitment of this area, which might counteract potential deficits in this task, seems to be at core here. This is in accordance with findings of compensatory activation during motor-tasks (Schoenfeld et al. 2005; Konrad et al. 2002) and tasks of executive function (Witiuk et al. 2014) in ALS patients. Also, hyperactivation in an area of the right precentral gyrus bordering the inferior frontal gyrus was associated with better performance in the ToM task.
To get further insights into the neural processing of ToM, we divided our ALS patients in two groups according to their performance in this task in the ECAS. In patients who performed well in this task, an increased neuronal activation within two brain areas – the right precentral/inferiofrontal gyrus and the left middle occipital gyrus – was detected when compared to low performing patients. The former has previously been implicated in this task (Uddin et al. 2007; Zaitchik et al. 2010), in further support of compensatory mechanisms in relevant brain regions depending on the degree of patients’ cognitive impairment. Even though low performers presented with cognitive impairment on the behavioral level, indicating that neuronal cell loss has exceeded a critical threshold up to which sufficient compensation is not possible anymore, there was one area in the right postcentral gyrus in which they showed an increased activation during the execution of the ToM task. This implicates that, although compensatory mechanisms might be at work during the execution of the ToM task in all ALS patients independent of their degree of cognitive impairment, there are neuronal differences between high and low performers. Interestingly, this pattern of differential activation is in congruence with a proposed mechanism of cortical disease spreading in ALS, which postulates a sequential four stage spreading pattern (Braak et al. 2013; Brettschneider et al. 2013). This model postulates the dissemination of the disease from primary motor areas spreading to more anterior portions of the brain and has already been replicated in a diffusion tensor imaging (DTI) study (Kassubek et al. 2014). It might therefore be that the neuronal activation underlying cognitive impairments in ALS patients is also affected in a corresponding fashion, i.e. cognitively less impaired patients maintain higher neuronal activation in more frontal areas, while these areas are stronger affected by the disease in cognitively more impaired patients, requiring them to compensate this potential loss by a hyperactivation in more parietal areas.
Verbal fluency
During the execution of the verbal fluency task, ALS patients presented with increased neuronal activation in right superior and inferior frontal areas, right thalamus, right inferior parietal lobule and left caudate, while again failing to exhibit any area of less activation than our sample of HC. As these areas have already been found to play a role during the execution of verbal fluency tasks in samples of ALS patients and HC (Abrahams et al. 2004; Wagner et al. 2014), this finding provides further credibility to our claim of widespread compensatory mechanisms during cognitive function tasks. Again, high performing ALS patients showed more activation in a cluster within the inferior frontal gyrus than low performers and there was a wide network of many distinct brain areas, especially in bilateral frontal regions, which correlated positively with patients’ behavioral performance in the ECAS version of this task. However, these results should be treated with caution as we have pooled participants’ neuronal activity of both verbal fluency tasks (“s-“-words and animals) and therefore have to be aware of the lack of a control condition for this task.
Alternation
In the alternation task our ALS patient sample showed greater activation in the right paracentral lobule when compared to HC. Yet, similarly as in the other two tasks, our healthy control sample did not exhibit any area of increased activation. Even though the right paracentral lobule has previously been described as part of a network activated when performing similar alternation tasks (Allen et al. 2011; Zakzanis et al. 2005), patients performed significantly worse than HC in the behavioral alternation task. This suggests that there is, as in the other tasks, neuronal reorganization and compensation, however, this activation is not sufficient in compensating our ALS patient samples’ cognitive deficits. As before, there was a significant correlation between activation and behavioral task performance in ALS patients. A better behavioral performance was associated with more activation in the left lingual and calcarine gyrus.
Behavioral impairments
Analyses of behaviorally impaired and behaviorally non-impaired ALS patients revealed a higher BOLD activity in the right orbital/rectal gyrus in patients without behavioral impairment. However, this activity was present in the ToM and the alternation task only and not in the verbal fluency task. These frontoorbital areas are associated with higher-level executive functioning and decision-related processes, relevant for socially adequate behavior (Andreasen et al. 1995; Wilson et al. 2014). This enables us to differentiate between behaviorally impaired and non-impaired ALS patients based on their neuronal activation during executive function tasks and supports previous findings about a relationship between orbitofrontal cortical atrophy and behavioral impairment in ALS (Tsujimoto et al. 2011). Interestingly, this task-specific orbitofrontal hypoactivation could also be reflective of more general difficulties in integrating available information about certain situations (Wilson et al. 2014), which might then express itself in behavioral abnormalities.
Limitations
Our study has several limitations. Due to breathing difficulties, which are a prominent feature in far advanced patients and an exclusion criterion for fMRI experiments, we were only able to include a patient sample with no patients in an advanced stage of the disease, so no data for final stages of ALS are available. However, previous studies have shown that cognitive and physical impairment are not necessarily associated (Jelsone-Swain et al. 2012; Keller et al. 2015). We did not record participants’ responses of the tasks while scanning, as it was not possible for some patients to give a response due to loss of speech and/or paralysis. This, of course, comes with the danger of potentially disturbing effects of some patients’ improper task performance, which we used to contain as good as possible though, by applying a post-hoc analysis of all fMRI data. Yet, by having subjects complete the tasks only mentally, we managed to exclude potentially confounding fMRI signals originating from movement. Of course this is at the cost of eventual difficulties in comparison of results to previous studies that did incorporate such a response (Chio et al. 2014). Also, we were not able to control for potential repetition effects, resulting from the similarity of the tasks in- and outside the scanner. For statistical analysis of our imaging data, we used small volume correction, i.e. we were only looking into pre-specified areas of the brain, potentially overlooking other areas (Poldrack 2007). The small sample size of our behaviorally impaired patient group might be considered as an additional shortcoming. Yet, obtaining reliable data on behavioral abnormalities was not possible for all patients as not all caregivers provided the necessary information. Therefore, analyses involving behavioral data could only be performed in a subset of our patient sample. An additional constraint on the group size of our behaviorally impaired sample is inherent to the nature of ALS, as behavioral impairments, which are not severe enough to be classified as frontotemporal dementia, typically affect less than one third of all patients (Kasper et al. 2015). Furthermore, the contrasts between high and low performing patients are in some conditions lacking statistical robustness, as the groups of low performing patients were rather small. To partially circumvent this limitation though, we have included regression analyses of patients’ behavioral performance scores on their BOLD-activation for all three tasks.
Future work is needed to further investigate the neural basis underlying changes in cognition and behavior of ALS patients. A longitudinal approach would be highly desirable as it would allow for analysis of the pattern alterations and eventual progress of neuronal changes and the pathogenesis of ALS as a whole, especially in the light of the proposed neuropathological staging system of ALS (Braak et al. 2013; Brettschneider et al. 2013; Kassubek et al. 2014). Furthermore, imaging contrasts between patients with familial and sporadic forms of ALS could provide even further insight into this topic.
Conclusion
In conclusion, this fMRI study demonstrates cortical reorganization and compensation in cognitive function tasks in ALS patients. An early adaptive process characterized by more activation in relevant brain areas during cognitive tasks seems to help overcome neuronal cell loss. In this context, clinical representation of cognitive and behavioral impairments manifests itself only when this process is no longer sufficient in compensating this loss. This finding therefore could provide an interesting and potentially useful biomarker for further fMRI research in this field (Filippi et al. 2015). Furthermore, the contrast between more and less cognitively impaired patients revealed a characteristic pattern of this cortical reorganization consistent with a recently proposed staging model of ALS.
References
Abrahams, S., Goldstein, L. H., Simmons, A., Brammer, M., Williams, S. C., Giampietro, V., et al. (2004). Word retrieval in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Brain, 127, 1507–1517.
Abrahams, S., Newton, J., Niven, E., Foley, J., & Bak, T. H. (2014). Screening for cognition and behaviour changes in ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 15, 9–14.
Abu-Akel, A., & Shamay-Tsoory, S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia, 49, 2971–2984.
Al-Chalabi, A., Jones, A., Troakes, C., King, A., Al-Sarraj, S., & van den Berg, L. H. (2012). The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathologica, 124, 339–352.
Allen, M. D., Owens, T. E., Fong, A. K., & Douglas, R. R. (2011). A functional neuroimaging analysis of the Trail making test-B: Implications for clinical application. Behavioural Neurology, 24, 159–171.
Andreasen, N. C., O'Leary, D. S., Cizadlo, T., Arndt, S., Rezai, K., Watkins, G. L., et al. (1995). Remembering the past: Two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry, 152, 1576–1585.
Ashburner, J., & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26, 839–851.
Ayaz, H., Shewokis, P. A., Scull, L., Libon, D. J., Feldman, S., Eppig, J., et al. (2014). Assessment of prefrontal cortex activity in amyotrophic lateral sclerosis patients with functional near infrared spectroscopy. Journal of Neuroscience and Neuroengineering, 3, 41–51.
Baron-Cohen, S. (1995). Mindblindness: An essay on autism and theory of mind. Cambridge: MIT Press.
Beeldman, E., Raaphorst, J., Klein Twennaar, M., de Visser, M., Schmand, B. A., & de Haan, R. J. (2016). The cognitive profile of ALS: A systematic review and meta-analysis update. Journal of Neurology, Neurosurgery and Psychiatry, 87, 611–619.
Böhm, S., Aho-Özhan, H. E. A., Keller, J., Dorst, J., Uttner, I., Ludolph, A. C., et al. (2016). Medical decisions are independent of cognitive impairment in amyotrophic lateral sclerosis. Neurology, 87, 1737–1738.
Bowie, C. R., & Harvey, P. D. (2006). Adminstration and interpretation of the Trail making test. Nature Protocols, 1, 2277–2281.
Braak, H., Brettschneider, J., Ludolph, A. C., Lee, V. M., Trojanowski, J. Q., & Del Tredici, K. (2013). Amyotrophic lateral sclerosis - a model of corticofugal axonal spread. Nature Reviews Neurology, 9, 708–714.
Brettschneider, J., Del Tredici, K., Toledo, J. B., Robinson, J. L., Irwin, D. J., Grossmann, M., et al. (2013). Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of Neurology, 74, 20–38.
Burke, T., Pinto-Grau, M., Lonergan, K., Elamin, M., Bede, P., Costello, E., et al. (2016). Measurement of social cognition in amyotrophic lateral sclerosis: A population based study. PloS One, 11, e0160850.
Cavallo, M., Adenzato, M., MacPherson, S. E., Karwig, G., Enrici, I., & Abrahams, S. (2011). Evidence of social understanding impairment in patients with amyotrophic lateral sclerosis. PloS One, 6, e25948.
Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B., et al. (1999). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). Journal of the Neurological Sciences, 169, 13–21.
Cerami, C., Dodich, A., Canessa, N., Crespi, C., Iannaccone, S., Corbo, M., et al. (2014). Emotional empathy in amyotrophic lateral sclerosis: A behavioural and voxel-based morphometry study. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 25, 21–29.
Chio, A., Pagani, M., Agosta, F., Calvo, A., Cistaro, A., & Filippi, M. (2014). Neuroimaging in amyotrophic lateral sclerosis: Insights into structural and functional changes. Lancet Neurology, 13, 1228–1240.
Deichmann, R., & Turner, R. (2002). Improvement of local BOLD sensitivities in the presence of susceptibility gradients by using tilted slices. International Society for Magnetic Resonance in Medicine, 10, 1414.
Filippi, M., Agosta, F., Grosskreutz, J., Benatar, M., Kassubek, J., Verstraete, E., et al. (2015). Progress towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurology, 14, 786–788.
Friston, K. J., Holmes, A. P., Worsley, K. J., Poline, J.-P., Frith, C. D., & Frackowiak, R. S. J. (1995). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2, 189–210.
Gibbons, Z. C., Snowden, J. S., Thompson, J. C., Happé, F., Richardson, A., & Neary, D. (2007). Inferring thought and action in motor neurone disease. Neuropsychologia, 45, 1196–1207.
Girardi, A., Macpherson, S. E., & Abrahams, S. (2011). Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology, 25, 53–65.
Goldstein, L. H., & Abrahams, S. (2013). Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurology, 12, 368–380.
Goldstein, L. H., Newsom-Davis, I. C., Bryant, V., Brammer, M., Leigh, P. N., & Simmons, A. (2011). Altered patterns of cortical activation in ALS patients during attention and cognitive response inhibition tasks. Journal of Neurology, 258, 2186–2198.
Jelsone-Swain, L., Persad, C., Votruba, K. L., Weisenbach, S. L., Johnson, T., Gruis, K. L., et al. (2012). The relationship between depressive symptoms, disease state, and cognition in amyotrophic lateral sclerosis. Frontiers in Psychology, 3, 542.
Jelsone-Swain, L., Persad, C., Burkard, D., & Welsh, R. C. (2015). Action processing and mirror neuron function in patients with amyotrophic lateral sclerosis: An fMRI study. PloS One, 10, e0119862.
Johnstone, T., Ores Walsh, K. S., Greischar, L. L., Alexander, A. L., Fox, A. S., Davidson, R. J., et al. (2006). Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Human Brain Mapping, 27, 779–788.
Kasper, E., Schuster, C., Machts, J., Bittner, D., Vielhaber, S., Benecke, R., et al. (2015). Dysexecutive functioning in ALS patients and its clinical implications. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 16, 160–171.
Kassubek, J., Müller, H. P., Del Tredici, K., Brettschneider, J., Pinkhardt, E. H., Lulé, D., et al. (2014). Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain, 137, 1733–1740.
Keller, J., Gorges, M., Horn, H. T., Aho-Özhan, H. E. A., Pinkhardt, E. H., Uttner, I., et al. (2015). Eye-tracking controlled cognitive function tests in patients with amyotrophic lateral sclerosis: A controlled proof-of-principle study. Journal of Neurology, 262, 1918–1926.
Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., Hardiman, O., et al. (2011). Amyotrophic lateral sclerosis. Lancet, 377, 942–955.
Konrad, C., Henningsen, H., Bremer, J., Mock, B., Deppe, M., Buchinger, C., et al. (2002). Pattern of cortical reorganization in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Experimental Brain Research, 143, 51–56.
Loose, M., Burkhardt, C., Aho-Özhan, H., Keller, J., Abdulla, S., Böhm, S., et al. (2016). Age and education-matched cut-off-scores for the revised German/Swiss-German version of ECAS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 17, 374–376.
Ludolph, A., Drory, V., Hardiman, O., Nakano, I., Ravits, J., Robberecht, W., et al. (2015). A revision of the El Escorial criteria – 2015. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 16, 291–292.
Lulé, D., Diekmann, V., Anders, S., Kassubek, J., Kübler, A., Ludolph, A. C., et al. (2007). Brain responses to emotional stimuli in patients with amyotrophic lateral sclerosis (ALS). Journal of Neurology, 254, 519–527.
Lulé, D., Burkhardt, C., Abdulla, S., Böhm, S., Kollewe, K., Uttner, I., et al. (2015). The Edinburgh cognitive and Behavioural amyotrophic lateral sclerosis screen: A cross-sectional comparison of established screening tools in a German-Swiss population. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 16, 16–23.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–1239.
Neumann, M., Sampathu, D. M., Kwong, L. K., Truax, A. C., Micsenyi, M. C., Chou, T. T., et al. (2006). Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science, 314, 130–133.
Ogawa, S., Lee, T. M., Kay, A. R., & Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America, 87, 9868–9872.
Phukan, J., Pender, N. P., & Hardiman, O. (2007). Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurology, 6, 994–1003.
Phukan, J., Elamin, M., Bede, P., Jordan, N., Gallagher, L., Byrne, S., et al. (2012). The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. Journal of Neurology, Neurosurgery, and Psychiatry, 83, 102–108.
Poldrack, R. A. (2007). Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience, 2, 67–70.
Poletti, B., Solca, F., Carelli, L., Madotto, F., Lafronzo, A., Faini, A., et al. (2016). The validation of the Italian Edinburgh cognitive and Behavioural ALS screen (ECAS). Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 17, 489–498.
Poujois, A., Schneider, F. C., Faillenot, I., Camdessanché, J. P., Vandenberghe, N., Thomas-Antérion, C., et al. (2013). Brain plasticity in the motor network is correlated with disease progression in amyotrophic lateral sclerosis. Human Brain Mapping, 34, 2391–2401.
Rascovsky, K., Hodges, J. R., Knopman, D., Mendez, M. F., Kramer, J. H., Neuhaus, J., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134, 2456–2477.
Ringholz, G. M., Appel, S. H., Bradshaw, M., Cooke, N. A., Mosnik, D. M., & Schulz, P. E. (2005). Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology, 65, 586–590.
Saxe, R., & Kanwisher, N. (2003). People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. NeuroImage, 19, 1835–1842.
Schoenfeld, M. A., Tempelmann, C., Gaul, C., Kühnel, G. R., Düzel, E., Hopf, J.-M., et al. (2005). Functional motor compensation in amyotrophic lateral sclerosis. Journal of Neurology, 252, 944–952.
Sebastian, C. L., Fontaine, N. M. G., Bird, G., Blakemore, S.-J., De Brito, S. A., McCrory, E. J. P., et al. (2012). Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Social Cognitive and Affective Neuroscience, 7, 53–63.
Sripada, C. S., Angstadt, M., Banks, S., Nathan, P. J., Liberzon, I., & Phan, K. L. (2009). Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. Neuroreport, 20, 984–989.
Stark, C., & Squire, L. (2001). When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America, 98, 12760–12766.
Strong, M. J., Abrahams, S., Goldstein, L. H., Woolley, S., McLaughlin, P., Snowden, J., et al. (2017). Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, online first, DOI:10.1080/21678421.2016.1267768.
Tsermentseli, S., Leigh, P. N., & Goldstein, L. H. (2012). The anatomy of cognitive impairment in amyotrophic lateral sclerosis: More than frontal lobe dysfunction. Cortex, 48, 166–182.
Tsujimoto, M., Senda, J., Ishihara, T., Niimi, Y., Kawai, Y., Atsuta, N., et al. (2011). Behavioral changes in early ALS correlate with voxel-based morphometry and diffusion tensor imaging. Journal of the Neurological Sciences, 307, 34–40.
Uddin, L. Q., Iacobini, M., Lange, C., & Keenan, J. P. (2007). The self and social cognition: The role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences, 11, 153–157.
Van der Hulst, E.-J., Bak, T. H., & Abrahams, S. (2015). Impaired affective and cognitive theory of mind and behavioural change in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 86, 1208–1215.
Wagner, S., Sebastian, A., Lieb, K., Tüscher, O., & Tadic, A. (2014). A coordinate based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neuroscience, 15, 19.
Wilson, R. C., Takahashi, Y. K., Schoenbaum, G., & Niv, Y. (2014). Orbitofrontal cortex as a cognitive map of task space. Neuron, 81, 267–279.
Witiuk, K., Fernandez-Ruiz, J., McKee, R., Alahyane, N., Coe, B. C., Melanson, M., et al. (2014). Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. Journal of Neuroscience, 34, 14260–14271.
Zaitchik, D., Walker, C., Miller, S., LaViolette, P., Feczkoi, E., & Dickerson, B. C. (2010). Mental state attribution and the temporoparietal junction: An fMRI study comparing belief, emotion, and perception. Neuropsychologia, 48, 2528–2536.
Zakzanis, K. K., Mraz, R., & Graham, S. J. (2005). An fMRI study of the Trail making test. Neuropsychologia, 43, 1878–1886.
Acknowledgments
The authors would like to thank Sonja Fuchs, Ines Schulthess (née Röll) and Jan Luitjens for their assistance with data collection as well as all those who participated in the study.
This is an EU Joint Programme – Neurodegenerative Disease Research (JPND; 01ED1405) project. The project is supported through the following organizations under the aegis of JPND – www.jpnd.eu, e.g. Germany, Bundesministerium für Bildung und Forschung (BMBF, FKZ), Sweden, Vetenskaprådet Sverige, and Poland, Narodowe Centrum Badan i Rozwoju (NCBR). This work was additionally supported by the BMBF (#01GM1103A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The experimental protocol was approved by the Ethics Committee of the University of Ulm (Statement No. 19/12).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have conflict of interest.
Rights and permissions
About this article
Cite this article
Keller, J., Böhm, S., Aho-Özhan, H.E.A. et al. Functional reorganization during cognitive function tasks in patients with amyotrophic lateral sclerosis. Brain Imaging and Behavior 12, 771–784 (2018). https://doi.org/10.1007/s11682-017-9738-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9738-3