Abstract
Alcohol Use Disorder (AUD) is a chronic relapsing condition characterized by excessive alcohol consumption despite its multifaceted adverse consequences, associated with impaired performance in several cognitive domains including decision-making. While choice deficits represent a core component of addictive behavior, possibly consecutive to brain changes preceding the onset of the addiction cycle, the evidence on grey-matter and white-matter damage underlying abnormal choices in AUD is still limited. To fill this gap, we assessed the neurostructural bases of decision-making performance in 22 early-abstinent alcoholic patients and 18 controls, by coupling the Cambridge Gambling Task (CGT) with quantitative magnetic resonance imaging metrics of grey-matter density and white-matter integrity. Regardless of group, voxel based morphometry highlighted an inverse relationship between deliberation time and grey-matter density, with alcoholics displaying slower choices related to grey-matter atrophy in key nodes of the motor control network. In particular, grey-matter density in the supplementary motor area, reduced in alcoholic patients, explained a significant amount of variability in their increased deliberation time. Tract-based spatial statistics revealed a significant relationship between CGT deliberation time and all white-matter indices, involving the most relevant commissural, projection and associative tracts. The lack of choice impairments other than increased deliberation time highlights reduced processing speed, mediated both by grey-matter and white-matter alterations, as a possible marker of a generalized executive impairment extending to the output stages of decision-making. These results pave the way to further studies aiming to tailor novel rehabilitation strategies and assess their functional outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol Use disorder (AUD) is a chronic relapsing condition characterized by excessive alcohol consumption despite its adverse consequences. AUD is associated with neurological alterations involving both brain metabolism (Volkow et al. 2017) and morphology (Pfefferbaum and Sullivan 2005; Xiao et al. 2015), and with neuro-cognitive impairments encompassing several domains, including decision-making (Le Berre et al. 2017; Oscar-Berman et al. 2014). Increasing evidence suggests that a critical characteristic of AUD such as the transition from voluntary and reward-related drinking to compulsive behavior depends on abnormal evaluations of action-reward instrumental associations (Beck et al. 2009; Galandra et al. 2018a). Novel insights into the neural bases of such deficits are coming from multi-modal MRI, associating specific cognitive impairments with regional alterations of brain structure and/or function. There is, however, limited evidence relating defective decision-making to grey-matter (GM) atrophy or white-matter (WM) disorganization.
The severity of decision-making deficits in early-abstinent AUD patients has been shown to reflect GM atrophy in key nodes of the reward network, such as ventro-medial prefrontal cortex, dorsal anterior cingulate cortex (dACC) and hippocampus (Le Berre et al. 2013). Conversely, GM atrophy in the amygdala, unrelated to altered decision-making, has been reported in long-term abstinent patients (Fein et al. 2006). Moreover, despite evidence of WM microstructural abnormalities associated with alcohol neurotoxic effects (Yeh et al. 2009; Konrad et al. 2012; Sorg et al. 2015), a relationship with decision-making impairments is debated. The available findings might reflect the different WM profiles observed in early vs. long-term abstainers. In the former, no relationship was found between abnormal decision-making and decreased axonal microstructural integrity, i.e. lower fractional anisotropy (FA) in the corpus callosum and in parietal, occipital, and frontal tracts (Zorlu et al. 2013). Instead, the lack of decision-making and FA alterations after long-term abstinence highlights possible compensatory repair processes of axonal injuries (Zorlu et al. 2014).
Overall, the evidence on GM and WM changes associated with decision-making impairments in AUD is limited. We thus aimed to characterize the possible relationship between alcoholics’ decision-making deficits, assessed with the Cambridge Gambling Task (CGT), and both GM density reduction and WM disorganization. While most previous studies have used the Iowa Gambling Task (Fein et al. 2006; Le Berre et al. 2013; Zorlu et al. 2013, 2014), the CGT overcomes some of its limitations by assessing decision-making outside a learning context, and minimizing the engagement of high-order cognitive functions. Consistent with the role of information processing speed in higher-order cognitive tasks (Salthouse 1996), previous evidence of increased deliberation time in alcoholic patients performing this task was discussed in terms of psychomotor slowing (Czapla et al. 2016; Lawrence et al. 2009). This proposal fits with recent evidence that alcoholics’ executive dysfunction, mostly involving processing speed in tasks such as the Trail Making Task Part A (TMT-A), reflects structural (Galandra et al. 2018b) and functional (Galandra et al. 2019) alterations in fronto-insular-striatal mechanisms supporting the salience-based access to cognitive resources required for cognitive control on goal-directed behavior and its motor implementation. On this basis, we assessed the hypothesis that slowed CGT performance in early-abstinent alcoholic patients reflects their decreased information processing speed at the TMT-A, as well as GM and WM alterations in networks underlying the production of behavioral responses, such as the motor control network and distinct features of white-matter microstructure of commissural bundles (e.g., body of corpus callosum).
Materials and methods
See Supplementary Materials for a full description of Materials and methods.
Participants
Twenty-two early-abstinent alcoholic patients and 18 healthy controls participated in the study. Patients, recruited during a 28-days alcohol withdrawal treatment, were interviewed to determine their drinking history, including the amount, type and lifetime duration of alcohol use (see Table 1a, b and Supplementary Methods for demographic, clinical, as well as nicotine and alcohol use, variables). They joined the protocol after being detoxified for at least 10 days via medically supported standard treatments, but had ceased benzodiazepine consumption at least 8 days before scanning (see Supplementary Methods for details on inclusion/exclusion criteria).
Participants provided written informed consent to the experimental procedure, which was approved by the ICS Maugeri (Pavia, Italy) Ethical Committee.
Decision-making assessment
Decision-making skills were assessed with the CGT (Rogers et al. 1999), providing six outcome measures reflecting different facets of decision-making: 1) deliberation time; 2) quality of decision-making; 3) delay aversion; 4) overall proportion bet; 5) risk adjustment; 6) risk taking.
MRI data acquisition
We used a 3 Tesla General Electrics Discovery MR750 scanner (GE Healthcare), equipped with a 16-channel head coil, to collect T1-weighted and Diffusion Tensor Imaging (DTI) images (See details in Supplementary Methods).
Statistical analysis of decision-making data
For each CGT measure we checked the normality of the score distribution across the whole sample. Based on this assessment, we examined age- and group-effects via non-parametric (Spearman’s correlation index and Mann-Whitney t-test, respectively) tests. Based on our previous reports of decreased processing speed at the TMT-A task (Galandra et al. 2018b), we assessed a correlation between performance in this task and CGT deliberation time. In patients, we also assessed a relationship between the CGT measure(s) showing a significant difference compared with controls and alcohol use variables (disease duration, length of sobriety before MRI and daily alcohol consumption). We applied a statistical threshold of p < 0.05, one-tailed due to a priori hypotheses of decision-making impairment in alcoholic patients vs. controls (Czapla et al. 2016; Lawrence et al. 2009).
VBM data preprocessing and statistical analysis
The preprocessing and statistical analysis of T1-weighted images was based on SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and the CAT12 toolbox r1447 (www.neuro.uni-jena.de/cat/). Pre-processing steps included image correction for bias-field inhomogeneities, spatial normalization using the DARTEL algorithm (Ashburner 2007), segmentation into GM, WM and cerebrospinal fluid (CSF) (Ashburner and Friston 2005), and smoothing (8 mm).
Statistical analyses included: a) two-sample t-tests, to compare groups; b) multiple regressions, to test correlations with behavioral variables; c) full factorial models (two-sample t-test plus a behavioral covariate) to test group differences in such correlations. Age and smoking status were modeled to remove their potentially confounding effects. We used conjunction-null analyses (Nichols et al. 2005) to assess the predicted overlap between regions in which GM density was both reduced in alcoholics, and related to decision-making performance. We applied threshold-free cluster enhancement (TFCE; Smith and Nichols 2009) with 5000 permutations per contrast and Family-Wise Error (FWE) correction for multiple comparisons at p < 0.05 (Radua et al. 2014).
Overlap with the motor control network
Based on VBM results, we assessed a spatial overlap between the regions displaying common effects of interest (atrophy in patients and correlation with defective CGT measure(s)) and those included in the motor control network. A meta-analytic map of this network was generated with the Neurosynth toolbox (http://neurosynth.org) (Yarkoni et al. 2011). We used the Marsbar toolbox (http://marsbar.sourceforge.net/) to create binary masks of the clusters displaying the aforementioned joint effects of interest, and the REX toolbox (http://web.mit.edu/swg/software.htm) to extract their average GM density at the single-subject level, for offline regression analyses (See details in Supplementary Methods).
DTI data preprocessing and whole-brain statistical analysis
We performed DTI data pre-processing with FSL (http://fsl.fmrib.ox.ac.uk/fsl/), and group analyses of fractional anisotropy (FA), mean (MD), axial (AD) and radial (RD) diffusivities with Tract-Based Spatial Statistics (TBSS) (Smith et al. 2006). We performed whole-brain voxelwise statistics via Randomise with TFCE (Smith and Nichols 2009), FWE corrected for multiple comparisons at p < 0.05. We assessed the presence of WM microstructural alterations in alcoholic patients vs. controls via a two-sample unpaired t-test (5000 random permutations per contrast) including age and smoking status as nuisance covariates. To constrain subsequent analyses, for each DTI invariant we created a binary mask reflecting the alteration pattern in alcoholic patients. We investigated whether the relationship between defective CGT measures and microstructural characteristics within such abnormal patterns reflects qualitative or quantitative group differences, via a linear relationship between CGT measures of interest and each DTI invariant, and task-by-group interactions, respectively (p < 0.05, TFCE corrected, 5000 permutations). The anatomical localization of significant clusters was performed using the JHU White-Matter Tractography Atlas and the JHU ICBM-DTI-81 White-Matter Labels (Hua et al. 2008). We extracted DTI invariant data from the thereby obtained significant clusters to perform off-line analyses relating neurostructural metrics of white-matter connectivity to clinical variables (p < 0.05 corrected with False-Discovery-Rate).
Results
Decision-making skills in alcoholic patients vs. controls
There were no significant group differences in demographic characteristics, smoking and nutritional status (Table 1a). Compared with controls, alcoholic patients displayed significantly worse CGT performance only in terms of deliberation time (Table 1c), which was also correlated with TMT-A response time (r = 0.303, p = 0.029). We found no significant correlation between deliberation time and any of the collected alcohol use variables (length of abstinence: r = −0.101, p = 0.328; disease duration: r = −0.165, p = 0.232; average daily alcohol use: r = 0.086, p = 0.351).
GM density reduction in alcoholic patients vs. controls
As previously reported (Galandra et al. 2018b), GM density was significantly reduced, in alcoholic patients vs. controls, in a wide midline cluster extending from the dorsomedial prefrontal cortex to the supplementary motor area (SMA) and middle cingulate cortex. GM atrophy additionally involved, in patients, the inferior frontal gyrus (IFG), rolandic operculum and posterior temporo-insular cortex, plus the thalamus, bilaterally but with a right-sided prevalence (Table 2a; Fig. 1). There was no significant correlation, in patients, between GM density and alcohol use variables.
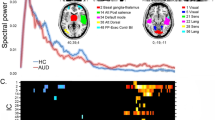
Grey-matter and white-matter damage in AUD. Significant group differences resulting from whole-brain voxelwise VBM and TBSS statistical analyses, highlighting grey-matter (GM: red) and white-matter (WM) changes in alcoholic patients compared with controls. In the case of WM changes, different colors depict alterations reflecting: a) a significant decrease of fractional anisotropy (FA: blue); b) a significant increase of diffusivity indices (MD: light blue, AD: green, RD: yellow) (p < 0.05 corrected for multiple comparisons).
Correlation between GM density and CGT deliberation time
Regardless of group, deliberation time was negatively correlated with GM density in a midline cluster encompassing the posterior fronto-medial cortex, SMA and middle cingulate cortex, as well as in the right fronto-insular cortex and thalamus bilaterally (Table 2b; Fig. 2a). We found no significant positive correlation between GM density and deliberation time.
Neurostructural correlates of slowed decision-making in AUD. GM and WM voxels showing a significant correlation with deliberation time (DT) in the whole group. Panel a depicts the regions in which GM density was negatively correlated with deliberation time. Within the tracts showing a significant WM damage in alcoholic patients, panel b depicts the voxels in which DT was positively related to mean (MD: light blue), axial (AD: green) and radial (RD: yellow) diffusivity (p < 0.05 corrected for multiple comparisons). The scatterplots show, in the whole sample, the relationship between CGT deliberation time (ms) and a) average GM density in the voxels depicted in red in the slices reported on the left; b) average DTI invariants in the voxels depicted in yellow (RD), light blue (MD) and green (AD) in the slices reported above.
Group differences in the correlation between GM density and CGT deliberation time
We found no significant task-by-group interactive effect on GM density.
Common neurostructural effects of AUD and correlation with CGT deliberation time
A conjunction-analysis confirmed the spatial overlap between the voxels associated with a) reduced GM density in alcoholic patients vs. controls, and b) slower performance in the CGT (Fig. 3a). Such common effects involved a cluster extending from the posterior fronto-medial to the middle cingulate cortex, plus the thalamus bilaterally (Table 2c).
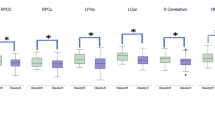
Slowed decision-making and GM atrophy in the Motor Control Network. The figure depicts the regions showing common effects of AUD and deliberation time (panel a), as well as the spatial overlap between the motor control network (green) and the regions where GM density was both correlated with deliberation time in the whole sample, and reduced in alcoholic patients (panel b). Average GM density in the yellow voxels explained 32.5% of individual differences in CGT deliberation time in the whole sample, with no significant group difference in the slope of this relationship. The scatterplot depicts the relationship between CGT deliberation time (ms) and average GM density in the SMA cluster highlighted by conjunction-analyses (i.e. in the voxels depicted in yellow color).
Overlap between GM-related findings and the motor control network
To test the hypothesis that slower performance on the CGT reflects GM atrophy in the motor control network, we assessed a relationship between deliberation time and GM density in the voxels which, besides showing a correlation with behavioral performance in the whole sample and atrophy in alcoholic patients, were additionally included in the Neurosynth “motor control” map (Fig. 3b). We found such overlap in the SMA (xyz: −4, −8, 50), with its average GM density explaining 32.5% of interindividual variability in deliberation time (F(1,38) = 18.312; p < 0.001) in the whole sample. A homogeneity-of-slopes model confirmed the lack of significant group differences in this relationship (F(1,36) = 1.190; p = 0.283).
WM microstructural changes in alcoholic patients vs. controls
Compared with controls, early-abstinent alcoholic patients displayed widespread alterations of microstructural properties in different white-matter bundles. Significant FA alterations were found in the body of corpus callosum. We also observed widespread and co-localized MD, AD and RD alterations in commissural fibers (genu and body of corpus callosum, tapetum, forceps minor and forceps major), projection fibers (all sectors of corona radiata and internal capsule, encompassing the corticospinal tract, and the anterior and posterior thalamic radiations), as well as associative fibers (bilateral inferior and superior longitudinal fasciculi, inferior fronto-occipital fasciculus, uncinate fasciculus, fornix and left cingulum bundle) (Fig. 1). There was no significant correlation between alcohol-related WM microstructural changes and alcohol use variables.
Correlation between WM microstructural changes and CGT deliberation time
We observed a significant linear relationship between deliberation time and distinct metrics of WM microstructure (MD, AD, RD) within the tracts which additionally displayed significant alterations in patients. Regardless of group, deliberation time was positively related to diffusivity invariants within a co-localized pattern including distinct sectors of corpus callosum (genu, body, splenium), forceps major and forceps minor, anterior and posterior thalamic radiations, all sectors of corona radiata and internal capsule, major associative bundles bilaterally (uncinate and inferior fronto-occipital fasciculi, superior and inferior longitudinal fasciculi), as well as left cingulum bundle (cingulate gyrus) and fornix/stria terminalis (Fig. 2b).
Group differences in the correlation between WM microstructural changes and CGT deliberation time
We found no significant task-by- group interactive effect on WM microstructure.
Discussion
We coupled the CGT with VBM and TBSS analyses of grey- and white-matter changes, respectively, to provide preliminary evidence on the neurostructural correlates of decision-making impairments in AUD vs. controls. The only significant group difference in behavioral performance involved increased choice latencies in early-abstinent alcoholics. Although at odds with previous reports of defective behavioral learning in AUD (Deserno et al. 2015; Park et al. 2010; see Galandra et al. 2018a), this result fits with available evidence of psychomotor slowing (Al-Zahrani and Elsayed 2009; Fama et al. 2012) and increased CGT deliberation time (Lawrence et al. 2009; Van Der Plas et al. 2009; Zois et al. 2014) in alcoholic patients. Moreover, the positive correlation between CGT decision latency and TMT-A response time (which is also increased in alcoholic patients; Galandra et al. 2018b, 2019), highlights the role of decreased information processing speed as an unspecific precursor of different types of high-order cognitive deficits in AUD (Nigg et al. 2017).
While impaired TMT-A performance has been recently discussed in terms of altered neural mechanisms of salience-detection (Galandra et al. 2018b, 2019), increased deliberation time is considered to reflect psychomotor slowing (Lawrence et al. 2009). A generalized decrease of processing speed indeed confirmed previous evidence of a prominent impairment, in AUD, in the “output” stage of decision-making (Czapla et al. 2016; Lawrence et al. 2009). To assess this hypothesis, we first used MRI metrics to unveil grey- and white-matter alterations in patients (Fortier et al. 2015; Pfefferbaum and Sullivan 2005; Xiao et al. 2015). Compared with controls, they displayed grey-matter atrophy in the subcortical and cortical components of the meso-cortico-limbic pathway (Fig. 1), alongside the fronto-insular nodes of the salience network (Menon and Uddin 2010) and the medial sector of the sensorimotor network. TBSS results confirmed diffuse alterations of WM microstructural integrity in alcoholic patients, involving the main commissural, projection and associative tracts, and particularly the body of corpus callosum where lower FA and higher diffusivity values provided multifaceted cues of WM damage (Fig. 1) (Crespi et al., 2019, 2020).
A non-significant task-by-group interaction indicates the lack of qualitative group differences in the relationship between deliberation time and GM density. Instead, correlation analyses highlighted quantitative group differences (Fig. 2a), with patients’ longer choice latency reflecting in decreased GM density in the medial sensorimotor cortex, right IFG and cerebellum. The extensive involvement of sensorimotor cortex suggests that alcoholic patients’ increased deliberation time might reflect the structural damage of the motor control network, previously associated with response selection and adjustment during cognitively demanding tasks (Sakai et al. 2000). This hypothesis was supported by the significant overlap between the meta-analytic map of this network and a SMA cluster in which GM was both negatively correlated with deliberation time in the whole sample, and decreased in patients vs. controls (Fig. 3b). The SMA role in the well-known speed-accuracy trade-off (SAT; Wood and Jennings 1976) has been shown by functional MRI (Ivanoff et al. 2008), magnetoencephalography (Wenzlaff et al. 2011) and electroencephalography (Perri et al. 2014) studies reporting its enhanced activity with faster (rather than accurate) responses. The present evidence of GM atrophy in the same SMA cluster reflecting deliberation time might thus highlight a specific neural signature of alcoholic patients’ deficits in accumulating evidence to produce fast choices. These results highlight a relatively simple measure such as choice speed as a marker of executive and decision-making impairment in AUD.
Successful processing speed depends also on the degree of WM integrity, supporting a coordinated communication among cortical brain networks (Mesulam 2000). Alcoholics’ cognitive impairments are thus likely to reflect the neurotoxic effects of chronic alcohol consumption on white-matter bundles, which in vivo MRI studies associate to demyelination and axonal structural alterations (Fortier et al. 2015; Monte and Kiril 2014; Pfefferbaum and Sullivan 2005). This hypothesis was supported by the present evidence of a relationship between DTI invariants of WM damage and increased deliberation time. The latter was positively correlated with all diffusivity invariants (AD, MD, RD) within a co-localized pattern encompassing the most relevant commissural, projection and associative tracts. As for VBM analyses, the lack of significant task-by-group interactions highlights no qualitative group difference with respect to this relationship.
A similar connection between effective processing speed and WM microstructural changes has been reported in healthy ageing (Borghesani et al. 2013). By impairing WM structural integrity, alcohol neurotoxic effects might accelerate the physiological age-related decline in processing speed, and promote the vicious circle linking alcohol abuse with defective decision-making and control over craving. While only few studies have addressed this issue, the available data fit with the present evidence of a significant relationship between decreased processing speed and WM microstructural changes, in alcoholic patients, mainly in the corpus callosum, alongside prefrontal and temporal bundles (Pfefferbaum et al. 2010; Rosenbloom et al. 2009).
A limitation of this study is represented by a small-to-moderate sample size. Although critical confounding factors such as age and education were matched across groups, the present data will require further support from larger samples. On the other hand, our alcoholic patients were carefully controlled for potentially confounding clinical variables, and might thus be considered as strongly representative of the AUD core features.
Conclusions
Our data show concurrent neuropathological changes slowing down information processing and motor responses during decision-making. Albeit preliminary, this evidence highlights several directions for future research. Increasing evidence suggests positive effects of cognitive remediation (Rupp et al. 2012) and neurostimulation (Herremans et al. 2016) on cognitive performance and craving in addictions. However, the efficacy of neurostimulation depends on the choice of target areas, in turn guided by evidence of significant relationships between their functional properties and cognitive performance. By highlighting decreased information processing speed as a precursor of more specific executive deficits, and its neural correlates, our results might thus help tailor remediation or neurostimulation protocols to target specific networks and their associated cognitive functions.
References
Al-Zahrani, M. A., & Elsayed, Y. A. (2009). The impacts of substance abuse and dependence on neuropsychological functions in a sample of patients from Saudi Arabia. Behavioral and Brain Functions, 5(1), 48–11. https://doi.org/10.1186/1744-9081-5-48.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007.
Ashburner, J., & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. https://doi.org/10.1016/j.neuroimage.2005.02.018.
Beck, A., Schlagenhauf, F., Wüstenberg, T., Hein, J., Kienast, T., Kahnt, T., et al. (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry, 66(8), 734–742. https://doi.org/10.1016/j.biopsych.2009.04.035.
Borghesani, P. R., Madhyastha, T. M., Aylward, E. H., Reiter, M. A., Swarny, B. R., Warner Schaie, K., & Willis, S. L. (2013). The association between higher order abilities, processing speed, and age are variably mediated by white-matter integrity during typical aging. Neuropsychologia, 51(8), 1435–1444. https://doi.org/10.1016/j.neuropsychologia.2013.03.005.
Crespi, C., Galandra, C., Manera, M., Basso, G., Poggi, P., & Canessa, N. (2019). Executive Impairment in Alcohol Use Disorder Reflects Structural Changes in Large-Scale Brain Networks: A Joint Independent Component Analysis on Gray-Matter and White-Matter Features. Frontiers in Psychology, 26. https://doi.org/10.3389/fpsyg.2019.02479.
Crespi, C., Galandra, C., Canessa, N., Manera, M., Poggi, P., & Basso, G. (2020). Microstructural damage of white-matter tracts connecting large-scale networks is related to impaired executive profile in alcohol use disorder. NeuroImage, 25. https://doi.org/10.1016/j.nicl.2019.102141.
Czapla, M., Simon, J. J., Richter, B., Kluge, M., Friederich, H. C., Herpertz, S., Mann, K., Herpertz, S. C., & Loeber, S. (2016). The impact of cognitive impairment and impulsivity on relapse of alcohol-dependent patients: implications for psychotherapeutic treatment. Addiction Biology, 21(4), 873–884. https://doi.org/10.1111/adb.12229.
Deserno, L., Beck, A., Huys, Q. J., Lorenz, R. C., Buchert, R., Buchholz, H. G., Plotkin, M., Kumakara, Y., Cumming, P., Heinze, H. J., Grace, A. A., Rapp, M. A., Schlagenhauf, F., & Heinz, A. (2015). Chronic alcohol intake abolishes the relationship between dopamine synthesis capacity and learning signals in the ventral striatum. European Journal of Neuroscience, 41(4), 477–486. https://doi.org/10.1111/ejn.12802.
Fama, R., Rosenbloom, M. J., Sassoon, S. A., Pfefferbaum, A., & Sullivan, E. V. (2012). Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcoholism: Clinical and Experimental Research, 36(10), 1738–1747. https://doi.org/10.1111/j.1530-0277.2012.01790.x.
Fein, G., Torres, J., Price, L. J., & Di Sclafani, V. (2006). Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research, 30(9), 1538–1544. https://doi.org/10.1111/j.1530-0277.2006.00185.x.
Fortier, C. B., Ph, D., Leritz, E. C., Ph, D., Salat, D. H., Ph, D., et al. (2015). Widespread effects of alcohol on white-matter microstructure. NIH Public Access, 38(12), 2925–2933. https://doi.org/10.1111/acer.12568.
Galandra, C., Basso, G., Cappa, S., & Canessa, N. (2018a). The alcoholic brain: neural bases of impaired reward-based decision-making in alcohol use disorders. Neurological Sciences. Springer Milan. https://doi.org/10.1007/s10072-017-3205-1
Galandra, C., Basso, G., Manera, M., Crespi, C., Giorgi, I., Vittadini, G., Poggi, P., & Canessa, N. (2018b). Salience network structural integrity predicts executive impairment in alcohol use disorders. Scientific Reports, 8(1), 14481. https://doi.org/10.1038/s41598-018-32828-x.
Galandra, C., Basso, G., Manera, M., Crespi, C., Giorgi, I., Vittadini, G., et al. (2019). Abnormal fronto-striatal intrinsic connectivity reflects executive dysfunction in alcohol use disorders. Cortex, 115, 27–42. https://doi.org/10.1016/j.cortex.2019.01.004.
Herremans, S. C., De Raedt, R., Van Schuerbeek, P., Marinazzo, D., Matthys, F., De Mey, J., & Baeken, C. (2016). Accelerated HF-rTMS protocol has a rate-dependent effect on dACC activation in alcohol-dependent patients: an open-label feasibility study. Alcoholism: Clinical and Experimental Research, 40(1), 196–205. https://doi.org/10.1111/acer.12937.
Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., et al. (2008). Tract probability maps in stereotaxic spaces: analyses of white-matter anatomy and tract-specific quantification. NeuroImage, 39(1), 336–347. https://doi.org/10.1016/j.neuroimage.2007.07.053.
Ivanoff, J., Branning, P., & Marois, R. (2008). fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PLoS One, 3(7). https://doi.org/10.1371/journal.pone.0002635.
Konrad, A., Vucurevic, G., Lorscheider, M., Bernow, N., Thümmel, M., Chai, C., et al. (2012). Broad disruption of brain white-matter microstructure and relationship with neuropsychological performance in male patients with severe alcohol dependence. Alcohol Alcohol, 47(2), 118–126. https://doi.org/10.1093/alcalc/agr157.
Lawrence, A. J., Luty, J., Bogdan, N. A., Sahakian, B. J., & Clark, L. (2009). Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction, 104(6), 1006–1015. https://doi.org/10.1111/j.1360-0443.2009.02533.x.
Le Berre, A. P., Rauchs, G., La Joie, R., Segobin, S., Mézenge, F., Boudehent, C., et al. (2013). Readiness to change and brain damage in patients with chronic alcoholism. Psychiatry Research - Neuroimaging, 213(3), 202–209. https://doi.org/10.1016/j.pscychresns.2013.03.009.
Le Berre, A. P., Fama, R., & Sullivan, E. V. (2017). Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcoholism: Clinical and Experimental Research. https://doi.org/10.1111/acer.13431.
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. NIH Public Access. https://doi.org/10.1007/s00429-010-0262-0.
Mesulam, M. (2000). Brain, mind, and the evolution of connectivity. Brain and Cognition, 6, 4–6. https://doi.org/10.1006/brcg.1999.1145.
Monte de la, S. M., & Kiril, J. (2014). Human alcohol - related neuropathology (pp. 71–90). https://doi.org/10.1007/s00401-013-1233-3.
Nichols, T., Brett, M., Andersson, J., Wager, T., & Poline, J. B. (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25(3), 653–660. https://doi.org/10.1016/j.neuroimage.2004.12.005.
Nigg, J. T., Jester, J. M., Stavro, G. M., Ip, K. I., Puttler, L. I., & Zucker, R. A. (2017). Specificity of executive functioning and processing speed problems in common psychopathology. Neuropsychology, 31(4), 448–466. https://doi.org/10.1037/neu0000343.
Oscar-Berman, M., Valmas, M. M., Sawyer, K. S., Ruiz, S. M., Luhar, R. B., & Gravitz, Z. R. (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. In Handbook of clinical neurology (Vol. 125, 1st ed., pp. 183–210). Elsevier B.V.. https://doi.org/10.1016/B978-0-444-62619-6.00012-4.
Park, S. Q., Kahnt, T., Beck, A., Cohen, M. X., Dolan, R. J., Wrase, J., & Heinz, A. (2010). Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. Journal of Neuroscience, 30(22), 7749–7753. https://doi.org/10.1523/JNEUROSCI.5587-09.2010.
Perri, R. L., Berchicci, M., Spinelli, D., & Di Russo, F. (2014). Individual differences in response speed and accuracy are associated to specific brain activities of two interacting systems. Frontiers in Behavioral Neuroscience, 8(July), 1–12. https://doi.org/10.3389/fnbeh.2014.00251.
Pfefferbaum, A., & Sullivan, E. V. (2005). Disruption of brain white-matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology, 30(2), 423–432. https://doi.org/10.1038/sj.npp.1300623.
Pfefferbaum, A., Rosenbloom, M. J., Fama, R., Sassoon, S. A., & Sullivan, E. V. (2010). Transcallosal white-matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcoholism: Clinical and Experimental Research, 34(7), 1201–1211. https://doi.org/10.1111/j.1530-0277.2010.01197.x.
Radua, J., Canales-Rodríguez, E. J., Pomarol-Clotet, E., & Salvador, R. (2014). Validity of modulation and optimal settings for advanced voxel-based morphometry. NeuroImage, 86, 81–90. https://doi.org/10.1016/j.neuroimage.2013.07.084.
Rogers, R. D., Owen, A. M., Middleton, H. C., Williams, E. J., Pickard, J. D., Sahakian, B. J., & Robbins, T. W. (1999). Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(20), 9029–9038 http://www.ncbi.nlm.nih.gov/pubmed/10516320.
Rosenbloom, M. J., Sassoon, S. A., Pfefferbaum, A., & Sullivan, E. V. (2009). Contribution of regional white-matter integrity to visuospatial construction accuracy, organizational strategy, and memory for a complex figure in abstinent alcoholics. Brain Imaging and Behavior, 3(4), 379–390. https://doi.org/10.1007/s11682-009-9080-5.
Rupp, C. I., Kemmler, G., Kurz, M., Hinterhuber, H., & Wolfgang Fleischhacker, W. (2012). Cognitive remediation therapy during treatment for alcohol dependence. Journal of Studies on Alcohol and Drugs, 73(4), 625–634. https://doi.org/10.15288/jsad.2012.73.625.
Sakai, K., Hikosaka, O., Takino, R., Miyauchi, S., Nielsen, M., & Tamada, T. (2000). What and when: parallel and convergent processing in motor control. The Journal of Neuroscience: the Official Journal of the Society for NEUROSCIENCE, 20(7), 2691–2700 http://www.ncbi.nlm.nih.gov/pubmed/10729350.
Salthouse, T. (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403–428.
Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024.
Sorg, S. F., Squeglia, L. M., Taylor, M. J., Alhassoon, O. M., Delano-Wood, L. M., & Grant, I. (2015). Effects of aging on frontal white-matter microstructure in alcohol use disorder and associations with processing speed. Journal of Studies on Alcohol and Drugs, 76(2), 296–306. https://doi.org/10.15288/jsad.2015.76.296.
Van Der Plas, E. A. A., Crone, E. A., Van Den Wildenberg, W. P. M., Tranel, D., & Bechara, A. (2009). Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. Journal of Clinical and Experimental Neuropsychology, 31(6), 706–719. https://doi.org/10.1080/13803390802484797.
Volkow, N. D., Wiers, C. E., Shokri-Kojori, E., Tomasi, D., Wang, G. J., & Baler, R. (2017). Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: studies with positron emission tomography. Neuropharmacology, 122, 175–188. https://doi.org/10.1016/j.neuropharm.2017.01.012.
Wenzlaff, H., Bauer, M., Maess, B., & Heekeren, H. R. (2011). Neural characterization of the speed – accuracy tradeoff in a perceptual decision-making task., 31(4), 1254–1266. https://doi.org/10.1523/JNEUROSCI.4000-10.2011.
Wood, C. C., & Jennings, J. R. (1976). Speed-accuracy tradeoff functions in choice reaction time: experimental designs and computational procedures. Perception & Psychophysics, 19(1), 92–102.
Xiao, P. R., Dai, Z. Y., Zhong, J. G., Zhu, Y. L., Shi, H. C., & Pan, P. L. (2015). Regional gray matter deficits in alcohol dependence: a meta-analysis of voxel-based morphometry studies. Drug and Alcohol Dependence, 153, 22–28. https://doi.org/10.1016/j.drugalcdep.2015.05.030.
Yarkoni, T., Poldrack, R. a., Nichols, T. E., Van Essen, D. C., Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8 (8), 665–670. https://doi.org/10.1038/nmeth.1635.
Yeh, P. H., Simpson, K., Durazzo, T. C., Gazdzinski, S., & Meyerhoff, D. J. (2009). Tract-based spatial statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Research - Neuroimaging, 173(1), 22–30. https://doi.org/10.1016/j.pscychresns.2008.07.012.
Zois, E., Kortlang, N., Vollstädt-Klein, S., Lemenager, T., Beutel, M., Mann, K., & Fauth-Bühler, M. (2014). Decision-making deficits in patients diagnosed with disordered gambling using the Cambridge gambling task: the effects of substance use disorder comorbidity. Brain and Behavior, 4(4), 484–494. https://doi.org/10.1002/brb3.231.
Zorlu, N., Gelal, F., Kuserli, A., Cenik, E., Durmaz, E., Saricicek, A., & Gulseren, S. (2013). Abnormal white-matter integrity and decision-making deficits in alcohol dependence. Psychiatry Research - Neuroimaging, 214(3), 382–388. https://doi.org/10.1016/j.pscychresns.2013.06.014.
Zorlu, N., Karavul Ucman, T., Gelal, F., Colak Kalayci, C., Polat, S., Saricicek, A., et al. (2014). Abnormal white-matter integrity in long-term abstinent alcohol dependent patients. Psychiatry Research - Neuroimaging, 224(1), 42–48. https://doi.org/10.1016/j.pscychresns.2014.07.006.
Acknowledgements
We wish to thank Dr. Giovanni Vittadini for his valuable contribution in patient recruitment and clinical assessment.
Author information
Authors and Affiliations
Contributions
C.G.: study design; data collection; data analysis; data interpretation; manuscript drafting and review; Dr. Galandra reports no disclosures.
C.C: data analysis; data interpretation; manuscript drafting and review; Dr. Crespi reports no disclosures.
G.B: study design; data collection; manuscript review; Prof. Basso reports no disclosures.
M.R.M.: study design; data collection; manuscript review; Dr. Manera reports no disclosures.
I.G.: study design; data collection; manuscript review; Dr. Giorgi reports no disclosures.
P.P: study design; data collection; manuscript review; Dr. Poggi reports no disclosures.
N.C: study design; data collection; data analysis; data interpretation; manuscript drafting and review; Prof. Canessa reports no disclosures.
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of ICS Maugeri (Pavia, Italy) and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Galandra, C., Crespi, C., Basso, G. et al. Decreased information processing speed and decision-making performance in alcohol use disorder: combined neurostructural evidence from VBM and TBSS. Brain Imaging and Behavior 15, 205–215 (2021). https://doi.org/10.1007/s11682-019-00248-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00248-8