Abstract
Visuospatial construction ability as used in drawing complex figures is commonly impaired in chronic alcoholics, but memory for such information can be enhanced by use of a holistic drawing strategy during encoding. We administered the Rey-Osterrieth Complex Figure Test (ROCFT) to 41 alcoholic and 38 control men and women and assessed the contribution of diffusion tensor imaging (DTI) measures of integrity of selected white matter tracts to ROCFT copy accuracy, copy strategy, and recall accuracy. Although alcoholics copied the figure less accurately than controls, a more holistic strategy at copy was associated with better recall in both groups. Greater radial diffusivity, reflecting compromised myelin integrity, in occipital forceps and external capsule was associated with poorer copy accuracy in both groups. Lower FA, reflecting compromised fiber microstructure in the inferior cingulate bundle, which links frontal and medial temporal episodic memory systems, was associated with piecemeal copy strategy and poorer immediate recall in the alcoholics. The correlations were generally modest and should be considered exploratory. To the extent that the inferior cingulate was relatively spared in alcoholics, it may have provided an alternative pathway to the compromised frontal system for successful copy strategy and, by extension, aided recall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Visuospatial deficits are a marked feature of chronic alcoholism and have been documented using a variety of behavioral tasks in chronic alcoholics who have been abstinent for weeks or even months (Brandt et al. 1983; Fama et al. 2004; Fein et al. 1990, 2006; Munro et al. 2000; Oscar-Berman and Marinkovic 2007; Sullivan et al. 2000, 2002; Wegner et al. 2001). Compromise of visuospatial functions are consistent with observations in fMRI studies of dampened occipital activation to visual stimulation in recently detoxified alcoholics (Hermann et al. 2007) and abnormally low visual P3b amplitudes and longer latencies in alcoholics (Fein and Chang 2006; Glenn et al. 1996; Porjesz et al. 1980). Spatial processing deficits have also been linked to volume deficits in parietal gray matter volume (Fein et al. 2009).
One useful test for assessing visuospatial constructional and mnemonic abilities is the Rey-Osterrieth Complex Figure Test (ROCFT) (Osterrieth and Rey 1944; Rey 1942), which has served to identify deficits in alcoholics in copying a complex geometric figure and reproducing it from memory (Beatty et al. 1996; Dawson and Grant 2000; Munro et al. 2000; Sullivan et al. 1992). A strength of the ROCFT is that it enables assessment of figure copying accuracy (visuospatial construction) and accuracy of short and long-term recall and recognition of nonverbal material. Furthermore, copy strategy, whether the subject approaches the task with an overall plan embracing the larger structure of the drawing (holistic) or in a disjointed and inefficient manner (piecemeal) can also be assessed and has been shown to affect accuracy of copy and of immediate and delayed recall of the drawing in healthy adults (Bennett-Levy 1984; Sullivan et al. 1992), alcoholics (Dawson and Grant 2000; Sullivan et al. 1992), and other patient groups (Heinrichs and Bury 1991; Kim et al. 2008; Lange et al. 2000; Shin et al. 2004).
Successful performance on the ROCFT involves multiple skills and multiple brain systems requiring integrity of each region, and of the white matter circuits linking them (Mesulam 1998). The visuoconstructional aspects of ROCFT copying involve occipitoparietal association networks for visual perception and frontostriatal motoric networks for copying. Studies have linked deficits in ROCFT copy to lesions in parietal-occipital regions (Astur et al. 2002; Pillon 1981; Taylor 1969). More generally, functional MRI (fMRI) studies frequently highlight activation in parietal-occipital cortical regions when participants are engaged in a visuospatial task (e.g., Tomasi et al. 2007). Thus candidate white matter circuits for enabling visuospatial perceptual processing would include the occipital forceps and superior and inferior longitudinal fasciculi, which link nodes of the parietal and occipital cortices known to participate in visuospatial processing. The strategic planning needed for successful copy may involve frontal circuits linked by the frontal forceps. The motoric aspects of the task may involve circuits linking the basal ganglia and caudate, such as the external capsule. Drawing the figure from memory involves declarative memory circuits involving medial temporal structures (Astur et al. 2002; Milner 2005; Pillon 1981; Taylor 1969), and working memory circuits involving inferior temporal and prefrontal structures (Muller and Knight 2006; Ranganath 2006; Ungerleider et al. 1998). Thus candidate white matter circuits for recall of visual stimuli would include the inferior cingulate bundle. A holistic approach to copying represents a form of planning and organization that involves regions of the frontal lobes for which a candidate tract is the frontal forceps (Ardila 2008; Owen et al. 1990; Stuss and Benson 1984). Thus frontal, parietal-occipital, and medial-temporal lobe structures are all likely invoked when earlier holistic encoding is used to reproduce a visual stimulus from memory as occurs during successful performance of the ROCFT recall task. Such a circuit is provided by the cingulum, an association fiber bundle that originates beneath the genu of the corpus callosum, courses around the corpus callosum, and terminates in the parahippocampal gyrus of the medial temporal lobe providing a critical link between the frontal cortex and the hippocampal system (Morris et al. 1999).
MR diffusion tensor imaging (DTI) of the brain enables identification and quantification of the integrity of white matter tracts in vivo (Pierpaoli and Basser 1996). Fractional anisotropy (FA) reflects the extent to which diffusion of water molecules is constrained within white matter tract barriers, reflecting the linear orientation of neural tracts; the apparent diffusion coefficient (ADC), or mean diffusivity (MD), are measures of the amount of freely diffusing water molecules in a brain sample. ADC can be decomposed into longitudinal (axial) and transverse (radial) diffusivity (λT), the latter increases with decline in myelin integrity (Song et al. 2005) and is affected in alcoholism (Pfefferbaum et al. 2009). DTI also enables reconstruction (Lehericy et al. 2004; Xu et al. 2002) of three-dimensional white matter fiber tracts shown in monkeys to be representative of the underlying anatomy (Schmahmann et al. 2007), and it is feasible to quantify (Gerig et al. 2005; Sullivan et al. 2006; Wakana et al. 2007) mean FA and diffusivity along their extent. Low FA and high λT are often interpreted as fiber tract integrity disruption.
DTI in patients with alcoholism has shown degradation of frontal and superior white matter circuits, along with relative preservation of posterior and inferior circuits (Pfefferbaum et al. 2009). In the current study, we used quantitative DTI tractography in alcoholic and comparison subjects to measure microstructural properties of selected white matter tracts and explore their association with different components of the ROCFT. We asked whether alcohol-related degradation of frontal (frontal forceps) and superior (external capsule, superior cingulate bundle, superior longitudinal fasciculus) fiber systems would be associated with visuospatial construction and holistic strategizing elements of ROCFT performance in the alcoholics. We also asked whether relative preservation of posterior (occipital forceps) and inferior (internal capsule, inferior cingulate bundle, inferior longitudinal fasciculus) circuits would provide alternative compensatory pathways to performance of visuoconstruction or memory elements of the ROCFT (cf., Ham and Parsons 1997; Ward 2005; Pfefferbaum et al. 2001).
Methods
Participants
Participants with alcoholism (n = 41) were recruited by referral from community outpatient substance abuse treatment centers. Healthy controls (n = 38) were recruited by advertising, referral from other study participants, and community outreach. Study participants underwent clinical assessment, DTI, and completed the ROCFT. Clinical, demographic (Rosenbloom et al. 2007), and brain (Pfefferbaum et al. 2007; Pfefferbaum et al. 2006, 2009) descriptions of a larger sample from which those with ROCFT data were available appear elsewhere. Informed consent was obtained following procedures approved by the Institutional Review Boards of SRI International and Stanford University School of Medicine.
Clinical, cognitive, and motor evaluation
Study criteria were based on the Structured Clinical Interview for DSM-IV (SCID) (First et al. 1998), administered by clinical psychologists to all subjects. Prospective subjects meeting lifetime criteria for schizophrenia or bipolar disorder or for non-alcohol substance dependence or abuse within the prior 3 months were excluded, as were prospective controls meeting DSM-IV criteria for any Axis I disorder. All alcoholics met DSM-IV criteria for alcohol dependence. Global Assessment of Functioning (GAF) was derived from the SCID (Endicott et al. 1976). Autobiographical history of alcohol consumption (Pfefferbaum et al. 1992; Skinner 1982; Skinner and Sheu 1982) yielded quantitative lifetime consumption of alcohol and time since last drink. Interviews and questionnaires assessed current depression symptoms using the Beck Depression Inventory–II (Beck et al. 1996); socioeconomic status (SES) using a two-factor scale based on education and occupation (Hollingshead and Redlich 1958); handedness (Crovitz and Zener 1962); history of smoking; and body mass index (height cm/weight kg2). General cognitive status was assessed using the Peabody Picture Vocabulary Test—3rd Edition (Dunn and Dunn 1997).
Simple motor function was assessed using the Fine Finger Movement Test (Corkin et al. 1986) in which subjects turned a knurled pin with their forefinger and thumb with left and right hands (Corkin et al. 1986). Three, 30-second trials for each hand were administered and the mean number of rotations across all trials for both hands was calculated. This test provided a control measure of simple motor function involved in ROCFT performance.
Demographic and clinical descriptors of the 79 men and women in this analysis appear in Table 1. Alcoholics were comparable to controls in age, gender representation, socioeconomic status, ethnic minority representation, body mass index, and simple motor dexterity, measured with the Fine Finger Movement Test, but had less education, more depressive symptoms, lower Global Assessment of Function (GAF) rating, higher lifetime alcohol consumption, and lower Peabody Scaled Score. Furthermore, alcoholics were more likely to be current or past smokers or have a family history of alcoholism. Characteristics on which alcoholics differed from controls were investigated as possible contributors to performance on the ROCFT including past dependence on or abuse of other substances or diagnosis of depressive or anxiety disorder.
Rey-osterrieth complex figure test administration
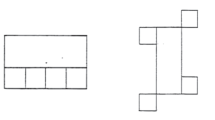
The Rey-Osterrieth Complex Figure Test (ROCFT) (Osterrieth and Rey 1944; Rey 1942) was administered using the procedure described by Meyers & Meyers (Meyers and Meyers 1995), with a pen-switching modification to track drawing strategy (Sullivan et al. 1992). The figure, a complex black and white line drawing, was placed before the subject who was instructed to make an exact copy as neatly and accurately as possible on an 8.5 x 11 inch sheet of white paper laid down in horizontal orientation. To track drawing strategy, the examiner gave the subject a different color pen, in fixed color order, every 30 s. If subjects were in the middle of drawing a line at the end of a 30-sec interval, the examiner waited until that line was finished before switching pens (Fig. 1). Drawing time was noted, and maximum time allowed for drawing was 5 min. Once the copy was completed, the figure card and the subject’s drawing were removed, and the subject was given a blank piece of paper and instructed to draw the figure from memory. An unannounced delayed recall condition was administered 30 min after immediate recall; unrelated tests (i.e., not involving nonverbal material to process or remember) were administered during the retention interval. Accuracy score for each condition was based on 18 elements of the figure for each of which subjects could score up to 2 points (Lezak et al. 2004; Taylor 1969). Strategy for each test condition was scored on a five-point scale based on the extent to which drawing proceeded systematically from the major framework of the figure to the individual details using continuous lines. The higher the score, the more holistic the strategy (Sullivan et al. 1992).
Examples of drawings copied by two controls (above) and two alcoholics (below). Figures on the left demonstrate holistic drawing strategy. Those on the right demonstrate piecemeal strategy. Strategy was assessed by providing subjects with different color pens in the order indicated to identify the extent to which drawing proceeded systematically from the major framework of the figure to the individual details using continuous lines
Image acquisition and processing
Imaging was performed on a 1.5 Tesla GE clinical whole body system. A dual-echo fast spin-echo (FSE) coronal structural sequence was acquired (47 contiguous, 4 mm thick slices; TR/TE1/TE2 = 7500/14/98 ms; matrix = 256 × 192). DTI was performed with the same slice location parameters as the dual-echo FSE, using a single shot spin-echo echo-planar imaging technique (47 contiguous, 4 mm thick slices, TR/TE = 10,000/103 ms, matrix = 128 × 128, in-plane resolution = 1.875 mm2, b-value = 860 s/mm2). Diffusion was measured along six non-collinear directions (6 NEX) with alternating signs to minimize the need to account for cross-terms between imaging and diffusion gradients (Neeman et al. 1991). For each slice, six images with no diffusion weighting (b = 0 s/mm2) were also acquired.
The structural data were passed through the FSL Brain Extraction Tool (Smith 2002) to extract the brain from the skull. Eddy current-induced image distortions in the diffusion-weighted images for each direction were minimized by alignment with an average made of all 12 diffusion-weighted images using a 2-D 6-parameter affine correction on a slice-by-slice basis (Woods et al. 1998). The DTI data were then aligned using the FSE data by a non-linear 3D warp (3rd-order polynomial), which provided in-plane and through-plane alignment compensating for subject motion. On a voxel-by-voxel basis, FA and ADC, the latter decomposed into its longitudinal (λL = λ1) and transverse (λT = [λ2 + λ3]/2) components, were computed. FA ranged from 0 to 1, and diffusivity was expressed in units of 10−6 mm2/s.
To achieve common anatomical coordinates across subjects, a population-average FA template (Sullivan et al. 2009) was constructed from the FA data of 120 control subjects (20–81 years old) with group-wise affine registration (Learned-Miller 2006) followed by iterative nonrigid averaging (Rohlfing and Maurer 2003). Each subject’s FA data set was registered to the population FA template with a 9-parameter affine transformation followed by nonrigid alignment using a multi-level, 3rd-order B-spline, with 5-mm final control point spacing (Rohlfing and Maurer 2003; Rueckert et al. 1999).
We followed a fiber tracking routine (Mori et al. 1999; Xu et al. 2002) that has been modified for use in our laboratory and described in detail elsewhere (Pfefferbaum et al. 2009; Sullivan et al. 2009). This approach applies a target-source convention that restricts the fibers to ones originating in source voxels and passing through target voxels that has demonstrated strong measurement reliability. For this study we examined eight white matter tracts: internal capsule, external capsule, frontal forceps, occipital forceps, superior cingulate bundle, inferior cingulate bundle, superior longitudinal fasciculus, inferior longitudinal fasciculus (see Fig. 2 for illustration of selected tracts).
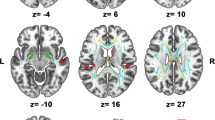
Regression plots of alcoholic (square) and control (dot) values for Copy Accuracy and Occipital Forceps λT, Frontal Forceps FA, External Capsule λT, and Superior Longitudinal Fasciculus λT. Location and extent of each tract is illustrated in axial and sagittal views (data from a 58 year old control man superimposed on the laboratory group average FA template of 120 controls) in the images adjacent to each plot
In common space each landmark was dilated with a morphological operator to produce a 9.375 mm cube as the fiber tracking target. Sources were defined as 5.625 mm thick planes: a) 5.625 mm anterior and 5.625 mm posterior to the frontal and occipital forceps, superior and inferior cingulate, superior and inferior longitudinal fasciculi; b) superior and inferior to the internal and external capsules. For each subject the targets and sources were mapped to that subject’s native image space with a numerical inversion of the transformation to common space for fiber tracking. To compensate for any registration inaccuracy and ensure proper localization for each target, the FA image was multiplied by the eigenvector value for the orientation orthogonal to the expected tract orientation (e.g., the z-axis for the internal capsule) and then a search was performed within a 3x3x3 voxel space allowing the target voxel to move +/− one 1.875 mm voxel in any direction. The targets, sources, and tensor images in native space were passed to the fiber tracking routine, the output of which is a 3D graphical model of the fiber paths, a table of locations and DTI metrics for each voxel in each fiber. Tracking parameters included white matter extraction threshold (minimum FA) of .17, fiber tracking threshold of .125, and maximum voxel-to-voxel coherence minimum transition smoothness threshold of .80 (~37° maximum deviation between voxels), with essentially no limit on the number of fibers. Fiber length minimum (11.25 mm) and fiber length maximum (45 mm) assured that only the data reflecting the mid-region of the fiber bundle at the target location was used. For each fiber bundle, mean FA, ADC, and λT of its designated length were the units of analysis.
Statistical analysis
Group differences for behavioral measures and DTI were tested with t-tests or Mann-Whitney U tests. The contribution of continuous demographic or clinical variables to group differences in behavioral measures was assessed using analyses of covariance (ANCOVA). Categorical clinical descriptors were analyzed using two-factor ANOVAs or t-tests as appropriate. A repeated measures (Copy, Immediate, and Delayed) analysis of variance (ANOVA) tested group differences in copy accuracy and immediate and delayed recall. Relationships between demographic, clinical, and cognitive variables and ROCFT scores were examined with Pearson Product-Moment or Spearman correlations separately for alcoholics and controls.
We examined association between ROCFT performance and FA and λT for the following tracts: superior and inferior cingulate bundle, superior and inferior longitudinal fasciculus, internal and external capsule, frontal and occipital forceps. Each measure was entered into a series of individual regression analyses with visuospatial ability (copy accuracy), episodic memory (immediate recall), and strategic thinking (copy strategy) as target dependent variables. Additional analyses were performed with Fine Finger Movement as a comparison dependent variable. FA or λT for all tracts showing an association with a given ROCFT score were entered into a follow-up multiple regression analysis to determine whether any made a further independent contribution to that score. Regression analyses were conducted for alcoholics and controls separately and the alpha level was set to 0.05 for all these exploratory analyses without correction for multiple analyses.
Results
Table 2 presents ROCFT and Fine Finger Movement descriptive statistics and group comparisons.
ROCFT copy accuracy, copy strategy, recall, and fine finger movement
Group differences
Alcoholics copied the figure less accurately and more slowly than controls. Adding copy time as a covariate enhanced the group difference in copy accuracy (F(1.76) = 6.2, p = .015). Alcoholics performed the Fine Finger Movement test as well as controls. On average, alcoholics achieved similar strategy scores. Among controls, a higher strategy score was associated with less time to copy the figure (rho = −.548, p = .0012) but not with higher copy accuracy (rho = .158, p = .3496). Among alcoholics, by contrast, copy strategy score and copy time were not associated (rho = −.245, p = .1263), although higher strategy scores tended to be associated with higher copy accuracy (rho = .304, p = .0575). Figure 1 presents examples of drawings and scores for copy accuracy and copy strategy.
To adjust memory scores for drawing ability, immediate and delayed drawing accuracy scores were expressed as a fraction of copy accuracy. Immediate drawing from memory was ~50% less accurate than initial copy in both groups, with little change in recall accuracy after a 30 min delay. A repeated measures ANOVA for Copy, Immediate recall, and Delayed recall yielded a trend towards an interaction between group and test time showing that alcoholics tended to lose more points relative to initial copy on delayed recall than controls (F (2,154) = 2.603, p = .0773). Initial copy accuracy was not associated with immediate or delayed recall in either group. Initial copy strategy score, however, influenced recall in both groups, although alcoholics showed weaker effects than controls in immediate (Alcoholics: rho = .36, p = .025; Controls: rho = .525, p = .0019) and delayed recall (Alcoholics: rho = .298, p = .064; Controls: rho = .629, p = .0002).
Demographic, clinical, and cognitive associates of ROCFT performance
Alcoholics
Among alcoholics, none of the ROCFT performance scores correlated with age, lifetime alcohol consumption, length of sobriety, or time since last abused or were dependent on other substance. Furthermore, those alcoholics with a past history of substance abuse or dependence, or of a depression or anxiety diagnosis did not differ significantly on any ROCFT performance score from those without such prior history. Years of education (r = 0.296, p = .06), socioeconomic status (r = 0.38, p = .06), and Peabody scores (r = 0.38, p = .026) were each modestly associated with copy accuracy, accounting for 11–13% of the variance but showed less robust associations with immediate recall and virtually no relation with copy strategy. When these variables were added as covariates in the group comparison for copy accuracy, alcoholics were no longer significantly different from controls on copy accuracy.
Depressive symptoms did not correlate with copy accuracy, but higher depression symptom scores were associated with poorer recall and strategy, accounting for 19% of immediate recall (r = −.438, p = .0075), 24% of delayed recall (r = −.49, p = .0024), and 13% of copy strategy score (r = −.367, p = .03). When depression symptom score was added as a covariate in group comparisons of recall and strategy, group differences were enhanced but did not reach significance.
Fine Finger Movement made a modest contribution to copy accuracy (r = .288, p = .07) and did not reduce significance of group difference from controls when entered as a covariate (F (1,75) = 4.61, p = .035). Of the categorical subject descriptors (sex, current or past smoker, family history for alcohol, ethnic minority status, or history of dependence/abuse of other drugs), only family history for alcoholism had an effect: those with a positive family history were less accurate in copying the figure than those without a family history of alcoholism (t (31) = 3.26, p = .0027). Family history was not associated with impaired recall or with copy strategy.
Controls
Among controls, older age was modestly associated with poorer immediate recall (r = −.317, p = .052). In addition, lower general intelligence (Peabody Test) was associated with poorer copy accuracy (r = .423, p = .0082) and immediate recall (r = .36, p = .026). Slower Fine Finger Movement was associated with poorer copy accuracy (r = .326, p = .046) and immediate recall (r = .406, p = .0115) and higher depressive symptom scores (range: 0–12 out of a possible maximum of 36) were associated with lower copy accuracy (r = −.378, p = .025) and more piecemeal copy strategy scores (r = −.378, p = .025).
White matter tracts
Alcoholics showed a similar regional pattern of microstructural compromise (Table 3) to that reported in the larger subject sample from which this subgroup was drawn (Pfefferbaum et al. 2009). In general, inferior (internal capsule, inferior cingulate bundle, inferior longitudinal fasciculus) and posterior (occipital forceps) tracts were relatively preserved in alcoholics. By contrast, FA was lower in the frontal forceps and diffusivity was higher in superior (external capsule, superior cingulate bundle, superior longitudinal fasciculus) white matter tracts in alcoholics than controls.
White matter tracts, visuospatial construction, strategy, and recall
Correlations are summarized in Table 4 and illustrated in Figs. 2 and 3.
Regression plots of alcoholic (square) and control (dot) values for Immediate Recall/Copy (left) and Copy Strategy (right) against Inferior Cingulate FA. Location and extent of this tract is illustrated in axial and sagittal views (data from a 58 year old control man superimposed on the group average FA image) in the images adjacent to the plots
Visuospatial construction
Among controls, three tracts showed significant individual associations with copy accuracy: frontal forceps (FA), occipital forceps (λT), and external capsule (λT). When all three metrics were entered into a multiple regression, together they accounted for only 14% of the variance and without overall significance (F(3,34) = 1.87, p = 0.1532); none showed a significant independent contribution to copy accuracy (Fig. 2).
Among alcoholics, three fiber tracts showed significant individual association with copy accuracy: occipital forceps (λT); external capsule (FA; λT); and superior longitudinal fasciculus (λT). When all three metrics were entered into a multiple regression, together they accounted for 16.8% of the variance with an overall trend for significance (F (3,37) = 2.48, p = .0759). However, no tracts survived as independent correlates (Fig. 2).
Strategy
Among controls, no tracts significantly correlated with copy strategy. Among alcoholics, only FA of the inferior cingulate bundle was associated with strategy . This effect was sustained with nonparametric analysis (rho = .473, p = .0031). A parallel analysis for λT showed a similar trend (p = .053; rho = −.268, n.s.) (Fig. 3).
Recall
Among controls, there were no significant correlations between any white matter tract or metric and either immediate or delayed memory scores. Among alcoholics, only FA of the inferior cingulate bundle was associated with immediate recall ( p = .028) with a weak trend for delayed recall (r = .272, p = .0856) (Fig. 3).
Multiple regression tested whether strategy or recall showed a unique relation with inferior cingulate FA in alcoholics and found that strategy remained a significant independent associate (t = 2.6, p = .0133) after accounting for immediate (t = 1.137, p = .26) and delayed recall (t = 0.41, p = .687).
Fine finger movement
Among controls, Fine Finger Movement was associated with the integrity of FA and λT of several tracts, including the internal (FA: r = .478, p = .003; λT: r = −.488, p = .002) and external (FA: r = .371, p = .022; λT: r = −.408, p = .01) capsules. By contrast, among alcoholics Fine Finger Movement was modestly associated only with the inferior cingulate bundle metrics (FA: r = .387, p = .014; λT: r = −.301, p = .059). Fine Finger Movement was associated with copy accuracy in controls (r = .326, p = .046) but only weakly in alcoholics (r = .288, p = .07). Multiple regression tested whether either Fine Finger Movement or copy accuracy showed a unique relationship with external capsule λT in each group and found that in controls, both variables together accounted for 20.3% of the variance with Fine Finger Movement remaining a significant associate (t = 2.14, p = .04) after accounting for copy accuracy (t = 1.26, p = .21). Among alcoholics, both variables together accounted for only 10.3% of the variance with copy accuracy remaining only a weak associate (t = 1.865, p = .07) after accounting for Fine Finger Movement (t = 0.315, p = .75).
Discussion
Accurately copying a complex geometric figure involves visually guided action, efficient motor processing for drawing, and a strategic approach for sequencing figure elements and reproducing their spatial localization. The associations revealed in our exploratory study between accurate performance of this visuospatial construction task in the controls and the occipital forceps linking bilateral regions of visual cortices, the external capsule linking basal ganglia and caudate, and the frontal forceps linking bilateral dorsolateral prefrontal cortices are all consistent with the visual, motoric, and planning demands of the task. As expected (c.f., Beatty et al. 1996; Sullivan et al. 1992), alcoholics demonstrated deficits in visuoconstruction, performing this task less accurately and more slowly than controls even though they were not impaired as a group in simple motor dexterity and scored on average as well as controls on copy strategy. Figure copying was less accurate in alcoholics with than without a family history of alcoholism, a finding consistent with earlier reports that family history of alcoholism is associated with poorer visuospatial learning skills in children of alcoholics (Schandler et al. 1988) and chronic alcoholics (Schandler et al. 1996). As in the controls, higher λT values of occipital forceps and external capsule were associated with poor copy accuracy in alcoholics. Lower frontal forceps FA showed an association with poor accuracy in controls but not in the alcoholics, perhaps because of alcohol-related disruption of integrity of this tract. Instead, the superior longitudinal fasciculus, which links intrahemispheric frontal, occipital, parietal and temporal lobes may have served the function performed by the frontal forceps in controls.
As with copying the figure, reproducing it from memory involves visually-guided action and motor processing, but these actions must now be guided by an internal mnemonic rather than an external representation and benefit from a holistic initial copy strategy. We observed limited behavioral differences between alcoholics and controls and confirmed earlier observations, based on a different cohort of alcoholics, that a more holistic initial copy strategy is associated with better recall, independent of the accuracy of the initial copy (Sullivan et al. 1992). Furthermore, our finding that depressive symptoms had a negative impact on strategy and immediate recall in alcoholics is consistent with differences in strategy and recall on the ROCFT observed between depressed and non-depressed elderly (Elderkin-Thompson et al. 2004).
Accurate copy of a complex visual figure was associated in both groups with integrity of predicted white matter tracts. Neither strategy nor immediate or delayed recall scores, however, were associated with the microstructural integrity of the tracts specifically predicted to link cortical sites known to play a role in strategy (frontal forceps) or recall (inferior cingulate) in the controls. The lack of association between frontal forceps integrity and strategy scores in controls suggests either that this particular tract does not in fact link the subregions of the frontal lobes involved in strategic planning in controls, or more likely that our 5-point score for strategy does not adequately represent the complexity of this construct. Nonetheless, frontal forceps FA association with copy accuracy suggests some frontal involvement in this visuospatial planning and constructional task in controls. The lack of association between frontal forceps integrity and strategy in alcoholics may be attributable to its degraded condition, thereby enabling other less affected tracts to play a role in strategy for construction.
Among alcoholics the FA of the inferior cingulate bundle emerged as the sole correlate of copy strategy and figure recall despite absence of abnormality in either the recall performance or the white matter measures. That the cingulate bundle plays a role in spatial memory has been shown in rats using lesion studies (Aggleton et al. 1995). In rhesus monkeys, tracer studies have shown how the cingulate bundle links the mid-dorsolateral frontal cortex with the hippocampal memory system (Morris et al. 1999). That integrity of the inferior cingulate tract was associated with strategy and recall is consistent with the behaviorally measured contribution of holistic strategy to enhanced recall in these patients. Although only moderately strong, these associations together may reflect the operation of a compensatory mechanism in which a relatively intact circuit takes over work poorly performed by the compromised frontal and superior associational circuits. Compensatory involvement of other brain regions by alcoholics to achieve comparable performance to controls has been observed in several fMRI studies (e.g., Desmond et al. 2003; Pfefferbaum et al. 2001; Tapert et al. 2001). Consistent with these speculations is an fMRI study revealing different patterns of activation in controls and alcoholics. Specifically, controls recruited a dorsal (“where”) circuit involving superior parietal and dorsolateral prefrontal cortex while performing a spatial working memory task; by contrast, alcoholics recruited a ventral (“what”) circuit involving lateral and medial temporal regions and ventral prefrontal cortex to perform the task at normal levels (Pfefferbaum et al. 2001). These broadly converging fMRI data and DTI observations of white matter sparing and loss findings may provide a neuroanatomical basis for revisiting earlier examinations of the compensatory hypothesis (Ham and Parsons 1997) based on behavioral data. The results of this exploratory study implicate possible compensatory mechanisms for holistic visual processing in alcoholics but require replication and further evaluation, ideally in a longitudinal design that permits evaluation of what is essentially a dynamic rather than a static process and addresses other neurobiological or cognitive factors that may influence ROCFT performance.
References
Aggleton, J. P., Neave, N., Nagle, S., & Sahgal, A. (1995). A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: evidence of a double dissociation between frontal and cingulum bundle contributions. Journal of Neuroscience, 15(11), 7270–7281.
Ardila, A. (2008). On the evolutionary origins of executive functions. Brain and Cognition, 68(1), 92–99.
Astur, R. S., Taylor, L. B., Mamelak, A. N., Philpott, L., & Sutherland, R. J. (2002). Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behavioral Brain Research, 132(1), 77–84.
Beatty, W. W., Hames, K. A., Blanco, C. R., Nixon, S. J., & Tivis, L. J. (1996). Visuospatial perception, construction and memory in alcoholism. Journal of Studies on Alcohol, 57, 136–143.
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation.
Bennett-Levy, J. (1984). Determinants of performance on the Rey-Osterrieth complex figure test: An analysis, and a new technique for single-case assessment. British Journal of Clinical Psychology, 23, 109–119.
Brandt, J., Butters, N., Ryan, C., & Bayog, R. (1983). Cognitive loss and recovery in long-term alcohol abusers. Archives of General Psychiatry, 40, 435–442.
Corkin, S., Growdon, J. H., Sullivan, E. V., Nissen, M. J., & Huff, F. J. (1986). Assessing treatment effects from a neuropsychological perspective. In L. Poon (Ed.), Handbook of clinical memory assessment in older adults (pp. 156–167). Washington DC: American Psychological Association.
Crovitz, H. F., & Zener, K. A. (1962). Group test for assessing hand and eye dominance. American Journal of Psychology, 75, 271–276.
Dawson, L. K., & Grant, I. (2000). Alcoholics’ initial organizational and problem-solving skills predict learning and memory performance on the Rey-Osterrieth complex figure. Journal of the International Neuropsychological Society, 6(1), 12–19.
Desmond, J. E., Chen, S. H., De Rosa, E., Pryor, M. R., Pfefferbaum, A., & Sullivan, E. V. (2003). Increased fronto-cerebellar activation in alcoholics during verbal working memory: an fMRI study. NeuroImage, 19, 1510–1520.
Dunn, L. M., & Dunn, E. S. (1997). Peabody picture vocabulary test (3rd ed.). Circle Pines, MN: American Guidance Service.
Elderkin-Thompson, V., Kumar, A., Mintz, J., Boone, K., Bahng, E., & Lavretsky, H. (2004). Executive dysfunction and visuospatial ability among depressed elders in a community setting. Archives of Clinical Neuropsychology, 19(5), 597–611.
Endicott, J., Spitzer, R. L., Fleiss, J. L., & Cohen, J. (1976). The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry, 33(6), 766–771.
Fama, R., Pfefferbaum, A., & Sullivan, E. V. (2004). Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcoholism: Clinical and Experimental Research, 28, 1657–1665.
Fein, G., & Chang, M. (2006). Visual P300s in long-term abstinent chronic alcoholics. Alcoholism Clinical and Experimental Research, 30(12), 2000–2007.
Fein, G., Bachman, L., Fisher, S., & Davenport, L. (1990). Cognitive impairments in abstinent alcoholics. Western Journal of Medicine, 152, 531–537.
Fein, G., Torres, J., Price, L. J., & Di Sclafani, V. (2006). Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research, 30(9), 1538–1544.
Fein, G., Shimotsu, R., Chu, R., & Barakos, J. (2009). Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcoholism Clinical and Experimental Research, on line 23 Jul 09.
First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1998). Structured clinical interview for DSM-IV axis I disorders (SCID) version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute.
Gerig, G., Corouge, I., Vachet, C., Krishnan, K. R., & MacFall, J. R. (2005). Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study. Paper presented at the 13th Proceedings of the International Society for Magnetic Resonance in Medicine, Miami, FL.
Glenn, S. W., Parsons, O. A., & Smith, L. T. (1996). ERP responses to target and nontarget visual stimuli in alcoholics from VA and community treatment programs. Alcohol, 13(1), 85–92.
Ham, H. P., & Parsons, O. A. (1997). Organization of psychological functions in alcoholics and nonalcoholics: a test of the compensatory hypothesis. Journal of Studies on Alcohol, 58(1), 67–74.
Heinrichs, R. W., & Bury, A. (1991). Copying strategies and memory on the Complex Figure Test in psychiatric patients. Psychological Reports, 69(1), 223–226.
Hermann, D., Smolka, M. N., Klein, S., Heinz, A., Mann, K., & Braus, D. F. (2007). Reduced fMRI activation of an occipital area in recently detoxified alcohol-dependent patients in a visual and acoustic stimulation paradigm. Addiction Biology, 12(1), 117–121.
Hollingshead, A., & Redlich, F. (1958). Social class and mental illness. New York: Wiley and Sons.
Kim, M. S., Namgoong, Y., & Youn, T. (2008). Effect of organizational strategy on visual memory in patients with schizophrenia. Psychiatry and Clinical Neurosciences, 62(4), 427–434.
Lange, G., Waked, W., Kirshblum, S., & DeLuca, J. (2000). Organizational strategy influence on visual memory performance after stroke: cortical/subcortical and left/right hemisphere contrasts. Archives of Physical Medicine and Rehabilitation, 81(1), 89–94.
Learned-Miller, E. G. (2006). Data driven image models through continuous joint alignment. IEEE Transactions on Pattern Analysis and Machine Intelligence, 28, 236–250.
Lehericy, S., Ducros, M., Van de Moortele, P. F., Francois, C., Thivard, L., Poupon, C., et al. (2004). Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology, 55(4), 522–529.
Lezak, M. D., Howieson, D. B., & Loring, D. W. (2004). Neuropsychological assessment (4th ed.). New York: Oxford University Press.
Mesulam, M. M. (1998). From sensation to cognition. Brain, 121(Pt 6), 1013–1052.
Meyers, J., & Meyers, K. (1995). Rey complex figure test and recognition trial: professional manual. Odessa, FL: Psychological Assessment Resources.
Milner, B. (2005). The medial temporal-lobe amnesic syndrome. Psychiatric Clinics of North America, 28(3), 599–611. 609.
Mori, S., Crain, B. J., Chacko, V. P., & van Zijl, P. C. (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45, 265–269.
Morris, R., Pandya, D. N., & Petrides, M. (1999). Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. Journal of Comparative Neurology, 407(2), 183–192.
Muller, N. G., & Knight, R. T. (2006). The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience, 139(1), 51–58.
Munro, C. A., Saxton, J., & Butters, M. A. (2000). The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcoholism: Clinical and Experimental Research, 24(10), 1510–1516.
Neeman, M., Freyer, J. P., & Sillerud, L. O. (1991). A simple method for obtaining cross-term-free images for diffusion anisotropy studies in NMR microimaging. Magnetic Resonance in Medicine, 21(1), 138–143.
Oscar-Berman, M., & Marinkovic, K. (2007). Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review, 17(3), 239–257.
Osterrieth, P., & Rey, A. (1944). Le test de copie d’une figure complex. Archives de Psychologie, 30, 205–221.
Owen, A. M., Downes, J. J., Sahakian, B. J., Polkey, C. E., & Robbins, T. W. (1990). Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia, 28, 1021–1034.
Pfefferbaum, A., Lim, K. O., Zipursky, R. B., Mathalon, D. H., Rosenbloom, M. J., Lane, B., et al. (1992). Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism: Clinical and Experimental Research, 16(6), 1078–1089.
Pfefferbaum, A., Desmond, J. E., Galloway, C., Menon, V., Glover, G. H., & Sullivan, E. V. (2001). Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. NeuroImage, 14, 7–20.
Pfefferbaum, A., Rosenbloom, M. J., Rohlfing, T., Adalsteinsson, E., Kemper, C. A., Deresinski, S., et al. (2006). Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. NeuroImage, 33(1), 239–251.
Pfefferbaum, A., Rosenbloom, M. J., Adalsteinsson, E., & Sullivan, E. V. (2007). Diffusion tensor imaging with quantitative fiber tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain, 130, 48–64.
Pfefferbaum, A., Rosenbloom, M. J., Rohlfing, T., & Sullivan, E. V. (2009). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biological Psychiatry, 65(8), 680–690.
Pierpaoli, C., & Basser, P. J. (1996). Towards a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine, 36, 893–906.
Pillon, B. (1981). Troubles visuo-constructifs et methodes de compensation: resultats de 85 patients atteints de lesions cerebrales. Neuropsychologia, 19, 375–383.
Porjesz, B., Begleiter, H., & Garozzo, R. (1980). Visual evoked potential correlates of information processing deficits in chronic alocholics. In H. Begleiter (Ed.), Biological effects of alcohol (pp. 603–622). New York: Plenum.
Ranganath, C. (2006). Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience, 139(1), 277–289.
Rey, A. (1942). L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie, 28, 286–340.
Rohlfing, T., & Maurer, C. R. (2003). Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Transactions on Information Technology in Biomedicine, 7(1), 16–25.
Rosenbloom, M. J., Sullivan, E. V., Sassoon, S. A., O’Reilly, A., Fama, R., Kemper, C. A., et al. (2007). Alcoholism, HIV infection and their comorbidity: factors affecting self-rated health-related quality of life. Journal of Studies on Alcohol and Drugs, 68, 115–125.
Rueckert, D., Sonoda, L. I., Hayes, C., Hill, D. L., Leach, M. O., & Hawkes, D. J. (1999). Nonrigid registration using free-form deformations: application to breast MR images. IEEE Transactions on Medical Imaging, 18(8), 712–721.
Schandler, S. L., Brannock, J. C., Cohen, M. J., Antick, J., & Caine, K. (1988). Visuospatial learning in elementary school children with and without a family history of alcoholism. Journal of Studies on Alcohol, 49(6), 538–545.
Schandler, S. L., Clegg, A. D., Thomas, C. S., & Cohen, M. J. (1996). Visuospatial information processing in intoxicated, recently detoxified, and long-term abstinent alcoholics. Journal of Substance Abuse, 8(3), 321–333.
Schmahmann, J. D., Pandya, D. N., Wang, R., Dai, G., D’Arceuil, H. E., de Crespigny, A. J., et al. (2007). Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain, 130(Pt 3), 630–653.
Shin, M. S., Park, S. J., Kim, M. S., Lee, Y. H., Ha, T. H., & Kwon, J. S. (2004). Deficits of organizational strategy and visual memory in obsessive-compulsive disorder. Neuropsychology, 18(4), 665–672.
Skinner, H. A. (1982). Development and validation of a lifetime alcohol consumption assessment procedure. Toronto, Canada: Addiction Research Foundation.
Skinner, H. A., & Sheu, W. J. (1982). Reliability of alcohol use indices: the lifetime drinking history and the MAST. Journal of Studies on Alcohol, 43, 1157–1170.
Smith, S. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155.
Song, S. K., Yoshino, J., Le, T. Q., Lin, S. J., Sun, S. W., Cross, A. H., et al. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage, 26(1), 132–140.
Stuss, D. T., & Benson, D. F. (1984). Neuropsychological studies of the frontal lobes. Psychological Bulletin, 95, 3–28.
Sullivan, E. V., Mathalon, D. H., Ha, C. N., Zipursky, R. B., & Pfefferbaum, A. (1992). The contribution of constructional accuracy and organizational strategy to nonverbal recall in schizophrenia and chronic alcoholism. Biological Psychiatry, 32, 312–333.
Sullivan, E. V., Rosenbloom, M. J., & Pfefferbaum, A. (2000). Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research, 24(5), 611–621.
Sullivan, E. V., Fama, R., Rosenbloom, M. J., & Pfefferbaum, A. (2002). A profile of neuropsychological deficits in alcoholic women. Neuropsychology, 16(1), 74–83.
Sullivan, E. V., Adalsteinsson, E., & Pfefferbaum, A. (2006). Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex, 16(7), 1030–1039.
Sullivan, E. V., Rohlfing, T., & Pfefferbaum, A. (2009). Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiology of Aging, online May 19 2008.
Tapert, S. F., Brown, G. G., Kindermann, S. S., Cheung, E., Frank, L. R., & Brown, S. A. (2001). fMRI measurement of brain dysfunction in alcohol dependent young women. Alcoholism: Clinical and Experimental Research, 25(2), 236–245.
Taylor, L. (1969). Localization of cerebral lesions by psychological testing. Clinical Neurosurgery, 16, 269–287.
Tomasi, D., Chang, L., Caparelli, E. C., & Ernst, T. (2007). Different activation patterns for working memory load and visual attention load. Brain Research, 1132(1), 158–165.
Ungerleider, L., Courtney, S., & Haxby, J. (1998). A neural system for human visual working memory. Proceedings of the National Academy of Science USA, 95(3), 883–890.
Wakana, S., Caprihan, A., Panzenboeck, M. M., Fallon, J. H., Perry, M., Gollub, R. L., et al. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage, 36(3), 630–644.
Ward, N. S. (2005). Neural plasticity and recovery of function. Progress in Brain Research, 150, 527–535.
Wegner, A. J., Gunthner, A., & Fahle, M. (2001). Visual performance and recovery in recently detoxified alcoholics. Alcohol and Alcoholism, 36(2), 171–179.
Woods, R. P., Grafton, S. T., Holmes, C. J., Cherry, S. R., & Mazziotta, J. C. (1998). Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography, 22(1), 139–152.
Xu, D., Mori, S., Solaiyappan, M., van Zijl, P. C., & Davatzikos, C. (2002). A framework for callosal fiber distribution analysis. NeuroImage, 17, 1131–1143.
Author information
Authors and Affiliations
Corresponding author
Additional information
Support: National Institute on Alcohol Abuse and Alcoholism (AA012388, AA005965, AA017347, AA010723, AA017168).
Rights and permissions
About this article
Cite this article
Rosenbloom, M.J., Sassoon, S.A., Pfefferbaum, A. et al. Contribution of Regional White Matter Integrity to Visuospatial Construction Accuracy, Organizational Strategy, and Memory for a Complex Figure in Abstinent Alcoholics. Brain Imaging and Behavior 3, 379–390 (2009). https://doi.org/10.1007/s11682-009-9080-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-009-9080-5