Abstract
Subtle cognitive and behavioral changes are common in early Parkinson’s disease. The cause of these symptoms is probably multifactorial but may in part be related to extra-striatal dopamine levels. 6-[18 F]-Fluoro-L-dopa (FDOPA) positron emission tomography has been widely used to quantify dopamine metabolism in the brain; the most frequently measured kinetic parameter is the tissue uptake rate constant, Ki. However, estimates of dopamine turnover, which also account for the small rate of FDOPA loss from areas of specific trapping, may be more sensitive than Ki for early disease-related changes in dopamine biosynthesis. The purpose of the present study was to compare effective distribution volume ratio (eDVR), a metric for dopamine turnover, to cognitive and behavioral measures in Parkinson’s patients. We chose to focus the investigation on anterior cingulate cortex, which shows highest FDOPA uptake within frontal regions and has known roles in executive function. Fifteen non-demented early-stage PD patients were pretreated with carbidopa and tolcapone, a central catechol-O-methyl transferase (COMT) inhibitor, and then underwent extended imaging with FDOPA PET. Anterior cingulate eDVR was compared with composite scores for language, memory, and executive function measured by neuropsychological testing, and behavior change measured using two informant-based questionnaires, the Cambridge Behavioral Inventory and the Behavior Rating Inventory of Executive Function-Adult Version. Lower mean eDVR (thus higher dopamine turnover) in anterior cingulate cortex was related to lower (more impaired) behavior scores. We conclude that subtle changes in anterior cingulate dopamine metabolism may contribute to dysexecutive behaviors in Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Loss of nigrostriatal dopaminergic projections is the major cause of the motor signs that define Parkinson’s disease (PD). However, PD patients also experience a variety of non-motor symptoms, many of which are related to extranigral pathology. Cognitive deficits in executive function, memory, and psychomotor speed are common in early PD (Muslimovic et al. 2005). Two informant-based questionnaires, the Cambridge Behavioral Inventory (CBI; Bozeat et al. 2000) and the Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A; Isquith et al. 2006), have been validated as measures of behavioral change in neurological diseases. The CBI, for example, is consistent with standardized neuropsychiatric interviews, but is more practical to use, and can distinguish the behavioral profiles of several neurodegenerative syndromes (Wedderburn et al. 2008). The purpose of the present study was to compare positron emission tomography (PET)-based estimates of dopamine turnover in anterior cingulate cortex to scores from these instruments in a group of non-demented PD patients.
[18F]-Fluoro-L-dopa (FDOPA) PET has been widely used to quantify dopamine metabolism in vivo. FDOPA follows the metabolic pathway of L-dopa, including decarboxylation by L-aromatic amino acid decarboxylase (AAAD) present in catecholaminergic and serotonergic terminals, vesicularization, and reuptake, as well as metabolism by catechol-O-methyl transferase (COMT; Brown et al. 1999; Firnau et al. 1988). Formation of COMT metabolites that cross the blood–brain barrier, reducing signal to noise, is a significant limitation of FDOPA. Quantification of COMT metabolites is particularly critical for estimation of tracer kinetics in regions outside of the striatum, where uptake is a fraction of striatal levels. However, measurement of the metabolite fraction requires serial arterial blood sampling, which increases the risk/benefit ratio of PET studies.
The rate constants for movement of radioactivity between plasma and tissue compartments are quantified from dynamic FDOPA PET images using graphical methods. Models widely used to estimate the uptake rate constant (Ki) typically assume irreversible FDOPA trapping (Patlak et al. 1983). The actual slowly reversible kinetics of FDOPA in the trapped compartment can be quantified by a modified graphical analysis (Patlak and Blasberg 1985), using PET data extended beyond the conventional 90-min imaging window. In addition to the uptake rate constant, Ki, the extended method estimates Kloss, a rate constant that quantifies the slow egress of trapped radiotracer due to neurotransmission. The ratio Ki /Kloss is effective distribution volume (eDV), the inverse of which is dopamine turnover (Doudet et al. 1998; Sossi et al. 2001). Dopamine turnover, measured biochemically, is the ratio of dopamine metabolites and dopamine; since this ratio cannot be measured using PET, in part because FDOPA is an analog of L-dopa and thus does not reflect tyrosine hydroxylase activity, effective dopamine turnover is an estimate of the relative strength of its uptake and elimination processes (Sossi et al. 2002). Effective dopamine turnover is elevated when Kloss is large relative to the tracer influx rate constant, Ki. In high turnover states, distribution volume is low, indicating that tracer is either coming into the trapped compartment slowly or leaving it quickly. Thus, eDV is analogous to the level of water in a bucket that has open filling and draining spigots—low eDV results from high flow through the draining spigot (Kloss) relative to the filling spigot (Ki). Previous studies have shown that low eDV appears to be a more sensitive marker than the uptake rate constant (Ki) for early cellular changes associated with PD (Sossi et al. 2002).
The above method can be extended to allow the use of a reference tissue input, in which case the estimated result is the effective distribution volume ratio (eDVR; Sossi et al. 2002). However, the fraction of the signal from the reference tissue that is due to FDOPA steadily declines with time, as it is replaced by its metabolite 3-O-methylFDOPA. This produces a well-known negative bias in the tissue-derive uptake rate constant (K*i). More importantly in the current context, the graphical method assumes the entire reference tissue signal to be FDOPA, and thus the failure of the expected amount of uptake to appear in the trapped compartment is interpreted by the model as loss of trapped tracer. This large apparent loss rate completely obfuscates the much smaller true rates of fractional loss of trapped tracer from the target tissue (Kloss) that is needed to accurately estimate eDVR (Holden et al. 2010). For the present study, we pretreated subjects with tolcapone, a central and peripheral COMT inhibitor, as well as carbidopa, to reduce the circulating metabolite fraction sufficient for estimation of eDVR. Non-human primate studies have shown that reduction of the COMT metabolite fraction corrects the downward bias of K*i, and upward bias of Kloss, resulting in a larger and more accurately measured eDVR (Holden et al. 1997).
The anterior cingulate cortex, which receives dopaminergic projections from the ventral tegmental area through the mesolimbic pathway, is the region of highest FDOPA uptake amongst frontal cortical regions (Moore et al. 2003; Li et al. 2014). Dopamine levels in prefrontal cortex are known to influence executive performance in non-PD populations (Meyer-Lindenberg et al. 2005). Anterior cingulate FDOPA uptake (K*i) is increased in early PD (Rakshi et al. 1999), and is inversely related to performance on the Stroop task (Bruck et al. 2005). For these reasons, we chose to focus the present investigation on anterior cingulate cortex; we hypothesized that mean anterior cingulate cortex eDVR would be related to measures of cognition and behavior in Parkinson’s patients.
Patients and methods
Fifteen non-demented early-stage PD subjects (mean age 60.3 years; SD, 6 years; 12 men) were recruited from local movement disorders clinics. All subjects passed a competency assessment and had MiniMental Status Exam scores of 28/30 or higher (Folstein et al. 1975, Dubois et al. 2007). Within a 2-month period, subjects underwent neuropsychological assessment, brain magnetic resonance imaging (MRI), and FDOPA PET. The study was approved by the local IRB and written informed consent was obtained from all participants.
For the study participants, mean duration of motor symptoms was 5.6 years (SD 5 years), mean Hoehn and Yahr stage was 1.63 (Hoehn and Yahr 1967), and mean Unified Parkinson’s disease Rating Scale (UPDRS) total and motor sub scores were 15.6 (SD 6), and 10 (SD 12) respectively. Five subjects were taking monoamine oxidase inhibitors, 7 dopamine agonists (pramipexole or ropinerole), and three carbidopa/levodopa (750–1200 mg of levodopa daily). Participants discontinued anti-Parkinson medications for 12–18 h prior to PET imaging and UPDRS scoring.
A neuropsychologist (BB) performed cognitive testing using a battery designed to evaluate language, verbal memory, and executive function (Morrison et al. 2000). Subjects’ caregivers also competed two questionnaires that have been validated as measures of disease-related change in executive function and behavior in neurological disease, the CBI (Bozeat et al. 2000) and the BRIEF-A (Isquith et al. 2006). During the CBI, the informant answers 39 questions about the research subject’s behavior, first indicating the frequency of observed mood or behavioral symptoms, and then indicating how much distress the symptom causes the carer. Scores for frequency range from 0 (never) to severe (several occurrences per week); the scores for distress range from 0 (not at all) to 3 (severely distressing). Cumulative scores are generated for frequency and distress to the career by summing the scores for all items. Factor analyses showed that the CBI addresses broad domains of executive function and self-care, stereotypic and eating behavior, mood, and loss of social awareness (Bozeat et al. 2000). The BRIEF-A is a 75-item questionnaire in which the informant indicates the frequency of observed behaviors—such as disorganization, difficulty concentrating, messiness, and forgetfulness—as occurring “never,” “sometimes,” or “often”. The responses to items in categories of inhibition, shift, emotional control, self-monitoring, initiate, plan/organize, task monitor, and organization of materials are summed to yield a metacognition index and behavior regulation index, as well as a global executive composite (GEC; Isquith et al. 2006). In the present study, the CBI scores for frequency and distress were highly correlated; therefore, the cumulative score for carer distress (CBI-dis) from the CBI, and the GEC from the BRIEF-A were used as summary measures for behavior change.
For the purpose of generating composite neuropsychological indices for comparison with PET, raw Z scores for the individual tests were computed with reference to means and standard deviations taken from a sample of 15 age- and education-matched controls participating in longitudinal studies of aging (Sager et al. 2005). This control sample did not complete behavioral questionnaires, HVLT, or FDOPA PET. Z scores from tests in which higher scores indicate worse performance (TMT B-A and WCST-64 perseverative errors) were multiplied by −1. Then, Z scores for individual tests were averaged to generate indices representing neuropsychological domains: For language, Boston Naming (Kaplan et al. 1983) and Controlled Oral Word Association tests (Benton et al. 1983); for memory, Hopkins Verbal Learning Test (HVLT; Benedict et al. 1998); for executive function, Stroop Color-Word score (Golden 1976), Trail Making Test part B minus part A time difference (TMT B-A) (Reitan 1992), and Wisconsin Card Sorting Test-64 (WCST-64) Perseverative Responses score (Paolo et al. 1996). Because the CBI and BRIEF-A GEC were highly correlated (r = 0.8), a composite Z score for behavior was generated by averaging Z scores for the CBI-dis and GEC, then multiplying by −1 to yield a behavioral index for which negative scores indicated greater than mean impairment and positive scores indicate greater than mean function.
Brain MRIs were acquired on a General Electric 3T SIGNA scanner using an 8-channel head coil, with higher-order shimming. These scans included a high-resolution magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted volume (TR 6.6 ms, TE 2.8 ms, flip angle 8°, inversion time 900 ms, field of view 260 mm, slice thickness 1.2 mm) that was used for PET coregistration and automated cortical segmentation. These T1-weighted MRI volumes were automatically segmented in Freesurfer 4.5 (surfer.nmr.mgh.harvard.edu) to yield standard regions of interest (ROIs) according to the Desikan-Killany atlas (Desikan et al. 2006). The regions were visually inspected for errors and corrected as necessary.
PET images were acquired on a Siemens ECAT EXACT HR + scanner. Subjects, off anti-Parkinson medication for 12–18 h, were pretreated with carbidopa, 2.5 mg/kg and tolcapone, a COMT inhibitor, 200 mg orally, 1 h prior to PET. Following intravenous injection of a mean of 5.2 mCi ±10 % of FDOPA, 18 (2 × 0.5, 3 × 1, 3 × 2, 4 × 5, 6 × 10 min) 3D dynamic frames were collected from 0 to 90 min; following a 45-min delay, 5 additional (10 min) frames were collected for extended kinetic modeling (Holden et al. 2010).
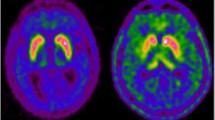
PET image sinograms were reconstructed by filtered back-projection into 23 volumes, which were corrected for scatter, attenuation, and F-18 decay. Each frame was realigned, within-subject, to the sum of the first 18 frames. The PET sum image was then coregistered to the T1-weighted MRI using an affine transformation with 12° of freedom in SPM8 (www.fil.ion.ucl.ac.uk/spm), which was then applied to each realigned PET volume. From the coregistered PET timeseries, a time-activity curve (TAC) was generated for each of the automatically defined cortical segments as well as a hand-drawn occipital cortex reference region (of mean volume 11,378, SD 2284 mm3). The ROI and reference region TACs were then entered into the modified graphical analysis (Patlak and Blasberg 1985) to generate eDVR (Sossi et al. 2001). Because the modified graphical method requires the time integral of the reference tissue time course, interpolation of the time course over the rest interval between the two data acquisition periods, was required; the measured data following the time course peak were fitted to the sum of two decaying exponential functions, and the fit was evaluated at the time points during the gap in the data to represent the unmeasured frames. Figure 1 shows a representative case; Kloss was then estimated as previously described (Holden et al. 1997).
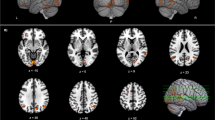
To generate a composite PET measure for comparison with the neuropsychological data, eDVR values for the right and left caudal and rostral anterior cingulate cortex (Freesurfer segments 1002, 2002,1026, 2026; Fig. 2, inset) were averaged to generate an index of dopamine turnover. We then used linear regression models to explore relationships between anterior cingulate eDVR and the cognitive and behavioral indices, with UPDRS III as a covariate to control for the effect of disease severity. Statistical analyses were conducted in SPSS (Version 21, IBM, Chicago). Significant relationships (P > 0.05) were plotted to exclude cases in which apparent relationships were driven by outliers.
Anterior cingulate cortex effective distribution volume ratio (eDVR) versus behavioral index (mean inverse Z score of BRIEF-A and CBI). Lower Z scores, corresponding to greater behavioral impairment, were related to lower eDVR, thus higher dopamine turnover. Inset Anterior cingulate regions within which eDVR was measured
Results
Subject characteristics and Z scores for cognitive and behavioral indices are shown in Table 1. The composite Z score for behavior was related to anterior cingulate eDVR (Fig. 2; t = 3.6, P = 0.004). Other neuropsychological indices (language, memory, or executive) were not convincingly related to anterior cingulate eDVR. An apparent trend towards relationship between the executive index and eDVR (t = 2.1, P = 0.06) was dependent on an outlying value (−4.15).
Discussion
In this study, higher cingulate cortex eDVR (corresponding to a lower rate of dopamine turnover) was related to lower caregiver perception of behavioral abnormalities amongst Parkinson’s patients. This relationship was not reflected in the neuropsychological indices — however, since the protocol enrolled early-stage, non-demented individuals, few had significant impairment on formal testing (Table 1; Gallagher et al. 2013). Previous work has suggested that some behavioral symptoms of PD, such as apathy, do not correlate well with formal executive measures (Robert et al. 2012). The CBI has been shown to be internally consistent, to have good agreement with other validated neuropsychiatric interviews, and to be sensitive to cognitive effects of disease progression in PD (Wedderburn et al. 2008; Athey and Walker 2006). In other neurodegenerative conditions such as Alzheimer’s disease, informant questionnaires have proved to be a useful addition to formal cognitive testing as predictors of disease progression (Tierney et al. 1996). We found scores derived from the two informant questionnaires to be highly correlated; this is interesting because although the BRIEF-A was sensitive to subtle executive changes associated with mild cognitive impairment or memory complaints (Rabin et al. 2006), it has not been used as extensively in neurodegenerative disease as the CBI. The Correlation between the BRIEF-A and CBI in this PD sample may reflect their similar structure and executive focus; however, it could also reflect informant biases. One limitation of informant reports is that they can suffer from rater bias because informants are not blinded to disease state.
The effects of extra-striatal dopamine levels on behavior in PD are not fully understood. Extra-striatal dopamine levels are influenced by disease factors, genetic factors, and exogenous dopamine supplementation. While levodopa or D2 receptor agonists improve mental flexibility, response inhibition, and working memory in PD, they do not improve memory encoding, and can adversely affect decision-making and compulsive behaviors (Kehagia et al. 2010). Research on COMT sequence variants in PD and other neuropsychological disorders suggests an inverted U-shaped relationship between frontal dopamine levels and executive function, such that both high and low dopamine levels adversely affect cognition (Wu et al. 2012). Thus, schizophrenic patients homozygous for a fast metabolizing COMT polymorphism (Val158Met) show improved executive function and cognitive efficiency with amphetamine, which increases frontal synaptic dopamine, while persons homozygous for the slow polymorphism (Met/Met) show the same or impaired performance with amphetamine (Meyer-Lindenberg et al. 2005). In PD, Met/Met homozygous show higher Ki in frontal cortex on delayed imaging in comparison to Val/Val, suggesting that frontal dopamine turnover is influenced by COMT genotype (Wu et al. 2012).
Several groups have described the tissue-derived FDOPA uptake rate constant (K*i) to be increased in prefrontal and limbic cortex in early PD, suggesting that influx of the tracer into cells projecting to these regions is more avid than normal (Bruck et al. 2005, Kaasinen et al. 2001, Rinne et al. 2000). Relationships between frontal FDOPA K*i and cognition have also been reported: Rinne et al. (2000) reported elevated FDOPA uptake in medial frontal cortex be correlated with improved verbal fluency; Bruck et al. (2005) reported higher FDOPA uptake in dorsolateral prefrontal cortex to be related to worse performance on vigilance task, but higher uptake in anterior cingulate and medial frontal cortex to be related to improved performance on the Stroop task. In the present study, patients whose informants reported little behavior change had higher cingulate cortex eDVR (K*i/Kloss), indicating that the profile that has been described in the early phases of neuronal loss in the striatum (declining K*i, elevated Kloss, and increased dopamine turnover) was less evident than in subjects with behavioral change. Findings from this study support previous evidence that behavior in PD patients is regulated in part by mesolimbic dopamine levels.
We chose to focus this investigation on anterior cingulate cortex in part because K*i in this region is highest of frontal cortical regions (Moore et al. 2003; Li et al. 2014), and has been related to executive tasks in other studies (Bruck et al. 2005). Anterior cingulate cortex is a major target of mesocortical dopaminergic projections arising from the ventral tegmental area (Paus 2001). Although autopsy studies have shown reduced neurotransmitter levels and Lewy bodies in limbic cortex of PD patients (Braak et al. 1995; Scatton et al. 1983), anterior cingulate FDOPA uptake is relatively preserved or even increased in early disease (Moore et al. 2008; Rakshi et al. 1999). Previous investigations in PD patients suggest that increased effective dopamine turnover in the striatum may be a more sensitive indicator of disease involvement than the uptake rate constant (Sossi et al. 2002). Therefore, increased dopamine turnover in this anterior cingulate cortex may also be an early sign of pathologic involvement.
Functional imaging studies have suggested that the supracallosal anterior cingulate cortex and dorsolateral prefrontal cortex participate in tasks that require cognitive control. Cognitive control “generally refers to a resource-limited system that guides voluntary, complex actions” and modification of habitual responses (MacDonald et al. 2000); within this system, the anterior cingulate cortex seems to be specifically involved in performance monitoring (Paus 2001). Lesions of the anterior cingulate and adjacent cortical regions cause apathy, amotivation, and akinetic mutism; apathy and amotivation are problematic disease symptoms in many Parkinson’s patients (Jordan et al. 2013). Therefore, increased eDVR may signify early changes in catecholamine synthesis and storage within limbic cortex that impact behavioral regulation.
This study is one of the first to measure effective dopamine turnover in limbic cortex in humans. The primary limitation of this study is the lack of a non-demented, non-PD, healthy elderly control sample. Another possible limitation is that some of the cognitive instruments used (or, the composite scores themselves) did not have the sensitivity required to detect behavior change or relationships with eDVR. Based on previous work in non-human primates (Doudet et al. 1997), we believe that the use of tolcapone improved the estimate of eDVR by reducing the COMT metabolite fraction; however, this has yet to be validated in humans. The single dose of tolcapone used in the protocol posed little risk for subjects (Gallagher et al. 2011a, b); however, we cannot recommend adoption of this procedure at other centers without a more systematic investigation of its effects on eDVR. Such an investigation would require acquisition of FDOPA PET in both PD and age-matched healthy subjects with and without tolcapone and would include arterial metabolite sampling.
This study presents new data suggesting that effective dopamine turnover can be measured in extra-striatal tissues in humans, and that anterior cingulate dopamine turnover is related to behavior in PD patients. Further work will need to be done to investigate whether this relationship is disease-specific, or might also be present in a healthy control sample.
References
Athey, R. J., & Walker, R. W. (2006). Demonstration of cognitive decline in Parkinson's disease using the Cambridge Cognitive Assessment (Revised) (CAMCOG-R). International Journal of Geriatric Psychiatry, 21(10), 977–982.
Benedict, R. H., Schretlen, D., Groninger, L., & Brandt, J. (1998). Hopkins Verbal Learning Test—Revised: Normative data and analysis of inter-form and test–retest reliability. Clinical Neuropsychologist, 12, 43–55.
Benton, A., Hamsher, D., & Sivan, A. (1983). Multilingual Aphasia Examination (3rd ed.). Iowa City: AJA Associates.
Bozeat, S., Gregory, C. A., Ralph, M. A., & Hodges, J. R. (2000). Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? Journal of Neurology, Neurosurgery, and Psychiatry, 69(2), 178–186.
Braak, H., Braak, E., Yilmazer, D., Schultz, C., de Vos, R. A., & Jansen, E. N. (1995). Nigral and extranigral pathology in Parkinson's disease. Journal of Neural Transmission, Supplement, 46, 15–31.
Brown, W. D., Taylor, M. D., Roberts, A. D., Oakes, T. R., Schueller, M. J., Holden, J. E., et al. (1999). FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology, 53(6), 1212–1218.
Bruck, A., Aalto, S., Nurmi, E., Bergman, J., & Rinne, J. O. (2005). Cortical 6-[18F]fluoro-L-dopa uptake and frontal cognitive functions in early Parkinson's disease. Neurobiology of Aging, 26(6), 891–898.
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980.
Doudet, D. J., Chan, G. L., Holden, J. E., Morrison, K. S., Wyatt, R. J., & Ruth, T. J. (1997). Effects of catechol-O-methyltransferase inhibition on the rates of uptake and reversibility of 6-fluoro-L-Dopa trapping in MPTP-induced parkinsonism in monkeys. Neuropharmacology, 36(3), 363–371.
Doudet, D. J., Chan, G. L., Holden, J. E., McGeer, E. G., Aigner, T. A., Wyatt, R. J., et al. (1998). 6-[18F]Fluoro-L-DOPA PET studies of the turnover of dopamine in MPTP-induced parkinsonism in monkeys. Synapse, 29(3), 225–232.
Dubois, B., Burn, D., Goetz, C., Aarsland, D., Brown, R. G., Broe, G. A., et al. (2007). Diagnostic procedures for Parkinson’s Disease dementia: recommendations for the Movement Disorder Society Task Force. Movement Disorders, 22(16), 2314–2324.
Firnau, G., Sood, S., Chirakal, R., Nahmias, C., & Garnett, E. S. (1988). Metabolites of 6-[18 F]fluoro-L-dopa in human blood. Journal of Nuclear Medicine, 29(3), 363–369.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198.
Gallagher, C., Oakes, T., Johnson, S., Chung, M., Holden, J., Bendlin, B., et al. (2011a). Rate of 6-[18 F]fluoro-L-dopa (FDOPA) uptake decline in striatal subregions in Parkinson’s disease. Movement Disorders, 4(26), 614–620.
Gallagher, C. L., Christian, B. T., Holden, J. E., Dejesus, O. T., Nickles, R. J., Buyan-Dent, L., et al. (2011b). A within-subject comparison of 6-[18 F]fluoro-m-tyrosine and 6-[18 F]fluoro-L-dopa in Parkinson's disease. Movement Disorders, 26(11), 2032–2038.
Gallagher, C. L., Bell, B., Bendlin, B., Palotti, M., Okonkwo, O., Sodhi, A., et al. (2013). White matter microstructural integrity and executive function in Parkinson's disease. Journal of the International Neuropsychological Society, 19(3), 349–354.
Golden, C. J. (1976). Identification of brain disorders by the Stroop Color and Word Test. Journal of Clinical Psychology, 32(3), 654–658.
Hoehn, M. M., & Yahr, M. D. (1967). Parkinsonism: onset, progression and mortality. Neurology, 17(5), 427–442.
Holden, J. E., Doudet, D., Endres, C. J., Chan, G. L., Morrison, K. S., Vingerhoets, F. J., et al. (1997). Graphical analysis of 6-fluoro-L-dopa trapping: effect of inhibition of catechol-O-methyltransferase. Journal of Nuclear Medicine, 38(10), 1568–1574.
Holden, J. E., Gallagher, C. L., Christian, B. T., & Sossi, V. (2010). COMT inhibition allows 6-[18 F]Fluoro-L-DOPA turnover assessment with reference tissue input functions in Parkinson Disease. NeuroImage, 52(Supplement 1), S224–S224.
Isquith, P., Roth, R., & Gioia, G. (2006). Behavior rating inventory of executive function - Adult Version. PAR, Inc.
Jordan, L. L., Zahodne, L. B., Okun, M. S., & Bowers, D. (2013). Hedonic and behavioral deficits associated with apathy in Parkinson's disease: potential treatment implications. Movement Disorders, 28(9), 1301–1304.
Kaasinen, V., Nurmi, E., Bruck, A., Eskola, O., Bergman, J., Solin, O., & Rinne, J. O. (2001). Increased frontal [18 F]fluorordopa uptake in early Parkinson’s disease: sex differences in the prefrontal cortex. Brain, 124, 1125–1130.
Kaplan, E., Goodglass, H., & Weintraub, S. (1983). Boston naming test. Philadelphia: Lee and Febiger.
Kehagia, A. A., Barker, R. A., & Robbins, T. W. (2010). Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurology, 9, 1200–1213.
Li, C. T., Palotti, M., Holden, J. E., Oh, J., Okonkwo, O., Christian, B. T., et al. (2014). A dual-tracer study of extrastriatal 6-[18 F]fluoro-m-tyrosine and 6-[18 F]-fluoro-L-dopa uptake in Parkinson's disease. Synapse, 68(8), 325–331.
MacDonald, A. W., 3rd, Cohen, J. D., Stenger, V. A., & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838.
Meyer-Lindenberg, A., Kohn, P. D., Kolachana, B., Kippenhan, S., McInerney-Leo, A., Nussbaum, R., et al. (2005). Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nature Neuroscience, 8(5), 594–596.
Moore, R. Y., Whone, A. L., McGowan, S., & Brooks, D. J. (2003). Monoamine neuron innervation of the normal human brain: an 18 F-DOPA PET study. Brain Research, 982(2), 137–145.
Moore, R. Y., Whone, A. L., & Brooks, D. J. (2008). Extrastriatal monoamine neuron function in Parkinson's disease: an 18 F-dopa PET study. Neurobiology of Disease, 29(3), 381–390.
Morrison, C. E., Borod, J. C., Brin, M. F., Raskin, S. A., Germano, I. M., Weisz, D. J., et al. (2000). A program for neuropsychological investigation of deep brain stimulation (PNIDBS) in movement disorder patients: development, feasibility, and preliminary data. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 13(3), 204–219.
Muslimovic, D., Post, B., Speelman, J. D., & Schmand, B. (2005). Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology, 65(8), 1239–1245.
Paolo, A. M., Axelrod, B. N., Troster, A. I., Blackwell, K. T., & Koller, W. C. (1996). Utility of a Wisconsin card sorting test short form in persons with Alzheimer's and Parkinson's disease. Journal of Clinical and Experimental Neuropsychology, 18(6), 892–897.
Patlak, C. S., & Blasberg, R. G. (1985). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. Journal of Cerebral Blood Flow and Metabolism, 5(4), 584–590.
Patlak, C. S., Blasberg, R. G., & Fenstermacher, J. D. (1983). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Journal of Cerebral Blood Flow and Metabolism, 3(1), 1–7.
Paus, T. (2001). Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews Neuroscience, 2(6), 417–424.
Rabin, L. A., Roth, R. M., Isquith, P. K., Wishart, H. A., Nutter-Upham, K. E., Pare, N., et al. (2006). Self- and informant reports of executive function on the BRIEF-A in MCI and older adults with cognitive complaints. Archives of Clinical Neuropsychology, 21(7), 721–32.
Rakshi, J. S., Uema, T., Ito, K., Bailey, D. L., Morrish, P. K., Ashburner, J., et al. (1999). Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease A 3D [(18)F]dopa-PET study. Brain, 122(Pt 9), 1637–1650.
Reitan, R. (1992). Trail Making Test. Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychological Laboratory.
Rinne, J. O., Portin, R., Ruottinen, H., Nurmi, E., Bergman, J., Haaparanta, M., & Solin, O. (2000). Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Archives of Neurology, 57(4), 470–475.
Robert, G., Le Jeune, F., Lozachmeur, C., Drapier, S., Dondaine, T., Peron, J., et al. (2012). Apathy in patients with Parkinson disease without dementia or depression. Neurology, 79, 1155–1160.
Sager, M. A., Hermann, B., & La Rue, A. (2005). Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. Journal of Geriatric Psychiatry and Neurology, 18(4), 245–249.
Scatton, B., Javoy-Agid, F., Rouquier, L., Dubois, B., & Agid, Y. (1983). Reduction of Cortical Dopamine, Noradrenaline, Serotonin, and Their Metabolites in Parkinson’s Disease. Brain Research, 275, 321–328.
Sossi, V., Doudet, D. J., & Holden, J. E. (2001). A reversible tracer analysis approach to the study of effective dopamine turnover. Journal of Cerebral Blood Flow and Metabolism, 21(4), 469–476.
Sossi, V., de La Fuente-Fernandez, R., Holden, J. E., Doudet, D. J., McKenzie, J., Stoessl, A. J., et al. (2002). Increase in dopamine turnover occurs early in Parkinson's disease: evidence from a new modeling approach to PET 18 F-fluorodopa data. Journal of Cerebral Blood Flow and Metabolism, 22(2), 232–239.
Tierney, M. C., Szalai, J. P., Snow, W. G., & Fisher, R. H. (1996). The prediction of Alzheimer disease. The role of patient and informant perceptions of cognitive deficits. Archives of Neurology, 53(5), 423–427.
Wedderburn, C., Wear, H., Brown, J., Mason, S. J., Barker, R. A., Hodges, J., et al. (2008). The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. Journal of Neurology, Neurosurgery, and Psychiatry, 79(5), 500–503.
Wu, K., O’Keeffe, D., Politis, M., O’Keeffe, G. C., Robbins, T. W., Bose, S. K., et al. (2012). The catechol-O-methyltransferase Val158Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson’s disease: a PET study. Brain, 135, 2449–2457.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development Service. It was also funded by the University of Wisconsin Institute for Clinical and Translational Research, through a National Institutes of Health Clinical and Translational Science Award, [grant number 1UL1RR025011]. This work was supported with use of facilities at the William S. Middleton Memorial Veterans Hospital Geriatric Research Education and Clinical Center and the Waisman Laboratory for Brain Imaging and Behavior, Madison, WI, USA.
Disclosures
Authors Gallagher, Bell, Palotti, Oh, Christian, Okonkwo, Sojkova, Buyan-Dent, Nickles, Harding, Stone, Johnson, and Holden declare no conflicts of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of this investigation. Informed consent was obtained from all study participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallagher, C.L., Bell, B., Palotti, M. et al. Anterior cingulate dopamine turnover and behavior change in Parkinson’s disease. Brain Imaging and Behavior 9, 821–827 (2015). https://doi.org/10.1007/s11682-014-9338-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-014-9338-4
Keywords
- Adult
- Aged
- Humans
- Brain mapping
- Cerebral cortex/metabolism/radionuclide imaging
- Dopamine agents/*diagnostic use/pharmacokinetics
- Dihydroxyphenylalanine/*analogs & derivatives/drug effects/pharmacokinetics
- Positron emission tomography
- Parkinson disease/*physiopathology/*radionuclide imaging
- Research support/U.S. Gov’t/P.H.S.