Abstract
Reducing greenhouse gas emissions is one of the major challenges in combating global warming. Carbon, including in the form of carbon dioxide (CO2), is considered an essential greenhouse gas under human control to demonstrate success in emission reductions. However, many carbon stock quantifications in forest ecosystems still rely on the estimated 50% carbon content instead of more precise species-, tissue- and site-specific values. Thus, this study aimed to thoroughly measure and analyze the carbon content and variability using the 14 major tree species in Northeast China. Over 600 trees were destructively sampled from three different major mountainous regions (i.e., the Changbai, Daxing’an, and Xiaoxing’an mountains), and the carbon contents of each species were precisely measured to the sub-tissue level. Carbon contents varied significantly between species, with foliage carbon mostly found to be the highest, while root carbon contents were the lowest. Average carbon contents can be ranked as: Ulmus laciniata (43.4%) < Phellodendron amurense (43.5%) < Acer mono (43.8%) < Tilia amurensis (44.2%) < Populus davidiana (44.5%) < Fraxinus mandshurica (44.7%) < Juglans mandshurica (44.9%) < Quercus mongolica (45.3%) < Betulla davurica (45.8%) < Betulla platyphylla (46.7%) < Picea koreansis (46.9%) < Larix gmelinii (47.4%) < Pinus koreansis (48.3%) < Abies nephrolepis (48.3%). Carbon contents were higher in conifers (47.7%) compared to broadleaf species (44.9%). In addition, both tree tissues and growing sites also had a significant effect on carbon content. At the sub-tissue level, only stem’s sub-tissues (i.e., bark, heartwood, and sapwood) carbon contents showed significant variations. The results suggest that bark should be separated from other stem sub-tissues and considered separately when determining carbon stocks. This research contributes to improving estimates of terrestrial carbon quantifications, and in particular, the values obtained can be used in China’s National Forest Inventory.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing atmospheric carbon dioxide (CO2) concentrations has a relatively high contribution to global warming (IPCC 2014). Forest ecosystems have the ability to reduce atmospheric CO2 by sequestering carbon in biomass which plays a crucial role in stabilizing the global climate system (Dixon et al. 1994). The United Nations Food and Agricultural Organization (FAO) has estimated that global forests store approximately 296 Gt of carbon in aboveground and belowground biomass, comprising nearly half of a forest’s total carbon stock (FAO 2015). A forest’s capacity to sequester carbon is greater compared with other biomes since the carbon stored within woody tissues is well protected from respiratory release and decomposition (Keenan and Williams 2018), while carbon stored in nonwoody tissues (i.e., root hairs and foliage) has a more rapid turnover. Thus, forests have a significant role in the world’s terrestrial carbon sink (Keenan and Williams 2018), and their biomass has been identified by the Intergovernmental Panel on Climate Change (IPCC) as one of the most promising forms to lessen atmospheric CO2 (IPCC 2007).
Carbon is among the most plentiful elements in living organisms (Dietze et al. 2014) and is converted from its gaseous form into biological compounds through photosynthesis, contributing to basic metabolic functions and structure of plants (Dietze et al. 2014; Martínez-Vilalta et al. 2016). The carbon stored from this process is determined by multiplying the plant’s dry weight with a carbon conversion factor, the most commonly used factor is 50% for almost all of carbon stock estimations, i.e., for local, regional and global carbon appraisals (Nizami 2012; Guerra-Santos et al. 2014; Tang et al. 2018). The 50% carbon conversion factor was originally determined from the average molecular formula, CH1.44O0.66, consisting of basic constituents such as 50% carbon, 44% oxygen, 6% hydrogen, and trace quantities of metal ions in live woody tissues (Pettersen 1984; Bert and Danjon 2006; Ma et al. 2018). This particular value has long been recommended as a generic carbon conversion factor by the IPCC to simplify the measurement process and to foster carbon stock quantifications worldwide (Houghton et al. 1990). However, its accuracy is uncertain (Zhang et al. 2009; Jones and O’Hara 2012; Widagdo et al. 2020a).
To-date, various international programs and mechanisms, such as the Reducing Emissions from Deforestation and Forest Degradation (REDD) program under the United Nations Framework Convention on Climate Change (UNFCCC), have been established to financially reward projects and countries that contribute to preventing carbon loss from forests (Ebeling and Yasué 2008; Mukama et al. 2012). To support these types of activities, forest carbon stock has to be accurately estimated in order to quantify the carbon benefits and compensation payments (Vieilledent et al. 2012). Moreover, a number of studies have reported that carbon concentrations vary within taxonomic groups (i.e., angiosperms and conifers; Thomas and Martin 2012; Martin et al. 2015; Ma et al. 2018), species (Pompa-García et al. 2017; Gillerot et al. 2018; Rodríguez-Soalleiro et al. 2018), origins (Elias and Potvin 2003; Widagdo et al. 2020b), provenances (Wang et al. 2015; Ying et al. 2019), growing sites (Ying et al. 2019; Widagdo et al. 2020a), tissues (Kim et al. 2017; Zhou et al. 2019; Dong et al. 2020), and even stem’s sub-tissues such as bark, heartwood, and sapwood (Lamlom and Savidge 2006; Castaño-Santamaría and Bravo 2012; Gao et al. 2016). Thus, investigation of variability in carbon content needs to be continued in order to improve the precision of carbon stock appraisals.
At the global scale, temperate mixed forests are largely situated in eastern Asia, northern North America, and northeastern Europe. In China, they are located in the northeast and are crucial for the nation’s carbon budgeting and climatic system (Wang 2006; State Forestry and Grassland Administration 2019). There are 14 major tree species which are mainly found in these temperate forests, namely: Manchurian Elm (Ulmus laciniata), Amur cork (Phellodendron amurense), Mongolian oak (Quercus mongolica), Manchurian walnut (Juglans mandshurica), Manchurian ash (Fraxinus mandshurica), Amur linden (Tilia amurensis), Maple (Acer mono), Dahurian poplar (Populus davidiana), Dahurian birch (Betula davurica), white birch (Betula platyphylla), Korean spruce (Picea koreansis), larch (Larix gmelinii), Korean pine (Pinus koreansis), and Khingan fir (Abies nephrolepis). This study aimed to: (1) accurately measure carbon contents of the 14 species to the sub-tissue level; and, (2) analyze both inter- and intra-specific carbon content variabilities, one of the key steps in forest carbon assessment.
Materials and methods
Study site
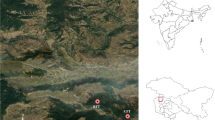
The data were collected from several natural secondary forests within the three major mountainous regions in northeast China (Fig. 1), the Changbai Mountains (CBM), the Daxing’an Mountains (DXM), and the Xiaoxing’an Mountains (XXM). These areas have a continental monsoon climate, and based on the Chinese soil taxonomic system, the soil type is dark brown forest soil (Haplumbrepts or Eutroboralfs). According to the Köppen-Geiger climate classification system, these regions are Dwa, Dwb, and Dwc, indicating dry winters with hot, warm, or cold summers, respectively (Beck et al. 2018). The elevations of these three mountainous regions are highly varied from 300 to 1500 ma.s.l., as well as mean annual temperatures (−4 to 6 °C) and mean annual rainfalls (500–800 mm). In this study, the natural secondary forests were the result of cutovers, and left to be naturally regenerated (Yu et al. 2011). To date, the area of secondary forests in China are managed by the Provincial Forestry and Grassland Unit, which comes under the supervision of the National Forestry and Grassland Department. The dominant species found within these forests are aspen (P. davidiana Dode.), Mongolian oak (Q. mongolica), white birch (Betula platyphylla), and Dahurian birch (Betula davurica).
Field sampling and sample preparation
Six hundred and three trees of the 14 most common broadleaf and conifer species were destructively sampled from 18 widespread sites. All had diameters at breast height (DBH) and tree total height (H) of 3.4–47.0 cm and 3.6–27.0 m, respectively. Only healthy trees with a straight bole without visual damage (i.e., major branch loss, fungal infections, or stem abrasions) were selected. In addition, each of the four primary tissues were divided into three sub-tissues in order to thoroughly analyze carbon concentration variability. Belowground biomass (roots) was separated based on its diameter (d); namely, RS—small (d < 2 cm), RM—medium (2 < d < 5 cm), and RL—large (d > 5 cm). Branches and foliage were classified according to their position within the crown; namely, lower (BL and FL), middle (BM and FM), and upper (BU and FU). Stems were divided based on three distinct layers; namely, heartwood (SH), sapwood (SS), and bark (SB). Heartwood and sapwood were differentiated according to Lachenbruch et al. (2011) in which the former is related to samples extracted from the radial position close to the pith, while the latter represents samples taken from the radial position close to the bark.
Basic inventory data were collected before felling, e.g., DBH, H, and length and width of crowns. Once felled, the aboveground section was separated into stem and crown at the point of the first live branch. The stem was then partitioned into one-meter sections and a 2-cm thick disc was taken from each portion. The crown section was divided equally into lower, middle, and upper layers, and 3–5 fresh branches and foliage samples were taken from each layer. All samples were carefully chosen, extracting only lignified green branches as samples. Roots were excavated within a 3-m circle. Apart from fine roots (<2 mm), approximately 100–200 g of each root class was sampled, weighed, recorded, and transferred to the laboratory. DBH, H and carbon concentrations for all samples are shown in Fig. 2.
Species-specific carbon concentrations for the 14 species; abbreviations on the left refer to species and numbers on the right indicate the total number of samples for each species. D DBH, H total height, ULC Ulmus laciniata, PAR Phellodendron amurense, AMN Acer mono, TAS Tilia amurensis, PDV Populus davidiana, FMS Fraxinus mandshurica, JMS Juglans mandshurica, QMC Quercus mongolica, BDV Betulla davurica, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, PKS Pinus koreansis, ANP Abies nephrolepis
Carbon concentration measurements
All samples were oven dried at 80 °C to constant weight. Each of the ±5 mg finely ground samples were put into a container for further weighing using an MS304S Mettler Toledo analytical balance (accuracy to 0.0001 g). Prior to measurements, all containers were washed with distilled water along with analytical grade acetone, vacuum desiccated overnight until dry (Lamlom and Savidge 2003). For determining carbon concentrations, each sample was burned at 1200 °C in a vial containing pure oxygen. The carbon concentration was then determined via a non-dispersion infrared ray (NDIR) analyzer (Multi N/C 3000 analyzer with 1500 Solids Module; Analytik Jena AG, Germany). The analyzer was calibrated and stabilized between runs using a 12% standard concentration of CaCO3 (standard curve, r2 > 99.99%), in which standards were included after every 15–20 samples to ensure that the readings were reliable. Volatile carbon was not measured by this method; thus, there might be a possible loss in the volatile carbon fraction during the measurements (Thomas and Malczewski 2007).
Statistical analysis
To assess the carbon content’s inter- and intra-species variability, PROC GLM was used with the statistical analysis software SAS version 9.3. The effect of seven variables on carbon concentration were determined; namely, DBH, H, growing regions (CBM, DXM, and XXM), taxonomic group (conifers and broadleaves), species, tissues and sub-tissues. Some variables are inherently nested within others, such as species within a taxonomic group and sub-tissues within a tissue. Thus, a partially nested analysis of variance (ANOVA) through a general linear model was used. The interaction of species and growing regions was intentionally excluded since not all species were present in all regions. To further analyze the effect of region and tree tissues on carbon contents within each species, a least significance difference (LSD) test was conducted.
Results
Overall variations in carbon concentrations
Apart from DBH and H, all variables (i.e., taxonomic group, species, region, and tissues) had significant variations in carbon contents (p < 0.05, Table 1). This indicates that, at least in our dataset, the differences in DBH and H did not significantly affect carbon contents. Average (±SE) carbon concentration across all taxonomic groups, species, regions, tissues, and sub-tissues was 45.7 ± 0.04%. A mean scatter diagram of the multiple comparison test (using the Tukey-Kramer adjustment method) was used to visualize the effect of the three growing regions (CBM, XXM, and DXM) and the four primary tissues—roots, stem, branches, and foliage, on carbon concentration values. The results show that both growing sites and tissue types significantly affect carbon concentrations (Fig. 3).
Inter-species variation in carbon concentration
In this research, carbon concentrations of the 14 species were measured to the sub-tissue level. Carbon concentration values for each species over nine sub-tissues are shown in Table 2 and varied significantly across species and sub-tissues (Tables 1 and 2), ranging from 39.6% within the bark of U. laciniata to 50.9% within the upper foliage of A. nephrolepis. Among all species, U. laciniata and A. nephrolepis consistently exhibited the lowest and highest average carbon concentration values over the nine sub-tissues at 43.4% and 48.3%, respectively. Mean carbon contents followed U. laciniata < P. amurense < A. mono < T. amurensis < P. davidiana < F. mandshurica < J. mandshurica < Q. mongolica < B. davurica < B. platyphylla < P. koreansis < L. gmelinii < P. koreansis < A. nephrolepis. This clearly shows that all conifer species had higher mean carbon contents than the 10 broadleaf species. Overall, carbon contents for all conifers was higher compared to those of broadleaf species across all sub-tissues (Table 2).
Carbon concentrations varied significantly among the nine sub-tissues (p < 0.05; Table 1). Table 3 presents the mean comparison (p value) of the nine sub-tissues across all species. The results show that most of variations were driven by the significant difference between the pairing comparisons of the sub-tissue across different tissues. The differences within the same tissue were insignificant for roots, branches, and foliage (Table 3). A significant difference within the same tissue was only found within the stem, in which the bark carbon content differed significantly from both sapwood and heartwood. Overall, carbon concentrations of the nine sub-tissues across the 14 species can be ranked as RS < RM < RL < SS < SH < BL < BM < BU < SB < FL < FM < FU (Table 2). The abbreviations are defined in Table 3.
Intra-species variation in carbon concentrations
Carbon concentrations differed significantly between the biomass tissues across all species (F-values = 129.16, p < 0.0001). Carbon concentrations of all species are as follows: foliage (46.7%) > branch (45.7%) > stem (45.6%) > root (44.7%). Both foliage and roots consistently showed the highest and lowest carbon concentrations for all four conifers (48.9% and 46.9%) and all ten broadleaf (45.9% and 43.9%) species, respectively. Average carbon content values for all species varied from 42.5% for roots of P. amurense to 50.7% for the foliage of A. nephrolepis. Apart from U. laciniata, the lowest carbon concentrations were always found in the roots, ranging from 42.5% to 47.6% (Fig. 4). The tissue with the highest carbon concentrations varied according to the species, in which foliage generally had the highest carbon content (9 out of 14 species, Fig. 4). The highest carbon concentrations for U. laciniata, F. mandshurica, P. koreansis, and P. koreansis were in the branches, while for P. amurense, it was within the stem. However, for four of these five species (excluding P. koraensis), most of the tissues with the highest carbon concentrations only had a significant difference with the roots (Fig. 4).
Comparisons of both species- and tissue-specific carbon concentrations for the 14 species; bars denote the standard error, letters represent the groups’ significant difference according to LSD test at 5% significance level. ULC Ulmus laciniata, PAR Phellodendron amurense, AMN Acer mono, TAS Tilia amurensis, PDV Populus davidiana, FMS Fraxinus mandshurica, JMS Juglans mandshurica, QMC Quercus mongolica, BDV Betulla davurica, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, PKS Pinus koreansis, ANP Abies nephrolepis
Between the stem’s sub-tissues across all species, the average carbon content of the bark is the highest (46.1%), sapwood the lowest (45.3%), and heartwood slightly higher (45.4%). The difference in carbon contents between the stem sub-tissues varied with species (Fig. 5). The difference between heartwood and sapwood carbon concentrations was insignificant for all species. Carbon contents of the bark were significantly different from either heartwood or sapwood, or even both, for seven of the 14 species. Among these, bark had the lowest carbon concentrations for U. laciniata, A. mono, and Q. mongolica, whereas the highest was found in B. davurica, B. platyphylla, L. gmelinii, and P. koreansis (Fig. 5). There was no significant difference in carbon content of lower, middle, and upper canopy positions for both branches and foliage, as well as among small, medium, and large roots (Table 3).
Comparisons of the stem tissue carbon concentration for the 14 species; bars denote the standard error, letters represent the groups’ significant difference according to LSD test at 5% significance level. ULC Ulmus laciniata, PAR Phellodendron amurense, AMN Acer mono, TAS Tilia amurensis, PDV Populus davidiana, FMS Fraxinus mandshurica, JMS Juglans mandshurica, QMC Quercus mongolica, BDV Betulla davurica, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, PKS Pinus koreansis, ANP Abies nephrolepis
The effects of the three sites on carbon concentrations were also analyzed for each species (Fig. 6). Four species were present on all three sites (CBM, DXM, and XXM), seven species on two sites (CBM and XXM), and the balance from one site (CBM). For eight of the 11 species sampled from either three or two sites, ANOVA revealed a significant difference between these regions (Fig. 6). Apart from T. amurensis, all species sampled from the CBM range consistently had the lowest carbon concentrations, ranging from 43.8% for U. laciniata to 47.8% for A. nephrolepis. The highest carbon concentrations varied with species, in which eight of 11 species were from the XXM range (Fig. 6). B. platyphylla and P. koreansis carbon concentrations were highest in the DXM, while T. amurensis carbon was highest in the CBM. Average carbon content of all species from the XXM site is the highest (47.0%) along with the DXM (47.0%), and the lowest in the CBM site (44.7%).
Comparisons of carbon concentrations on the three sites for the 14 species; bars denote standard error, letters represent the groups’ significant difference according to LSD test at 5% significance level. PDV Populus davidiana, BPL Betulla platyphylla, PCK Picea koreansis, LGM Larix gmelinii, ANP Abies nephrolepis, BDV Betulla davurica, FMS Fraxinus mandshurica, JMS Juglans mandshurica, QMC Quercus mongolica, TAS Tilia amurensis, ULC Ulmus laciniata
Discussion
Chemical traits of both foliage and roots have received considerable attention in comparative studies of ecosystem function and ecology (Reich et al. 1997; Wright et al. 2004; Tsunoda and van Dam 2017). However, many measurements of carbon contents are only on stems or boles (Martin and Thomas 2011; Castaño-Santamaría and Bravo 2012; Azeem et al. 2019). This is perhaps because woody stems account for the largest biomass component in a tree (Laiho and Laine 1997) and net primary production at the stand level (Gower et al. 2001; Zhang et al. 2009). Hence, tree stems are regarded as a major carbon sinks contributing to mitigating the effects of climate change. Our results show that the average stem carbon concentrations of the 14 species (45.6%) was less than the 50% generic conversion factor. Even after calculating average stem carbon contents based on taxonomic group (47.0% for conifers and 45.0% for broadleaf species), the values are still below the widely used carbon conversion factor. It is worth noting that the oven-dried method adopted in this study might result in a loss in the volatile carbon fraction, possibly contributing to underestimating carbon contents. Previous studies show that volatile losses vary among tree species and oven temperatures. Lamlom and Savidge (2003), Thomas and Malczewski (2007), and Martin and Thomas (2011) demonstrated that volatile carbon loses were ~ 2.0%, ~2.2% and ~ 2.5% when samples were oven-dried at 93 °C, 105 °C, and 110 °C, respectively, for 14 temperate species in China, 41 temperate species in Canada, and 59 tropical species in Panama. If the carbon losses occur linearly with oven temperature, the carbon contents obtained in this study would have been 1 or 2% higher if the kiln- or freeze-dried method was used instead (oven temperature in this study was 80 °C). However, a direct cross-study comparison is difficult and unreliable due to discrepancies in species, methods, sample treatments, and laboratory equipment. All samples were oven-dried to the same 80 °C temperature, and thus volatile losses could be considered as systematic and common to all data.
Several studies have reported that the largest biomass (≈60%) within an individual tree are found in the stems (Zhu et al. 2013; Ozdemir et al. 2019; Widagdo et al. 2020b). However, to precisely measure the carbon stock of an individual tree, a measure of each tree tissue’s carbon content is needed since type of tissue significantly affects carbon concentrations (Table 1, Figs. 3 and 4). This result also corroborates the work of Rodríguez-Soalleiro et al. (2018), Zhou et al. (2019), and Dong et al. (2020). In this study, root carbon concentrations across all species (44.7%) was always the lowest compared to other tree tissues, while foliage had the highest carbon concentrations (46.7%), in line with previous studies (Zhang et al. 2009; Ma et al. 2018). Variations between sub-tissues were also analyzed. The stem’s sub-tissues (bark, heartwood, and sapwood) carbon contents varied significantly, while differences between root’s, branch’s, and foliage’s sub-tissues were insignificant (Table 3). Research by Bert and Danjon (2006) has shown that some changes in carbon concentration were related with the size of the tree tissue or its position within the tree. However, different results were obtained in this study; diameter and height did not have any significant effect on the carbon concentrations. Moreover, Table 3 shows that root size and both branch and foliage position within the crown did not have any significant effect on carbon contents. These discrepancies might be related to environmental variations and experimental differences.
Tree bark has long been recognized to have chemical divergences from other tree tissues (Srivastava 1964; Vidensek et al. 1990). In spite of several general chemical constituents (polyphenols, fats, sterols, hemicellulose, cellulose), bark has also developed unique polymeric materials (Bert and Danjon 2006) and contains half of the cellulose of the trunk (Labosky 1979; Vázquez et al. 1987). In this study, bark carbon concentrations were always higher than that of the heartwood and sapwood for all conifers. Among the 10 broadleaf species, bark carbon concentrations of six species (U. laciniata, Q. monglica, J. mandshurica, F. mandshurica, T. amurensis, and A. mono) were the lowest compared to the other two sub-tissues. This is consistent with the literature which showed that greater carbon contents in the bark might be caused by higher levels of tannins, suberin, lignin, and extractives (Hergert 1960; Porter 1974; Bert and Danjon 2006), particularly for conifer species (Srivastava 1964; Vidensek et al. 1990; Nemli et al. 2006). From an ecological perspective, this pattern is related with bark functions in controlling water deficiency and protecting the tree from fire (Hengst and Dawson 1994) and insects (Franceschi et al. 2005). The current study also noted that the difference between carbon contents of the heartwood and sapwood were insignificant for all species (Fig. 5).
In the present research, average carbon concentrations of the four conifers were higher than those of the 10 broadleaf species, both on average (47.7% cf. 44.9%) and separately across the roots (46.9% cf. 43.8%), stems (47.0% cf. 45.0%), branches (47.8% cf. 44.9%), and foliage (48.9% cf. 45.8%). The reasons for this is because conifers have approximately 10% higher lignin content than broadleaf species (Savidge 2000). Of all the macromolecules within a tree’s woody tissues, lignin has the highest carbon percent (Savidge 2000; Lamlom and Savidge 2003). We also found that the carbon concentrations varied across the species and the growing regions, as confirmed by a number of studies (Elias and Potvin 2003; Azeem et al. 2019; Ying et al. 2019). Kozlowski (1992) reported that individual trees with different growth and metabolism characteristics had various differences in carbon compounds; hence, intra- and inter-specific variations in carbon concentrations would be influenced by silvicultural practices, stand characteristics (i.e., age of tree and position within the crown), and growing conditions. Using species-, tissue-, and site-specific carbon content instead of the common 50% conversion factor will provide more accurate results on terrestrial carbon stock estimations, as has been demonstrated by previous studies (Zhang et al. 2009; Martin and Thomas 2011; Dong et al. 2016; Widagdo et al. 2020a). Thus, the continuity of research on carbon contents of species, tissues of species (stem, branches, roots, foliage), and regional-specific carbon contents needs to continue in order to increase the accuracy of carbon stock estimations. The data obtained in this research are useful for accurate carbon stock estimates of the following species, particularly for those in northeastern China: U. laciniata, P. amurense, Q. mongolica, J. mandshurica, F. mandshurica, T. amurensis, A. mono, P. davidiana, B. davurica, B. platyphylla, P. koreansis, L. gmelinii, P. koreansis, and A. nephrolepis.
Conclusions
This research highlights the importance of considering intra- and inter-species variation in carbon contents, which has broad impacts for increasing the accuracy of global carbon quantifications. Carbon contents varied significantly across the growing regions, taxonomic groups, species, and tissues. Both DBH and tree total height did not have any effect on carbon contents. Among the nine sub-tissues analyzed, carbon contents of the stem’s sub-tissues (bark, heartwood, and sapwood) differed significantly, while carbon contents of the root’s, branch’s, and foliage’s sub-tissues were insignificant. Based on these results, it is recommended that bark should be separated from heartwood and sapwood and considered separately when measuring carbon stock of an individual tree. More attention is required to improve the estimates of forest carbon inventories.
References
Azeem F, Ahmed B, Atif RM, Ali MA, Nadeem H, Hussain S, Rasul S, Manzoor H, Ahmad U, Afzal M (2019) Drought affects aquaporins gene expression in important pulse legume chickpea (Cicer arietinum L.). Pak J Bot 51(1):81–88. https://doi.org/10.30848/PJB2019
Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF (2018) Present and future köppen-geiger climate classification maps at 1-km resolution. Sci Data 5:180214. https://doi.org/10.1038/sdata.2018.214
Bert D, Danjon F (2006) Carbon concentration variations in the roots, stem and crown of mature Pinus pinaster (Ait.). For Ecol Manag 222:279–295. https://doi.org/10.1016/j.foreco.2005.10.030
Castaño-Santamaría J, Bravo F (2012) Variation in carbon concentration and basic density along stems of sessile oak (Quercus petraea (Matt.) Liebl.) and Pyrenean oak (Quercus pyrenaica Willd.) in the Cantabrian Range (NW Spain). Ann For Sci 69(6):663–672. https://doi.org/10.1007/s13595-012-0183-6
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65(1):667–687. https://doi.org/10.1146/annurev-arplant-050213-040054
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263(5144):185–190. https://doi.org/10.1126/science.263.5144.185
Dong L, Zhang L, Li F (2016) Allometry and partitioning of individual tree biomass and carbon of Abies nephrolepis Maxim in northeast China. Scand J For Res 31(4):399–411. https://doi.org/10.1080/02827581.2015.1060257
Dong L, Widagdo FRA, Xie L, Li F (2020) Biomass and volume modeling along with carbon concentration variations of short-rotation poplar plantations. Forests 11(7):780. https://doi.org/10.3390/f11070780
Ebeling J, Yasué M (2008) Generating carbon finance through avoided deforestation and its potential to create climatic, conservation and human development benefits. Philos Trans R Soc B 363(1498):1917–1924. https://doi.org/10.1098/rstb.2007.0029
Elias M, Potvin C (2003) Assessing inter- and intra-specific variation in trunk carbon concentration for 32 neotropical tree species. Can J For Res 33(6):1039–1045. https://doi.org/10.1139/x03-018
FAO (2015) Global Forest Resources Assessment 2015: how are the world’s forests changing? Food and Agriculture Organization of The United Nations, Rome, p 44
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167(2):353–376. https://doi.org/10.1111/j.1469-8137.2005.01436.x
Gao B, Taylor AR, Chen HYH, Wang J (2016) Variation in total and volatile carbon concentration among the major tree species of the boreal forest. For Ecol Manag 375:191–199. https://doi.org/10.1016/j.foreco.2016.05.041
Gillerot L, Vlaminck E, De Ryck DJR, Mwasaru DM, Beeckman H, Koedam N (2018) Inter- and intraspecific variation in mangrove carbon fraction and wood specific gravity in Gazi Bay, Kenya. Ecosphere 9(6):e02306 https://doi.org/10.1002/ecs2.2306
Gower ST, Krankina O, Olson RJ, Apps M, Linder S, Wang C (2001) Net primary production and carbon allocation patterns of boreal forest ecosystems. Ecol Appl 11(5):1395. https://doi.org/10.2307/3060928
Guerra-Santos JJ, Cerón-Bretón RM, Cerón-Bretón JG, Damián-Hernández DL, Sánchez-Junco RC, Carrió ECG (2014) Estimation of the carbon pool in soil and above-ground biomass within mangrove forests in Southeast Mexico using allometric equations. J For Res 25(1):129–134. https://doi.org/10.1007/s11676-014-0437-2
Hengst GE, Dawson JO (1994) Bark properties and fire resistance of selected tree species from the central hardwood region of North America. Can J For Res 24(4):688–696. https://doi.org/10.1139/x94-092
Hergert HL (1960) Chemical composition of tannins and polyphenols from conifer wood and bark. For Prod J 10(1):610–617
Houghton JT, Jenkins GJ, Ephraums JJ (1990) Climate change: The IPCC Scientific Assessment. Cambridge University Press, Cambridge, p 365
IPCC (2007) Climate Change 2007—Mitigation of Climate Change: Working Group III contribution to the Fourth Assessment Report of the IPCC. Cambridge University Press, Cambridge, p 851
IPCC (2014) Climate Change 2014—Synthesis Report: Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, p 151
Jones DA, O’Hara KL (2012) Carbon density in managed coast redwood stands: Implications for forest carbon estimation. Forestry 85(1):99–110. https://doi.org/10.1093/forestry/cpr063
Keenan TF, Williams CA (2018) The terrestrial carbon sink. Annu Rev Environ Resour 43(1):219–243. https://doi.org/10.1146/annurev-environ-102017-030204
Kim C, Yoo BO, Jung SY, Lee KS (2017) Allometric equations to assess biomass, carbon and nitrogen content of black pine and red pine trees in southern Korea. IForest 10(2):483–490. https://doi.org/10.3832/ifor2164-010
Kozlowski TT (1992) Carbohydrate sources and sinks in woody plants. The Bot Rev 58(2):107–222. https://doi.org/10.1007/BF02858600
Labosky PJ (1979) Chemical constituents of four Southern pine barks. Wood Sci 12(2):80–85
Lachenbruch B, Moore JR, Evans R (2011) Radial variation in wood structure and function in woody plants, and hypotheses for its occurrence. In: Meinzer FC et al (eds) Size- and age-related changes in tree structure and function, tree physiology, vol 4, pp 121–164. https://doi.org/10.1007/978-94-007-1242-3_5
Laiho R, Laine J (1997) Tree stand biomass and carbon content in an age sequence of drained pine mires in southern Finland. For Ecol Manag 93:161–169
Lamlom SH, Savidge RA (2003) A reassessment of carbon content in wood: variation within and between 41 North American species. Biomass Bioenergy 25(4):381–388. https://doi.org/10.1016/S0961-9534(03)00033-3
Lamlom SH, Savidge RA (2006) Carbon content variation in boles of mature sugar maple and giant sequoia. Tree Physiol 26(4):459–468. https://doi.org/10.1093/treephys/26.4.459
Ma S, He F, Tian D, Zou D, Yan Z, Yang Y, Zhou T, Huang K, Shen H, Fang J (2018) Variations and determinants of carbon content in plants: a global synthesis. Biogeosciences 15(3):693–702. https://doi.org/10.5194/bg-15-693-2018
Martin AR, Thomas SC (2011) A reassessment of carbon content in tropical trees. PLoS One 6(8):e23533. https://doi.org/10.1371/journal.pone.0023533
Martin AR, Gezahegn S, Thomas SC (2015) Variation in carbon and nitrogen concentration among major woody tissue types in temperate trees. Can J For Res 45(6):744–757. https://doi.org/10.1139/cjfr-2015-0024
Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecol Monogr 86(4):495–516. https://doi.org/10.1002/ecm.1231
Mukama K, Mustalahti I, Zahabu E (2012) Participatory forest carbon assessment and REDD+: learning from Tanzania. Int J For Res 2012:1–14. https://doi.org/10.1155/2012/126454
Nemli G, Gezer ED, Yildiz S, Temiz A, Aydin A (2006) Evaluation of the mechanical, physical properties and decay resistance of particleboard made from particles impregnated with Pinus brutia bark extractives. Bioresour Technol 97(16):2059–2064. https://doi.org/10.1016/j.biortech.2005.09.013
Nizami SM (2012) The inventory of the carbon stocks in sub tropical forests of Pakistan for reporting under Kyoto Protocol. J For Res 23(3):377–384. https://doi.org/10.1007/s11676-012-0273-1
Ozdemir E, Makineci E, Yilmaz E, Kumbasli M, Caliskan S, Beskardes V, Keten A, Zengin H, Yilmaz H (2019) Biomass estimation of individual trees for coppice-originated oak forests. Eur J For Res 138(4):623–637. https://doi.org/10.1007/s10342-019-01194-2
Pettersen RC (1984) The chemical composition of wood. In: Rowell R (ed) The chemistry of solid wood. ACS, Seattle, pp 57–126. https://doi.org/10.1021/ba-1984-0207.ch002
Pompa-García M, Sigala-Rodríguez JA, Jurado E, Flores J (2017) Tissue carbon concentration of 175 Mexican forest species. IForest 10(4):754–758. https://doi.org/10.3832/ifor2421-010
Porter LJ (1974) Extractives of Pinus radiata bark. 2. Procyanidin constitu ents. N Z J Sci 17:213–218
Reich Peter B, Walters Michael B, Ellsworth David S (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci U S A 94:13730–13734. https://doi.org/10.1073/pnas.94.25.13730
Rodríguez-Soalleiro R, Eimil-Fraga C, Gómez-García E, García-Villabrille JD, Rojo-Alboreca A, Muñoz F, Oliveira N, Sixto H, Pérez-Cruzado C (2018) Exploring the factors affecting carbon and nutrient concentrations in tree biomass components in natural forests, forest plantations and short rotation forestry. For Ecosyst 5(1):35. https://doi.org/10.1186/s40663-018-0154-y
Savidge RA (2000) Biochemistry of seasonal cambial growth and wood formation—an overview of the challenges. In: Savidge RA, Barnett J, Napier R (eds) Cell & molecular biology of wood formation. BIOS Scientific, Oxford, pp 1–30
Srivastava LM (1964) Anatomy, chemistry, and physiology of bark. International Review of Forestry Research 1:203–277. https://doi.org/10.1016/B978-1-4831-9975-7.50010-7
State Forestry and Grassland Administration (2019) The Ninth Forest Resources Survey Report (2014–2018). China Forestry Press, Beijing, p 451
Tang W, Zheng M, Zhao X, Shi J, Yang J, Trettin CC (2018) Big geospatial data analytics for global mangrove biomass and carbon estimation. Sustain 10(2):1–17. https://doi.org/10.3390/su10020472
Thomas SC, Malczewski G (2007) Wood carbon content of tree species in Eastern China: interspecific variability and the importance of the volatile fraction. J Environ Manag 85(3):659–662. https://doi.org/10.1016/j.jenvman.2006.04.022
Thomas SC, Martin AR (2012) Carbon content of tree tissues: a synthesis. Forests 3(2):332–352. https://doi.org/10.3390/f3020332
Tsunoda T, van Dam NM (2017) Root chemical traits and their roles in belowground biotic interactions. Pedobiologia (Jena) 65:58–67
Vázquez G, Antorrena G, Parajó JC (1987) Studies on the utilization of Pinus pinaster bark. Wood Sci Technol 21(1):65–74
Vidensek N, Lim P, Campbell A, Carlson C (1990) Taxol content in bark, wood, root, leaf, twig, and seedling from several taxus species. J Nat Prod 53(6):1609–1610. https://doi.org/10.1021/np50072a039
Vieilledent G, Vaudry R, Andriamanohisoa SFD, Rakotonaviro OS, Randrianasolo HZ, Razafindrabe HN, Rakotoarivony CB, Ebeling J, Rasamoelina M (2012) A universal approach to estimate biomass and carbon stock in tropical forests using generic allometric models. Ecol Appl 22(2):572–583. https://doi.org/10.1890/11-0039.1
Wang C (2006) Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For Ecol Manag 222:9–16. https://doi.org/10.1016/j.foreco.2005.10.074
Wang XW, Weng YH, Liu GF, Krasowski MJ, Yang CP (2015) Variations in carbon concentration , sequestration and partitioning among Betula platyphylla provenances. For Ecol Manag 358:344–352. https://doi.org/10.1016/j.foreco.2015.08.029
Widagdo FRA, Li F, Zhang L, Dong L (2020a) Aggregated biomass model systems and carbon concentration variations for tree carbon quantification of natural mongolian oak in northeast China. Forests 11(4):397. https://doi.org/10.3390/F11040397
Widagdo FRA, Xie L, Dong L, Li F (2020b) Origin-based biomass allometric equations, biomass partitioning, and carbon concentration variations of planted and natural Larix gmelinii in northeast China. Glob Ecol Conserv 23:e01111. https://doi.org/10.1016/j.gecco.2020.e01111
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M (2004) The worldwide leaf economics spectrum. Nature 428 (6985): 821. https://doi.org/10.1038/nature02403
Ying J, Weng Y, Oswald BP, Zhang H (2019) Variation in carbon concentrations and allocations among Larix olgensis populations growing in three field environments. Ann For Sci 76(4):99. https://doi.org/10.1007/s13595-019-0877-0
Yu D, Zhou L, Zhou W, Ding H, Wang Q, Wang Y, Wu X, Dai L (2011) Forest management in Northeast China: history, problems, and challenges. Environ Manag 48(6):1122–1135. https://doi.org/10.1007/s00267-011-9633-4
Zhang Q, Wang C, Wang X, Quan X (2009) Carbon concentration variability of 10 Chinese temperate tree species. For Ecol Manag 258:722–727. https://doi.org/10.1016/j.foreco.2009.05.009
Zhou L, Li S, Liu B, Wu P, Heal KV, Ma X (2019) Tissue-specific carbon concentration, carbon stock, and distribution in Cunninghamia lanceolata (Lamb.) Hook plantations at various developmental stages in subtropical China. Ann For Sci 76(3):70. https://doi.org/10.1007/s13595-019-0851-x
Zhu HY, Weng YH, Zhang HG, Meng FR, Major JE (2013) Comparing fast- and slow-growing provenances of Picea koraiensis in biomass, carbon parameters and their relationships with growth. For Ecol Manag 307:178–185. https://doi.org/10.1016/j.foreco.2013.06.024
Acknowledgements
The researchers thank the faculty and students of the Department of Forest Management, Northeast Forestry University (NEFU), P. R. China, who provided and collected the data for this study. We also highly appreciate the help of Nathan J. Roberts (NEFU) for final polishing of the English text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Zhu Hong.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was supported financially by the Heilongjiang Province Applied Technology Research and Development Program Key Project (GA19B201), National Natural Science Foundation of China (31971649), Provincial Funding for National Key Research and Development Program of China in Heilongjiang Province (GX18B041), the Fundamental Research Funds for the Central Universities (2572019CP08) and the Heilongjiang Touyan Innovation Team Program (Technology Development Team for High-efficient Silviculture of Forest Resources).
The online version is available at http://www.springerlink.com.
Rights and permissions
About this article
Cite this article
Widagdo, F.R.A., Li, F., Xie, L. et al. Intra- and inter-species variations in carbon content of 14 major tree species in Northeast China. J. For. Res. 32, 2545–2556 (2021). https://doi.org/10.1007/s11676-020-01264-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01264-x