Abstract
Decomposition dynamics were compared among green tree leaves, partially decomposed tree leaf litter (i.e., decayed tree leaf litter on forest floor) and a mixture of the two in a warm temperate forest ecosystem in central China to test the influence of litter chemical quality on the degree of decomposition. The study was conducted in situ at two contrasting forest sites, an oak forest dominated by Quercus aliena var. acuteserrata Maxim., and a mixed pine and oak forest dominated by Pinus armandii Franch. and Q. aliena var. acuteserrata. We found marked differences in the rate of decomposition among litter types at both forest sites; the litter decomposition constant, k, was about 39 % greater at the oak forest site and more than 70 % greater at the pine-oak forest site, for green leaves than for partially decomposed leaf litter. The decomposition dynamics and temporal changes in litter chemistry of the three litter types also greatly differed between the two forest sites. At both forest sites, the higher rate of decomposition for the green leaves was associated with a higher nitrogen (N) content and lower carbon to N ratio (C/N) and acid-unhydrolyzable residue to N ratio (AUR/N). We did not find any non-additive effects when mixing green leaves and partially decomposed leaf litter. Our findings support the contention that litter chemical quality is one of the most important determinants of litter decomposition in forest ecosystems at the local or regional scale, but the effect of litter chemical quality on decomposition differs between the contrasting forest types and may vary with the stage of decomposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litter decomposition is a critical link in nutrient and carbon cycling in terrestrial ecosystems (Swift et al. 1979; Sun et al. 2004) and plays a key role in maintaining soil fertility and ecosystem functioning (Moore et al. 2004). Quantification of the dynamics of forest-floor litter decomposition is of great importance for assessing the carbon balance and understanding the patterns of nutrient cycling in forest ecosystems (Prescott 2010). It is generally recognized that at a global scale, variations in litter decomposition is strongly shaped by changes in climate, whereas regionally litter decomposition is primarily a function of both litter chemical quality and site microenvironments (Hoorens et al. 2003; Parton et al. 2007; Polyakova and Billor 2007). Under certain conditions, litter chemical quality alone can be the best predictor of the rate of decomposition, with soil properties being of secondary importance (Meentemeyer 1978; Aerts 1997).
Litter chemical quality may be defined in different ways, e.g., as a measure of chemical recalcitrance (i.e., litter lignin content) or as a measure of nutritional value for decomposers (i.e., litter N content or C/N ratio) (Melillo et al. 1982). It specifically refers to the degradability of plant litter under natural conditions. The chemical factor in the litter that best predicts the rate of decomposition may also vary with habitats or ecosystem types (Smith and Bradford 2003); findings on the chemical aspect of the litter that is the best predictor of decomposition rate have been variable among different studies (Loranger et al. 2002). The initial N content and C/N ratio of litter are often considered as the primary chemical parameters for predicting the rate of decomposition (Hector et al. 2000). Based on a comprehensive global database of litter decomposition compiled from 110 research sites, Zhang et al. (2008) found that a combination of total nutrient elements and C/N ratio accounted for as much as 70.2 % of the variation in the rate of litter decomposition. In contrast, other studies have demonstrated a strong negative linear relationship between the rate of litter decomposition and the lignin content (Meentemeyer 1978) or the lignin/N ratio of the decomposing litter (Aerts 1997; Chapman and Koch 2007).
The dynamics of carbon turnover of the forest-floor litter in most ecosystem carbon balance models is typically represented by estimates of the litter decomposition constant, k, derived from the litterbag method starting with either green or newly senescent leaves. However, in the real world, the forest-floor litter layer is often made up of plant-derived organic materials of mixed decomposition stages and forms. Potential bias in adopting the estimates of k value based on decomposition of green leaves to represent the decomposition dynamics of forest-floor litter layer has rarely been tested. The decomposition of green leaves could be more rapid than that of the older or partly decomposed litter due to chemical changes in the substrate because green leaves contain more labile organic compounds that are lost quickly in the early phase of decomposition while the more recalcitrant organic compounds such as lignin would remain stable for much longer and thus be present in greater fraction in the later phase of decomposition (Herbert et al. 1999; Berg 2000; Fonte and Schowalter 2004).
In this study, we tested the effects of litter chemical quality as influenced by the degree of decay on decomposition dynamics and temporal changes in litter chemistry. Litter chemical quality refers to both chemical recalcitrance represented by lignin content and nutritional value to decomposers represented by the absolute and relative contents of litter N. We used the litterbag method with treatments involving green tree leaves, partially decomposed tree leaf litter, and an even mixture between green leaves and partially decomposed leaf litter at two forest sites in central China: a pure oak forest dominated by Quercus aliena var. acuteserrata Maxim., and a mixed pine-oak forest dominated by Pinus armandii Franch. and Q. aliena var. acuteserrata. We hypothesized that (1) differences in the chemical quality of contrasting litter samples would result in differential decomposition dynamics, with green leaves decomposing much faster than the partially decomposed litter as a result of lower chemical recalcitrance of the green leaves compared with the partially decomposed leaf litter on the forest floor, and (2) mixing green leaves and partially decomposed leaf litter would lead to non-additive effects of mixed-litter decomposition similar to that found in litter decomposition of mixed plant species.
Materials and methods
Study area
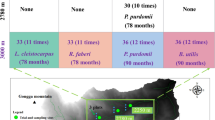
The study was located in the Baotianman Long-Term Forest Ecosystem Research Station in the Baotianman Nature Reserve (latitude 33°20′–33°36′N, longitude 111°46–112°04′E, elevation 600–1860 m a.s.l.), in the eastern Qinling Mountains in central China. It is in a transitional zone from warm temperate to northern subtropical climatic region. The climate is of a continental eastern monsoon type, with annual mean air temperature of 15.1 °C and mean annual precipitation of 900 mm. Rainfall occurs mainly in June through August (55–62 % of annual precipitation). The soils are of dystric cambisols (IUSS Working Group 2014) developed on weathered arenites. The zonal vegetation and environmental conditions at the study sites have been described in detail by You et al. (2014) and Wang et al. (2015).
Experimental setup and procedures
The litter decomposition experiment was conducted in situ on a 20 m × 20 m plot in a pure oak forest stand dominated by Q. aliena var. acuteserrata and in a mixed pine-oak forest stand dominated by P. armandii and Q. aliena var. acuteserrata. Both stand types are mature in tree age and situated on gentle slopes with closed canopy (Wang et al. 2015).
The treatments included green tree leaves (LGREEN), partially decomposed tree leaf litter (LLITTER), and an even mixture of green leaves and partially decomposed leaf litter (LMIX) of the dominant tree species at each site. Green leaf samples were collected from the mid-canopy of at least three individual Q. aliena var. acuteserrata trees in the pure oak stand and three individual trees for each of the P. armandii and Q. aliena var. acuteserrata species in the mixed pine-oak stand in August 2011. Partially decomposed tree leaf litter samples were collected from the surface of the litter layer on the forest floor within the experimental plots representing the two forest types; they were predominantly leaf-fall of the previous season from trees on each plot, i.e., oak leaves at the oak forest site and a mixture of oak leaves and pine needles at the mixed pine-oak forest site.
Collected samples of green leaves and leaf litter were quickly dried in a ventilation oven at a low temperature (<65 °C) before being placed in litterbags for deployment at the field study sites. Each 15 cm × 20 cm litterbag was made of 1.0 mm mesh nylon and contained 6 g dry mass (DW) of desiccated green leaves or partially decomposed leaf litter, or evenly mixed samples of green leaves and partially decomposed leaf litter, either as single tree species of Q. aliena var. acuteserrata for testing decomposition in the pure oak forest stand, or an even mixture between P. armandii and Q. aliena var. acuteserrata for testing decomposition in the mixed pine-oak forest stand. There were five replicate litterbags for each of five separate retrieval samplings during the litter decomposition study.
All litterbags were deployed at the field sites in August 2011, with the existing forest-floor litter removed and the lower side of the litterbags in direct contact with the soil surface. Litterbags were retrieved on 27 October 2011, 18 April, 9 August and 26 October 2012, and 28 March 2013. The litter samples at each retrieval time were weighed after oven-drying at 65 °C to constant mass, then analyzed for C (%C), N (%N) and acid-unhydrolyzable residue (%AUR).
Litter chemical analysis
Litter %C was measured using the K2Cr2O7-H2SO4 calefaction method (Nelson and Sommers 1982). Litter %N was analyzed following the Kjeldahl digestion procedure (Gallaher et al. 1976). Litter %AUR was determined as Klason lignin by the method of Parton et al. (2007). Ash content (%Ash) was determined following the procedure described in Giese et al. (2009).
Data computation
The litter mass remaining (RM) at each retrieval time was determined and compared with the initial mass values using the equation:
where X i is the litter mass at retrieval time t i , and X 0 is the initial litter mass. The litter decomposition constant, k, was computed with a single exponential decomposition model as (Olson 1963):
where X t is the remaining litter mass after a given time period t.
The relative N and AUR retentions at each retrieval time as compared with the initial values were also calculated using Eq. 1.
To determine the effect of litter mixture of different initial states on decomposition, we computed the differences between the observed percentage of mass remaining of LMIX and the expected percentage of mass remaining of LMIX. The expected mass remaining of LMIX was calculated using the formula of Hoorens et al. (2003):
where R 1 and R 2 are the remaining mass (%) of LGREEN and LLITTER, respectively, and M 1 and M 2 are the initial dry mass of these litter types in LMIX. In this study, M 1 = M 2, so the expected mass remaining was calculated as:
The expected k for LMIX was calculated based on the expected mass remaining and exponential decomposition model.
Statistical analysis
Six litter quality indicators were used in the analysis of the litter quality factors affecting decomposition, including %C, %N, C/N, %AUR, AUR/N, and %Ash. Differences in decomposition rate and initial litter chemistry among treatments were examined with a one-way ANOVA. Differences between observed and expected mass remaining of LMIX were also tested with a one-way ANOVA. Pearson’s correlation analysis was used to examine the relationships of decomposition rate, as expressed by the value of k, with the six litter quality indicators. Only two variables were significantly (p < 0.05) related to litter decomposition (i.e., %N and C/N). Before the analysis, data were tested for normality using the Shapiro–Wilk’s test. Variables that did not meet normal distribution were log-transformed before the analysis. All analyses were performed using the SPSS 18.0 software package (SPSS Inc., Chicago, USA) for Windows. Means of the main effects were compared by Duncan’s multi-range test at p < 0.05.
Results
Differences in the initial chemistry among litter types
At the oak forest site, there were significant (p < 0.05) differences in the initial litter %N, C/N and %Ash among the three litter types (Table 1). While LGREEN and LMIX had similar %N and C/N, both litter types were significantly (p < 0.05) more than 26 % higher in %N, and 17 % lower in C/N, than LLITTER (Table 1). The %Ash markedly contrasted among the three litter types; LGREEN and LLITTER differed more than 3-fold in %Ash, whereas, LMIX had twice the %Ash of LGREEN and two-thirds of the %Ash of LLITTER (Table 1).
At the pine-oak forest site, the three litter types also differed significantly (p < 0.05) in the initial litter %N, C/N, and %Ash, but the patterns differed from those at the oak forest site (Table 1). The three litter types markedly contrasted in %N and C/N, with LGREEN having nearly twice the %N and half the C/N of LLITTER (Table 1). The %Ash differed much less among the three litter types at the pine–oak site compared with the oak forest site, albeit there was a significant (p < 0.05) effect of litter type (Table 1). AUR/N was significantly (p < 0.05) more than 36 % greater in LLITTER than in the other two litter types but was similar between LGREEN and LMIX (Table 1).
Temporal dynamics of litter decomposition and changes in litter chemistry
The rate of decomposition was greatest for LGREEN, smallest for LDECOM, and intermediate for LMIX at both forest sites, and was consistently greater at the oak forest site than at the pine-oak forest site for both LLITTER and LMIX but not LGREEN (Fig. 1; Table 2). The differences in litter decomposition among the three litter types were greater at the pine-oak forest site than at the oak forest site (Table 2). On average, some 50 % of the mass for LGREEN and less for LMIX and about 30 %of the mass for LLITTER, were turned over in the first year of litterbag deployment at the oak forest site (Fig. 1a), where the value of k was about 39 % greater for LGREEN than for LLITTER (Table 2). At the pine-oak forest site, mass turnover in the first year was about 60 % for LGREEN, 30 % for LLITTER, and 40 % for LMIX (Fig. 1b); the value of k was more than 70 % greater for LGREEN than for LLITTER (Table 2).
At both forest sites, no significant differences were found between the observed and expected values of k for LMIX (Table 2).
The temporal changes in litter chemistry mostly differed between the two forest sites and among the three litter types within sites (Figs. 1, 2). At the oak forest site, N retention followed patterns similar to the temporal changes in mass remaining for both LLITTER and LMIX, whereas LGREEN had the highest N retention among the three litter types 2 months after the initial litterbag deployment, and was lowest in N retention by 8 months continuing to the end of the study (Fig. 1a). At the pine-oak forest site, N retention for LDECOM did not change much during the study period, but it varied with sampling times with a general downward trend for both LGREEN and LMIX, with LGREEN having lowest N retention throughout the experiment (Fig. 1b). The patterns of temporal changes in AUR retention were similar between the two forest sites for the three litter types, with fastest decline in LGREEN and slowest decline in LLITTER (Fig. 1). The rate of decline in AUR retention was clearly differentiated among the three litter types at the oak forest site (Fig. 1a), but did not differ between LLITTER and LMIX at the pine-oak forest site (Fig. 1b).
Patterns of change in C/N and AUR/N during decomposition distinctly differed between the two forest sites and among the three litter types within sites (Fig. 2). At the oak forest site, the C/Nin LGREEN remained mostly steady throughout the experiment, whereas it significantly (p < 0.05) increased in both LLITTER and LMIX 2 months after the initial deployment of litterbags followed by a trend to decline until approaching steady values comparable to the initial stages at end of the experiment (Fig. 2a; Table 1); the AUR/N significantly (p < 0.05) and markedly increased compared with the initial values in all three litter types 2 months after the initial deployment of litterbags followed by a trend in linear decline, with values remaining at or above the initial stages (Fig. 2a; Table 1). At the pine-oak forest site, changes in C/N and AUR/N during decomposition were inconsistent among litter types, but with a general trend to decline and converge at levels lower than the initial stages (Fig. 2b; Table 1).
Correlations of decomposition rate with litter chemistry
Pearson correlation analyses identified %N and C/N as the two most prominent factors linked to the rate of litter decomposition among the litter chemical variables examined (Table 3). The %N had the greatest positive effect on the k value, followed by a weaker negative effect of C/N; the other four explanatory variables (%C, %AUR, AUR/N and %Ash) were not significantly correlated with the rate of litter decomposition (Table 3).
Discussion
In forest ecosystems, litter decomposition is largely mediated by biological processes, specifically through the action of microbial communities (Freschet et al. 2012), and leaf litter of different forest tree species decomposes at different rates because of chemical differences. Therefore, the recalcitrance of decomposing materials and factors affecting microbial activities all potentially impose significant influences on the rate of litter decomposition. In this study, the initial litter chemistry markedly differed among green tree leaves, partially decomposed tree leaf litter and their mixture within and between two forest sites. In agreement with our first hypothesis, the chemical quality and recalcitrance strongly constrained the decomposition dynamics such that the rate of decomposition was highest for green leaves and lowest for partially decomposed leaf litter at both forest sites, reflecting the effects of litter chemical quality (Wang et al. 2009; Talbot et al. 2012). At both forest sites, the higher rate of decomposition for the green leaves was associated with a higher value of N content and lower values of C/N and AUR/N. In contrast, the lower rate of decomposition for the partially decomposed leaf litter corresponded to lower chemical quality (Wang et al. 2015). However, contrary to our second hypothesis, here we did not find any non-additive effects of litter mixture of different decays.
Previous studies have shown that green leaves decompose at a higher rate than senescent litter (Berg 2000; Fonte and Schowalter 2004), which is consistent with findings in this study at the two contrasting forest sites. The higher N content and lower lignin/N are attributed to a faster rate of decomposition in green leaves as compared with senescent litter (Herbert et al. 1999; Wang et al. 2015). The faster rate of decomposition at the oak forest site than at the pine-oak forest site for partially decomposed leaf litter and mixed green leaves and partially decomposed leaf litter could well be explained by differences in litter chemical quality (Prescott et al. 2000; Wang et al. 2015). The greater differences in initial chemistry and rate of decomposition among the three litter types at the pine-oak forest site than at the oak forest site might reflect differential nutrient resorption and recycling owing to contrasting traits between pine needles and oak leaves (Prescott et al. 2000; Fonte and Schowalter 2004). In a decomposition study involving needle-leaf litter, broadleaf litter and different combinations of the two litter types, Chapman and Koch (2007) found that differences in mass loss among different decay stages were also greater at a pine site than at an aspen site. The presence of more chemically recalcitrant compounds such as lignin and phenolics in pine needles than in broadleaf litter might explain the much slower rate of decomposition at the pine-oak forest site (Parton et al. 2007; Pérez-Suárez et al. 2012). Moreover, partially decomposed needle-leaf litter might protect some soluble substances from the effects of abrasion and leaching through a stability mechanism by interaction between recalcitrant compounds and soluble compounds, thereby providing a more persistent habitat for decomposers than broadleaf litter (Ostrofsky 2007). The temporal patterns in N, AUR, C/N and AUR/N all greatly differed between the two forest sites in our study, suggesting differential controls of litter quality (especially the nitrogen content) and decay stage on decomposition dynamics between contrasting forest types (Vivanco and Austin 2011; Wang et al. 2015).
In this study, we found a significant positive correlation (p < 0.05) between the k value and initial N content and a significant negative correlation (p < 0.05) between the k value and C/N, in line with similar studies (Hoorens et al. 2003; Smith and Bradford 2003; Tripathi et al. 2006; Pandey et al. 2007; Wang et al. 2009), reinforcing that initial N content or C/N of plant litter can be a strong predictor of the rate of decomposition.
Most studies determining the non-additive effects of litter mixture between contrasting litter species have found that the occurrence of nutrient-rich litter may speed up the decomposition of nutrient-poor litter when they co-occur (Hoorens et al. 2003; Pérez-Suárez et al. 2012; Ostrofsky 2007). In this study, however, non-additive effects were not apparent when green leaves were mixed with partially decomposed leaf litter at both forest sites, implicating a complex interplay between the effects of litter chemical quality and interactions with decomposers (Pandey et al. 2007; Purahong et al. 2014). Although green leaves are generally rich in N, they also contain secondary chemical compounds such as phenols that can inhibit soil microbial activities. In contrast, partially decomposed leaf litter is characterized by larger surface area for microbial action than newly senescent leaves because of the greater fragmentation brought about by decomposition. Moreover, different stages of litter decomposition may involve different types of decomposers. For example, as found in the study of Pandey et al. (2007), fungi and bacteria are the active decomposer organisms responsible for the early phase of litter decay, and the actinomycetes population is enhanced as compared with bacteria and the fungi as decay proceeds. The effects of litter chemistry and interactions with decomposers might operate in different directions when green leaves and partially decomposed leaf litter are mixed, hence the absence of non-additive effect over the duration of experiment (Herbert et al. 1999; Fonte and Schowalter 2004). Further studies are needed to address the role of decomposers in regulating the non-additive effects of mixed litter types.
We emphasize that our results are from two contrasting forest types under similar habitat conditions. Extrapolation of the outcome from this study to forest types with different habitat conditions should be treated with caution.
Conclusion
Marked differences were found in the rate of decomposition among the green tree leaves, partially decomposed tree leaf litter and their mixture at an oak forest site and a pine-oak forest site in this study. For green leaves, the value of the litter decomposition constant, k, was about 39 % greater than the k value for partially decomposed leaf litter at the oak forest site and more than 70 % greater at the mixed pine-oak forest site. The decomposition dynamics and temporal changes in litter chemistry of the three litter types also greatly differed between the two forest sites. At both forest sites, the higher rate of decomposition for the green leaves was associated with greater nitrogen (N) content and a lower ratio of carbon (C) to N (C/N) and acid-unhydrolyzable residue (AUR) to N (AUR/N). Non-additive effects were absent when green leaves were mixed with partially decomposed leaf litter.
Overall, our findings support the contention that litter chemical quality, in terms of both chemical recalcitrance and nutritional value to decomposers, is one of the most important determinants of leaf litter decomposition in forest ecosystems at the local or regional scale (Loranger et al. 2002), but the controls of litter chemical quality on decomposition differ between contrasting forest types and may vary with decomposition stages. Therefore, evaluating litter decomposition dynamics based on experiments with green or newly senescent leaves may potentially bias the prediction on forest floor litter turnover because of the unquantifiable effects of mixing litter that has decomposed to different degrees.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449. doi:10.2307/3546886
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133:13–22. doi:10.1016/S0378-1127(99)00294-7
Chapman SK, Koch GW (2007) What type of diversity yields synergy during mixed litter decomposition in a natural forest ecosystem? Plant Soil 299:153–162. doi:10.1007/s11104-007-9372-8
Fonte SJ, Schowalter TD (2004) Decomposition of greenfall vs. senescent foliage in a tropical forest ecosystem in Puerto Rico. Biotropica 36:474–482. doi:10.1111/j.1744-7429.2004.tb00343.x
Freschet GT, Aerts R, Cornelissen JHC (2012) Multiple mechanisms for trait effects on litter decomposition: moving beyond home-field advantage with a new hypothesis. J Ecol 100:619–630. doi:10.1111/j.1365-2745.2011.01943.x
Gallaher RN, Weldon CO, Boswell FC (1976) A semiautomated procedure for total nitrogen in plant and soil samples. Soil Sci Soc Am J 40:887–889. doi:10.2136/sssaj1976.03615995004000060026x
Giese M, Gao Y, Zhao Y, Pan Q, Lin S, Peth S, Brueck H (2009) Effects of grazing and rainfall variability on root and shoot decomposition in a semi-arid grassland. Appl Soil Ecol 41:8–18. doi:10.1016/j.apsoil.2008.08.002
Hector A, Beale AJ, Minns A, Otway SJ, Lawton JH (2000) Consequences of the reduction of plant diversity for litter decomposition effects through litter quality and microenvironment. Oikos 90:357–371. doi:10.1034/j.1600-0706.2000.900217.x
Herbert DA, Fownes JH, Vitousek PM (1999) Hurricane damage to a Hawaiian forest: nutrient supply rate affects resistance and resilience. Ecology 80:908–920. doi:10.1890/0012-9658(1999)080[0908:HDTAHF]2.0.CO;2
Hoorens B, Aerts R, Stroetenga M (2003) Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 137:578–586. doi:10.1007/s00442-003-1365-6
IUSS Working Group WRB (2014) World Reference Base for Soil Resources 2014. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome
Loranger G, Ponge JF, Imbert D, Lavelle P (2002) Leaf decomposition in two semi-evergreen tropical forest: influence of litter quality. Biol Fertil Soils 35:247–252. doi:10.1007/s00374-002-0467-3
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472. doi:10.2307/1936576
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626. doi:10.2307/1936780
Moore JC, Berlow EL, Coleman DC, de Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, Nadelhoffer K, Rosemond AD, Post DM, Sabo JL, Scow KM, Vanni MJ, Wall DH (2004) Detritus, trophic dynamics and biodiversity. Ecol Lett 7:584–600. doi:10.1111/j.1461-0248.2004.00606.x
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy and Soil Science Society of American, Madison, pp 101–129
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331. doi:10.2307/1932179
Ostrofsky ML (2007) A comment on the use of exponential decay models to test nonadditive processing hypotheses in multispecies mixtures of litter. J North Am Benthol Soc 26:23–27. doi:10.1899/0887-3593(2007)26[23:ACOTUO]2.0.CO;2
Pandey RR, Sharma G, Tripathi SK, Singh AK (2007) Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in northeastern India. For Ecol Manag 240:96–104. doi:10.1016/j.foreco.2006.12.013
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364. doi:10.1126/science.1134853
Pérez-Suárez M, Arredondo-Moreno JT, Huber-Sannwald E (2012) Early stage of single and mixed leaf-litter decomposition in semiarid forest pine-oak: the role of rainfall and microsite. Biogeochemistry 108:245–258. doi:10.1007/s10533-011-9594-y
Polyakova O, Billor N (2007) Impact of deciduous tree species on litterfall quality, decomposition rates and nutrient circulation in pine stands. For Ecol Manag 253:11–18. doi:10.1016/j.foreco.2007.06.049
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149. doi:10.1007/s10533-010-9439-0
Prescott CE, Zabek LM, Staley CL, Kabzems R (2000) Decomposition of broadleaf and needle litter in forests of British Columbia: influences of litter type, and litter mixtures. Can J For Res 30:1742–1750. doi:10.1139/x00-097
Purahong W, Schloter M, Pecyna MJ, Kapturska D, Däumlich V, Mital S, Buscot F, Hofrichter M, Gutknecht JLM, Krüger D (2014) Uncoupling of microbial community structure and function in decomposing litter across beech forest ecosystems in Central Europe. Sci Rep 4:7014. doi:10.1038/srep07014
Smith VC, Bradford MA (2003) Do non-additive effects on decomposition in litter-mix experiments result from differences in resource quality between litters? Oikos 102:235–243. doi:10.1034/j.1600-0706.2003.12503.x
Sun OJ, Campbell J, Law BE, Wolf V (2004) Dynamics of carbon stocks in soils and detritus across chronosequences of different forest types in the Pacific Northwest, USA. Glob Change Biol 10:1470–1481. doi:10.1111/j.1365-2486.2004.00829.x
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Blackwell, Oxford
Talbot JM, Yelle DJ, Nowick J, Treseder KK (2012) Litter decay rates are determined by lignin chemistry. Biogeochemistry 108:279–295. doi:10.1007/s10533-011-9599-6
Tripathi SK, Sumida A, Shibata H, Ono K, Uemura S, Kodama Y, Hara T (2006) Leaf litterfall and decomposition of different above- and belowground parts of birch (Betula ermanii) trees and dwarf bamboo (Sasa kurilensis) shrubs in a young secondary forest in Northern Japan. Biol Fertil Soils 43:237–246. doi:10.1007/s00374-006-0100-y
Vivanco L, Austin AT (2011) Nitrogen addition stimulates forest litter decomposition and disrupts species interactions in Patagonia, Argentina. Glob Change Biol 17:1963–1974. doi:10.1111/j.1365-2486.2010.02344.x
Wang Q, Wang S, Huang Y (2009) Leaf litter decomposition in the pure and mixed plantations of Cunninghamia lanceolata and Michelia macclurei in subtropical China. Biol Fertil Soils 45:371–377. doi:10.007/s00374-008-0338-7
Wang J, You Y, Tang Z, Liu S, Sun OJ (2015) Variations in leaf litter decomposition across contrasting forest stands and controlling factors at local scale. J Plant Ecol 8:261–272. doi:10.1093/jpe/rtu019
You Y, Wang J, Huang X, Tang Z, Liu S, Sun OJ (2014) Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol Evol 4:633–647. doi:10.1002/ece.969
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:1–9. doi:10.1093/jpe/rtn002
Acknowledgments
This study was supported by the National Basic Research Program of China (Grant No. 2011CB403205). We gratefully acknowledge the Baotianman Long-Term Forest Ecosystem Research Station and Baotianman Bureau of the National Nature Reserve for logistic support and site access permission. Rongcheng Li and Qiongda Pu assisted in field sampling and laboratory analyses. We thank an anonymous reviewer for constructive comments and suggestions for revision of an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: This study was supported by the National Basic Research Program (Grant No. 2011CB403205).
The online version is available at http://www.springerlink.com
Corresponding editor: Yu Lei
Rights and permissions
About this article
Cite this article
Wang, J., You, Y., Tang, Z. et al. A comparison of decomposition dynamics among green tree leaves, partially decomposed tree leaf litter and their mixture in a warm temperate forest ecosystem. J. For. Res. 27, 1037–1045 (2016). https://doi.org/10.1007/s11676-016-0248-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-016-0248-8