Abstract
Phase equilibria in the quasi-ternary system Ag2Se–GeSe2–As2Se3 were investigated by direct synthesis, x-ray phase, differential thermal and microstructural analysis methods. Isothermal section of the system at 513 K (240 °C) was constructed, the existence of ternary compounds Ag8GeSe6, Ag3AsSe3, AgAsSe2, AgAs3Se5 was confirmed, and the existence of quaternary compounds was not found. Three quasi-binary phase diagrams Ag2Se–As2Se3, Ag8GeSe6–AgAsSe2, GeSe2–AgAsSe2, three vertical sections Ag8GeSe6–Ag3AsSe3, Ag8GeSe6–As2Se3, GeSe2–AgAs3Se5, and the liquidus surface projection onto the concentration triangle were constructed. The regions of primary crystallization of phases, character, temperature, and coordinates of mono- and invariant equilibria were determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The binary compounds Ag2Se, GeSe2, As2Se3 melt congruently at 1170 K (897 °C), 1013 K (740 °C), and 648 K (375 °C)[1], respectively, possess insignificant homogeneity regions and may serve as components of the quasi-ternary system Ag2Se–GeSe2–As2Se3. The study of this quasi-ternary system is of current interest because it is formed by binary compounds with important semiconducting properties. Complex chalcogenide semiconductors attract growing interest in materials science due to their prospects for use as materials in the fields of nonlinear optics, optoelectronics, and acousto-optics.[2,3,4,5,6]
Phase equilibria in the quasi-binary system Ag2Se–GeSe2 were studied in Ref 7,8,9,10. The authors of Ref 10 confirmed the existence of one ternary compound Ag8GeSe6 which melts congruently at 1175 K (902 °C) and undergoes two polymorphous transformations at 269 K (–4 °C)[7] and 321 K (48 °C),[7,9,10] respectively. The eutectic points coordinates were ascertained as 1103 K (830 °C), 15 mol.% GeSe2 and 843 K (570 °C), 56 mol.% GeSe2,[10] and agree well with Ref 7. γ-Ag8GeSe6 which is stable at room temperature crystallizes in space group, S.G. Pmn21, with lattice parameters a = 0.7823 nm, b = 0.7712 nm, c = 1.0885 nm.[11]
The Ag2Se–As2Se3 phase diagram was described in Ref 12,13,14. According to Ref 14, the existence of three compounds, Ag3AsSe3, AgAsSe2 and AgAs3Se5, was established in the system. The AgAs3Se5 compound forms incongruently at 643 K (370 °C) and has a eutectic with As2Se3 at 87 mol.% As2Se3 and 630 K (357 °C). The AgAsSe2 compound melts congruently at 673 K (400 °C) and does not have a polymorphous transformation (whereas according to Ref 13 there is a polymorphous transformation of AgAsSe2 at 658 K (385 °C)). The peritectic reaction L + Ag2Se ↔ Ag3AsSe3 takes place at 663 K (390 °C). The coordinates of the eutectic of Ag3AsSe3 − AgAsSe2 are 33 mol.% As2Se3 and 653 K (380 °C).
The crystal structure of the Ag3AsSe3 compound was determined in Ref 13, 15. The compound is an analogue of the proustite mineral (Ag3AsS3), crystallizes in S.G. R3c; and has lattice parameters a = 1.1285 nm, c = 0.8803 nm [13], or a = 1. 1298 nm, c = 0.8757 nm[15]. For the high-temperature modification (HTM) of AgAsSe2, the authors of Ref 13 determined the crystal structure S.G. R \(\overline{3}\) m, structure type NaCrS2, a = 0.3915 nm, c = 2.0375 nm. The diffraction pattern of the low-temperature modification (LTM) of AgAsSe2 was indexed by the authors of Ref 13 in the primitive tetragonal cell with a = 1.2548 nm, c = 1.1140 nm. The compound AgAs3Se5 crystallizes in S.G. R \(\overline{3}\) m, a = 0.38195(1) nm, c = 5.0082(2) nm[16].
Phase diagram of the GeSe2–As2Se3 system of the eutectic type, with the eutectic point at 20 mol.% GeSe2 and 618 K (345 °C)[17].
2 Experimental Methods
Phase equilibria in the quasi-ternary system Ag2Se–GeSe2–As2Se3 were studied using 122 samples. The alloys were synthesized by direct single-temperature method from high-purity elements (Ag 99.995, Ge 99.99, Se 99.9997, As 99.9999 wt.%) in quartz containers that were evacuated to a residual pressure of 0.133 Pa and sealed. The synthesis was performed in a shaft-type furnace with temperature control with an accuracy of ± 5 K (± 5 °C). The maximum synthesis temperature was 1373 K (1100 °C), the heating and cooling rate was 10 K/h (10 °C/h). Homogenization annealing at 513 K (240 °C) was held for 600 h, after which the samples were quenched into 25% aqueous NaCl solution.
The as-obtained samples were investigated by x-ray diffraction (XRD) method (DRON 4–13 diffractometer, CuKα radiation, 10(20)° < 2θ < 90°, 0.05° scan step, 1–5 s exposure in each point) and differential thermal analysis (DTA) ("Thermodent H307/1" furnace with a PDA-1 XY-recorder, Pt/Pt–Rh thermocouple). The two-phase or three-phase composition of the samples was also checked by the microstructure analysis (MSA), which was performed on a PMT-3 M microhardness tester.
3 Results and Discussion
3.1 Isothermal Section of the Quasi-Ternary System Ag 2 Se–GeSe 2 –As 2 Se 3 at 513 K (240 °C)
According to XRD and MSA results of 122 samples (Fig. 1), isothermal section of the Ag2Se – GeSe2 – As2Se3 system at 513 K was plotted (Fig. 2). Diffraction patterns of the binary compounds were indexed (Fig. 3): GeSe2 by S.G. P21/c, a = 0.7007(2) nm, b = 1.6819(5) nm, c = 1.1806(3) nm, β = 90.74(2)º; As2Se3 in S.G. P21/n, a = 1.2794(7) nm, b = 0.9874(5) nm, c = 0.4267(2) nm, α = 90.96(4)º, and agree well with the literature data from Ref 18,19,20, respectively. The existence of the four ternary compounds Ag8GeSe6, Ag3AsSe3, LTM-AgAsSe2, AgAs3Se5 was confirmed (Fig. 3). The diffraction pattern of the Ag8GeSe6 compound was indexed with the orthorhombic S.G. Pmn21, a = 0.78443(5) nm, b = 0.77372(5) nm, c = 1.09141(7) nm, which is consistent with Ref 11. The diffraction pattern of Ag3AsSe3 was indexed with the hexagonal S.G. R3c, structural type proustite Ag3AsS3, a = 1.1270(6) nm, c = 0.8765(9) nm, which agrees with Ref 13. The LTM-AgAsSe2 was identified by comparing our diffraction pattern with the one indexed with a tetragonal structure (a = 1.2548 nm, c = 1.1140 nm) presented in Ref 13. The crystal structure of the AgAs3Se5 compound was determined by the powder diffraction method. It was found that the compound crystallizes in S.G. R \(\overline{3}\) m, a 0.38195(1) nm, c = 5.0082(2) nm)[16]. Ag2Se was obtained as a LTM (S.G. P212121, a = 0.4337(5) nm, b = 0.7072(5) nm, c = 0.7773(5) nm).
The presence of the four two-phase equilibria, Ag8GeSe6–Ag3AsSe3, Ag8GeSe6–AgAsSe2 (Fig. 4), GeSe2–AgAsSe2, and GeSe2–AgAs3Se5, was established in the studied quasi-ternary system according to x-ray phase analysis and MSA of the synthesized samples. They divide the system into the respective regions of three-phase equilibria Ag2Se + Ag8GeSe6 + Ag3AsSe3, Ag3AsSe3 + Ag8GeSe6 + AgAsSe2, AgAsSe2 + Ag8GeSe6 + GeSe2, AgAs3Se5 + AgAsSe2 + GeSe2 and As2Se3 + AgAs3Se5 + GeSe2. No significant solid solubility ranges were found for the binary compounds or intermediate ternary compounds.
3.2 The Quasi-Binary System Ag 2 Se–As 2 Se 3
Due to somewhat differing literature data in Ref 12,13,14, we re-investigated the Ag2Se–As2Se3 system (Fig. 5). The compositions of the samples for the investigation are given in Table 1. Phase diagram of the system was plotted from the results of XRD, DTA (Table 1) and MSA. The existence and formation mechanism of three compounds Ag3AsSe3, AgAsSe2 and AgAs3Se5, and the polymorphous transformation of AgAsSe2 were confirmed. Ag3AsSe3 melts incongruently at 660 K (387 °C), AgAsSe2 melts congruently at 683 K (410 °C), and AgAs3Se5 melts incongruently at 644 K (371 °C). The horizontal line at 635 K (362 °C) corresponds to the eutectic reaction Le1 ↔ AgAs3Se5 + As2Se3 with the eutectic point at 88 mol.% As2Se3. The temperatures of invariant reactions practically do not differ from Ref 15. The phase diagram of the Ag2Se – As2Se3 system shows a horizontal line at 630 K (357 °C) which indicates a polymorphous transformation of the AgAsSe2 compound; obtained temperature value is slightly lower than indicated in Ref 12, 13.
3.3 The Quasi-Binary System Ag 8 GeSe 6 –AgAsSe 2
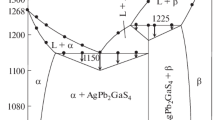
Phase diagram of the Ag8GeSe6 – AgAsSe2 system (Fig. 6) is based on DTA, XRD and MSA results. The axes of the isothermal section (Fig. 1 and 2) and the liquidus projection (Fig. 12) are in mol.% of the components, Ag2Se, As2Se3 and GeSe2, while the axes of the vertical sections (Figs. 6-11) are in mol.% of the compounds constituting these sections. For easier correlation with the isothermal section and the liquidus projection, a secondary axis with mol.% of As2Se3 is added to the diagram. The compositions of the synthesized samples as well as DTA results are given in Table 2. Some of the DTA curves are shown in Fig. 7. They were measured from and till the temperature of annealing of the samples, 513 K. The investigated system is of the eutectic type. The eutectic point coordinates were determined by plotting the Tammann triangle according to Ref 21. Point e5 corresponds to the composition of 4 mol.% Ag8GeSe6–96 mol.% AgAsSe2 (55.2 mol.% Ag2Se–3.4 mol.% GeSe2–41.4 mol.% As2Se3), 660 K (387 °C), where the invariant reaction Le5 ↔ Ag8GeSe6 + HTM-AgAsSe2 takes place. The liquidus is represented by the curves of the primary crystallization of Ag8GeSe6 and the solid solution HTM-AgAsSe2. The eutectoid reaction HTM-AgAsSe2 ↔ LTM-AgAsSe + Ag8GeSe6 takes place at 590 K (317 °C). Below this temperature the samples are two-phase, Ag8GeSe6 + LTM-AgAsSe2, which was established by XRD (Fig. 4) and MSA methods. No significant solid solubility based on the starting compounds was observed.
3.4 The Vertical Section Ag 8 GeSe 6 –Ag 3 AsSe 3
The compositions and DTA data of the alloys are given in Table 3. Based on these the vertical section was built. The liquidus (Fig. 8) crosses the primary crystallization regions of Ag8GeSe6 (field 2) and Ag2Se (field 4). The regions of the secondary crystallization of the monovariant eutectic reaction Le3-U1 ↔ Ag2Se + Ag8GeSe6 (field 3) and the peritectic reaction Lp2-U1 + Ag2Se ↔ Ag3AsSe3 (field 5) descend to the plane of the invariant reaction LU1 + Ag2Se ↔ Ag8GeSe6 + Ag3AsSe3 which occurs at 650 K (377 °C) and ends with the exhaustion of L and Ag2Se. Therefore, below 650 K (377 °C) the samples are two-phase Ag8GeSe6 + Ag3AsSe3 (field 6), which was determined by XRD and MSA data. No solid solubility based on the starting compounds was found.
3.5 The Quasi-Binary System GeSe 2 – AgAsSe 2
Phase diagram of the GeSe2–AgAsSe2 system (Fig. 9) is based on DTA (Table 4), XRD and MSA results for 16 samples, compositions of which are given in Table 4. Phase diagram is of the eutectic type, the liquidus consists of the curves of the primary crystallization of GeSe2 (ab) and the solid solution HTM-AgAsSe2 (bc), respectively. The horizontal line at 670 K (397 °C) corresponds to the invariant eutectic reaction Le6 ↔ GeSe2 + HTM-AgAsSe2. The coordinates of the eutectic point e6 are 7.7 mol.% GeSe2–92.3 mol.% AgAsSe2 (46 mol.% Ag2Se–8 mol.% GeSe2–46 mol.% As2Se3) as determined by plotting the Tammann triangle. The eutectoid reaction HTM-AgAsSe2 ↔ LTM-AgAsSe2 + GeSe2 takes place at 610 K (337 °C). Below this temperature, the samples are two-phase GeSe2 + LTM-AgAsSe2 that is consistent with Fig. 2. No significant solid solubility based on the starting compounds was detected.
3.6 The Vertical Section Ag 8 GeSe 6 –As 2 Se 3
The Ag8GeSe6 – As2Se3 section (Fig. 10) is based on XRD, MSA and DTA results of 20 samples. Their compositions and DTA data are shown in Table 5. The liquidus of the vertical section is represented by the primary crystallization curves of Ag8GeSe6 (ab), GeSe2 (bcd), HTM-AgAsSe2 (df), AgAs3Se5 (fg) and As2Se3 (gj). The section crosses two subsystems of the studied quasi-ternary system, AgAsSe2 + Ag8GeSe6 + GeSe2 (II) and AgAsSe2 + GeSe2 + As2Se3 (III). The section crosses the plane of the invariant eutectic reaction LE2 ↔ HTM-AgAsSe2 + Ag8GeSe6 + AgAs3Se5 at 630 K (357 °C) in subsystem II. This ends with the exhaustion of liquid, therefore the alloys are three-phase (field 12) below 630 K (357 °C) in the region between 2 and 75 mol.% As2Se3. The horizontal at 590 K (317 °C) represents the reaction HTM-AgAsSe2 ↔ LTM-AgAsSe2 + Ag8GeSe6 + GeSe2 in the subsolidus region, and alloys below 590 K contain LTM-AgAsSe2 and are three-phase (LTM-AgAsSe2 + Ag8GeSe6 + GeSe2).
The vertical section Ag8GeSe6–As2Se3: 1–L, 2–L + Ag8GeSe6, 3–L + GeSe2, 4–L + HTM-AgAsSe2, 5–L + AgAs3Se5, 6–L + As2Se3, 7–L + Ag8GeSe6 + GeSe2, 8–L + HTM-AgAsSe2 + GeSe2, 9–L + HTM-AgAsSe2 + AgAs3Se5, 10–L + GeSe2 + AgAs3Se5, 11–L + AgAs3Se5 + As2Se3, 12–Ag8GeSe6 + HTM-AgAsSe2 + GeSe2, 13–GeSe2 + HTM-AgAsSe2, 14–GeSe2 + HTM-AgAsSe2 + LTM-AgAsSe2, 15–HTM-AgAsSe2 + GeSe2 + AgAs3Se5, 16–Ag8GeSe6 + LTM-AgAsSe2 + GeSe2, 17–GeSe2 + LTM-AgAsSe2, 18–LTM-AgAsSe2 + GeSe2 + AgAs3Se5, 19–GeSe2 + AgAs3Se5, 20–GeSe2 + AgAs3Se5 + As2Se3
The sample with 80 mol.% As2Se3 is two-phase at the annealing temperature (LTM-AgAsSe2 + GeSe2) because it falls on the quasi-binary section GeSe2–AgAsSe2. The section in subsystem III crosses the reaction plane at 634 K (361 °C) which reflects the invariant reaction LU2 + HTM-AgAsSe2 ↔ AgAs3Se5 + GeSe2. This reaction ends in samples with higher Ag8GeSe6 content with the disappearance of the liquid, so that the alloys of this subsystem below 634 K (361 °C) are three-phase (field 15). The horizontal line at 630 K (357 °C) corresponds to the reaction HTM-AgAsSe2 + AgAs3Se5 ↔ LTM-AgAsSe2 + GeSe2 in the subsolidus region, thus the alloys below 630 K (357 °C) contain LTM-AgAsSe2 and are three-phase (AgAs3Se5 + LTM-AgAsSe2 + GeSe2). This reaction ends in point k with the exhaustion of both L and HTM-AgAsSe2, so that the alloys are two-phase GeSe2 + AgAs3Se5 below 630 K (357 °C) (field 19). Samples with higher As2Se3 content end in the invariant reaction at 634 K (361 °C) with the exhaustion of HTM-AgAsSe2. Therefore, the alloys below 634 K (361 °C) are three-phase L + GeSe2 + AgAs3Se5 (field 10). This field together with the region of the secondary crystallization L + As2Se3 + AgAs3Se5 (field 11) descends to the plane of the invariant reaction LE3 ↔ GeSe2 + As2Se3 + AgAs3Se5 at 600 K (327 °C). This reaction ends with the exhaustion of the liquid; thus, the alloys are three-phase (GeSe2 + As2Se3 + AgAs3Se5) below 600 K (327 °C). This agrees with the results shown in Fig. 2. The phase composition of the samples was determined by XRD and MSA results.
3.7 The Vertical Section GeSe 2 – AgAs 3 Se 5
The section (Fig. 11) was investigated by DTA, XRD and MSA of 17 samples with compositions given in Table 6. The liquidus consists of the curves of the primary crystallization of GeSe2 (ab) and HTM-AgAsSe2 (bc). The section crosses the plane of the invariant reaction LU2 + HTM-AgAsSe2 ↔ AgAs3Se5 + GeSe2 at 634 K (361 °C), which ends with the exhaustion of both L and HTM-AgAsSe2, so that the alloys are two-phase below 634 K (361 °C) as confirmed by XRD and MSA results.
3.8 Liquidus Surface Projection of the Quasi-Ternary System Ag 2 Se–GeSe 2 –As 2 Se 3
The liquidus surface projection of the quasi-ternary system Ag2Se–GeSe2–As2Se3 (Fig. 12 and Table 7) is based on literature data and our own results from studies of three phase diagrams Ag2Se–As2Se3, Ag8GeSe6–AgAsSe2 and GeSe2–AgAsSe2 and three vertical sections Ag8GeSe6–Ag3AsSe3, Ag8GeSe6–As2Se3 and GeSe2–AgAs3Se5, as well as the isothermal section of the system at 513 K (240 °C).
The system’s liquidus consists of the fields of the primary crystallization of Ag2Se (Ag2Se-p2-U1-e3-Ag2Se), Ag3AsSe3 (e2-E1-U1-p2-e2), HTM-AgAsSe2 (p1-U2-e6-E2-e5-E1-e2-p1), AgAs3Se5 (e1-E3-U2-p1-e1), As2Se3 (As2Se3-e7-E3-e1-As2Se3), Ag8GeSe6 (e3-U1-E1-e5-E2-e4-e3) and GeSe2 (GeSe2-e7-E3-U2-e6-E2-e4-GeSe2). The largest fields correspond to the primary crystallization of Ag8GeSe6 and GeSe2. The fields of the primary crystallization are separated by 13 monovariant curves of binary eutectic and peritectic reactions and 14 invariant points (Table 7).
The system undergoes five quaternary invariant reactions (Table 7): transition LU1 + Ag2Se ↔ Ag8GeSe6 + Ag3AsSe3 at 650 K (377 °C), eutectic LE1 ↔ Ag8GeSe6 + Ag3AsSe3 + HTM-AgAsSe2 at 640 K (367 °C), eutectic LE2 ↔ Ag8GeSe6 + GeSe2 + HTM-AgAsSe2 at 630 K (357 °C), transition LU2 + HTM-AgAsSe2 ↔ AgAs3Se5 + GeSe2 at 634 K (361 °C) and eutectic LE3 ↔ GeSe2 + As2Se3 + AgAs3Se5 at 600 K (327 °C).
4 Conclusions and Future Work
Phase equilibria in the Ag2Se–GeSe2–As2Se3 system were investigated for the first time by XRD and MSA. At 513 K (240 °C) four ternary compounds Ag8GeSe6, Ag3AsSe3, AgAsSe2, AgAs3Se5 were identified and an isothermal section of Ag2Se–GeSe2–As2Se3 system was constructed. The liquidus surface projection of the quasi-ternary system Ag2Se–GeSe2–As2Se3 was plotted according to the investigation of the vertical sections Ag8GeSe6–Ag3AsSe3, Ag8GeSe6–As2Se3, GeSe2–AgAs3Se5 and phase diagrams Ag2Se–As2Se3, Ag8GeSe6–AgAsSe2, GeSe2 – AgAsSe2. There are seven fields of the primary crystallization of Ag2Se, Ag3AsSe3, HTM-AgAsSe2, AgAs3Se5, As2Se3, Ag8GeSe6 and GeSe2 on the liquidus surface projection with the largest fields of Ag8GeSe6 and GeSe2 compounds. In the investigated system there are two transition reactions: LU1 + Ag2Se ↔ Ag8GeSe6 + Ag3AsSe3 at 650 K (377 °C), LU2 + HTM-AgAsSe2 ↔ AgAs3Se5 + GeSe2 at 634 K (361 °C) and three eutectic ones: LE1 ↔ Ag8GeSe6 + Ag3AsSe3 + HTM-AgAsSe2 at 640 K (367 °C), LE2 ↔ Ag8GeSe6 + GeSe2 + HTM-AgAsSe2 at 630 K (357 °C) and LE3 ↔ GeSe2 + As2Se3 + AgAs3Se5 at 600 K (327 °C).
This work was performed as part of the effort to gather more data for a thermodynamic assessment of the Ag2Se–BIVSe2–As2Se3 systems, where BIV–Si, Ge, Sn, which is still in progress.
References
T. B. Massalski, Binary Alloy Phase Diagrams, ASM International, Materials Park, OH, 1990, Vols. 1–3
G. Z. Vinogradova, Glass Formation and Phase Equilibria in Chalcogenide Systems. Binary and Ternary Systems, Nauka, Moscow, 1984
D. I. Bletskan, Crystalline and Glassy Semiconductors of Si, Ge, Sn and Their Alloys, Zakarpattia, Uzhgorod, 2004
M.A. Popesku, Non-Crystalline Chalcogenides. Kluwer Academie Publishers, New York, 2002.
A. Zakery, and S.R. Elliott, Optical Properties and Applications of Chalcogenide Glasses: A Review, J. Non-Cryst. Solids, 2003, 330, p 1–12.
C. Goncalves, M. Kang, B. Sohn, G. Yin, J. Hu, D.T.H. Tan, and K. Richardson, New Candidate Multicomponent Chalcogenide Glasses for Supercontinuum Generation, Appl. Sci, 2018, 8, p 2082.
O. Gorochov, Lescomposes Ag8MX6 (M = Si, Ge, Sn et X – S, Se, Te), Bull. Soc. Chim. France, 1968, 6, p 2263–2275.
A.A. Movsun-zade, ZYu. Salaeva, and M.R. Allazov, The Ag–Ge–Se System, Zhurn. Neorg. Khimii, 1987, 32(7), p 1705–1709.
O. P. Kokhan, Interaction in the Ag2X – BIVX2 Systems (BIV – Si, Ge, Sn; X – S, Se) and Properties of Compounds, Ph.D. (Chemistry) thesis, Uzhgorod State Univ., Uzhgorod, 1996
I. Olekseyuk, O. Parasyuk, L. Piskach, G. Gorgut, O. Zmiy, O. Krykhovetz, L. Sysa, E. Kadykalo, O. Strok, O. Marchuk, and V. Halka, Quasi-Ternary Chalcogenide Systems, Vezha, 1999, Vol. 1
D. Carre, R. Ollitrault-Fichet, and J. Flahaut, Structure de Ag8GeSe6 Beta, Acta Cryst., 1980, 36, p 245–249.
S.A. Tarasevich, Z.S. Medvedeva, I.S. Kovaleva, and L.I. Antonova, On Interaction in the Ternary System Ag–As–Se Along the Section Ag2Se – As2Se3, Zhurn. Neorg. Khimii, 1972, 17, p 1475–1478.
Y.V. Voroshilov, M.P. Golovey, and M.V. Potoriy, X-ray Investigations of the Compounds AgAsSe2 and Ag3AsSe3, Z. Kristallographie, 1976, 21(3), p 595–596.
D. Houphouet-Boigny, R. Ollitrault-Fichet, R. Eholie, and J. Flahaut, Sur le systeme Ag2Se–As2Se3: Compose AgAs3Se5 et polimorphisme de AgAsSe2, CR Acad Sci Ser, 1981, 292(6), p 513–516.
K. Sakai, T. Koide, and T. Matsumoto, Silver Orthoselenoarsenite, Acta Crystallogr., 1978, 34, p 3326–3328.
O.S. Klymovych, O.F. Zmiy, L.D. Gulay, and T.A. Ostapyuk, Phase Diagram of the Ag2Se–As2Se3 System and Crystal Structure of the AgAs3Se5 Compound, Chem. Met. Alloys, 2008, 1, p 288–292.
O. Klymovych, I. Ivashchenko, I. Olekseyuk, O. Zmiy, and Z. Lavrynyuk, Quasi-Ternary System Cu2Se–GeSe2–As2Se3, J. Phase Equilib. Diffus, 2020, 41(2), p 157–163.
P. Rahlfs, Über die kubischen Hochtemperaturmodifikationen der Sulfide und Telluride des Silbers und des einwertigen Kupfers, Phase Transit, 1992, 38, p 127–220.
G. Dittmar, and H. Schäfer, The Crystal Structure of Germanium Dieselenide, Acta Cryst., 1976, 32, p 2726–2728.
A. Renninger, and B. Averbach, Crystalline Structures of As2Se3 and As4Se4, Acta Cryst., 1973, 29, p 1583–1589.
V.Y. Anosov, M.I. Ozerova, and Y.Y. Fialkov, Fundamentals of Physico-chemical Analysis. Nauka, Moscow, 1976.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivashchenko, I.A., Klymovych, O.S., Olekseyuk, I.D. et al. Quasi-Ternary System Ag2Se–GeSe2–As2Se3. J. Phase Equilib. Diffus. 43, 483–494 (2022). https://doi.org/10.1007/s11669-022-00987-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-022-00987-0