Abstract
Direct single-temperature method has been employed to synthesize 137 samples of the Cu2Se-GeSe2-As2Se3 system. The samples have been investigated by x-ray diffraction and differential thermal analysis. The isothermal section at 513 K (240 °C), two phase diagrams GeSe2-As2Se3 and Cu2GeSe3-As2Se3, four vertical sections Cu2GeSe3-CuAsSe2, Cu8GeSe6-CuAsSe2, Cu8GeSe6-As2Se3, A-B (A = 85 mol.% GeSe2-15 mol.% Cu2Se; B = 85 mol.% As2Se3-15 mol.% Cu2Se), and the liquidus surface projection onto the concentration triangle have been constructed. The existence of the ternary compounds Cu2GeSe3, Cu8GeSe6 and CuAsSe2 have been confirmed. The fields of the primary crystallization of phases, character, temperatures and coordinates of nonvariant points have been determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The binary compounds Cu2Se, GeSe2, As2Se3 melt congruently at 1421 K (1148 °C), 1013 K (740 °C), and 648 K (375 °C),[1] respectively, possess insignificant homogeneity regions and may serve as components of the quasi-ternary system Cu2Se-GeSe2-As2Se3. The study of this quasi-ternary system is of current interest because it is formed by binary compounds with important semiconducting properties.[2,3]
Phase equilibria in the quasi-binary system Cu2Se-GeSe2 were studied in Ref 4,5,6,7. Two ternary compounds were found in the system, Cu2GeSe3 and Cu8GeSe6.[4,5,6,7] Cu2GeSe3 melts congruently at 1053 K (780 °C), and Cu8GeSe6 is formed in the peritectic reaction at 1083 K (810 °C) and has two polymorphous transformations at 983 K (710 °C) and 333 K (60 °C).[7] The interactions of Cu8GeSe6 and Cu2GeSe3, Cu2GeSe3 and GeSe2 are eutectic. The eutectic points have the following coordinates: e1-38 mol.% GeSe2 and 1033 K (760 °C); e2-83 mol.% GeSe2 and 960 K (687 °C). The Cu2GeSe3 compound crystallizes in space group Imm2, a = 1.1860(3) nm, b = 0.3960(1) nm, c = 0.5485(2) nm;[8] Cu8GeSe6 in space group P63cm, a = 1.2648(5) nm, c = 1.176(4) nm.[9]
The Cu2Se-As2Se3 phase diagram was described in Ref 10,11,12,13. The system features one ternary compound CuAsSe2 which melts incongruently at 725 K[10] and crystallizes in space group R3 (Cu7As6Se13 structure type) with the cell parameters a = 1.4025(3) nm, c = 0.961(3) nm.[14]
The GeSe2-As2Se3 system is a section of the ternary system Ge-As-Se that was studied in terms of its glass-forming ability.[15,16,17] According to these studies, no intermediate phases form in the GeSe2-As2Se3 system. No phase diagram of this system has been reported in the literature.
2 Experimental Methods
Phase equilibria in the quasi-ternary system Cu2Se-GeSe2-As2Se3 were studied on 137 samples. The alloys were synthesized by direct single-temperature method from high-purity elements (Cu 99.99, Ge 99.99, Se 99.9997, As 99.9999 wt.%) in evacuated to the residual pressure 0.133 Pa and sealed quartz containers. The synthesis was performed in a shaft-type furnace with temperature control with an accuracy of ± 5 K (± 5 °C). The maximum synthesis temperature was 1453 K (1180 °C), the heating and cooling rate was 10 K/h (10 °C/h). Homogenizing annealing at 513 K (240 °C) was held for 600 h, after which the samples were quenched into 25% aqueous NaCl solution.
Obtained samples were investigated by x-ray diffraction (XRD) method (DRON 4-13 diffractometer, CuKα radiation, 10° < 2θ < 80°, 0.05° scan step, 1 s exposure in each point) and differential thermal analysis (DTA) (“Thermodent H307/1” furnace with a PDA-1 XY-recorder, Pt/Pt-Rh thermocouple). The two-phase or three-phase composition of the samples was also checked by the microstructural analysis (MSA), which was performed on a PMT-3M microhardness tester.
3 Results and Discussion
3.1 The Quasi-Binary System GeSe2-As2Se3
Phase diagram of the GeSe2-As2Se3 system was investigated by DTA and XRD methods (Fig. 1). The liquidus consists of the curves of the primary crystallization of α-solid solutions of GeSe2 and β-solid solutions of As2Se3, which at 513 K (240 °C) extend to less than 4 mol.%. The system solidus is the eutectic horizontal at 618 K (345 °C). The eutectic point was determined by the Tamman triangle according to the literature[18] as 20 mol.% GeSe2, 80 mol.% As2Se3. The alloys below the eutectic horizontal are two-phase.
3.2 The Quasi-Binary System Cu2GeSe3-As2Se3
The phase diagram of the Cu2GeSe3-As2Se3 system was constructed from XRD and DTA results (Fig. 2). The system liquidus consists of the curves of the primary crystallization of η-solid solutions of Cu2GeSe3 and β-solid solutions of As2Se3. Nonvariant eutectic process L ↔ η + β takes place at 633 K (360 °C). The eutectic point e4 was determined by the Tamman triangle as the composition of 11.7 mol.% Cu2GeSe3, 88.3 mol.% As2Se3. The alloys below the eutectic horizontal are two-phase, β-solid solutions of As2Se3 and η-solid solutions of Cu2GeSe3. The solid solubility at 513 K (240 °C) is less than 2 mol.% Cu2GeSe3 and 2 mol.% As2Se3, respectively.
3.3 The Vertical Section Cu2GeSe3-CuAsSe2
The Cu2GeSe3-CuAsSe2 section was investigated by DTA and XRD methods (Fig. 3). The section liquidus is represented by the curves of the primary crystallization: ab of η-solid solutions of Cu2GeSe3, bc of LTM-Cu8GeSe6, cd of γ-solid solutions of Cu2Se. The section crosses the plane of the nonvariant peritectic process LU1 + γ ↔ LTM-Cu8GeSe6 + δ at 700 K (427 °C) (δ—solid solutions of CuAsSe2), where the volumes of monovariant peritectic processes L + γ ↔ LTM-Cu8GeSe6 and L + γ ↔ δ converge. The plane of the nonvariant peritectic process LU2 + LTM-Cu8GeSe6 ↔ η + δ lies at 650 K (377 °C) and the volumes of monovariant eutectic processes L ↔ η + LTM-Cu8GeSe6 and L ↔ LTM-Cu8GeSe6 + δ converge to this plane. The nonvariant peritectic process at 650 K (377 °C) ends with the exhaustion of both L and the crystals of LTM-Cu8GeSe6, since this section is the connecting line of the plane of this peritectic process. Therefore, the section alloys below 650 K (377 °C) are two-phase as confirmed by XRD data (Fig. 3).
The vertical section Cu2GeSe3-CuAsSe2: 1-L, 2-L + η, 3-η, 4-η + LTM-Cu8GeSe6, 5-L + η + LTM-Cu8GeSe6, 6-L + LTM-Cu8GeSe6, 7-L + LTM-Cu8GeSe6 + γ, 8-L + γ, 9-L + γ + δ, 10-δ + γ, 11-L + LTM-Cu8GeSe6 + δ, 12-η + δ, 13-δ (where γ-solid solutions of Cu2Se, δ-solid solutions of CuAsSe2, η-solid solutions of Cu2GeSe3)
3.4 The Vertical Section Cu8GeSe6-CuAsSe2
The Cu8GeSe6-CuAsSe2 section was investigated from DTA and XRD results (Fig. 4). Liquidus of the section is the curve of primary crystallization of γ-solid solutions of Cu2Se. The section then crosses the plane of the nonvariant process HTM-Cu8GeSe6 ↔ LTM-Cu8GeSe6 + γ + LU3 at 970 K (697 °C) which is due to the polymorphous transition of Cu8GeSe6 in the Cu2Se-GeSe2 system. The section crosses the plane of the nonvariant peritectic process LU1 + γ ↔ LTM-Cu8GeSe6 + δ at 700 K (427 °C). The process in the alloys of this section at this temperature ends with the exhaustion of both liquid and the crystals of γ-solid solutions of Cu2Se because the section coincides with the connecting diagonal of the plane of the nonvariant peritectic process. Therefore, the alloys below 700 K (427 °C) are two-phase, LTM-Cu8GeSe6 and δ, which is confirmed by XRD results (Fig. 4).
3.5 The Vertical Section Cu8GeSe6-As2Se3
The Cu8GeSe6-As2Se3 section was investigated by DTA and XRD methods (Fig. 5). The section liquidus consists of the curves ab (of the primary crystallization of γ-solid solutions of Cu2Se), bc LTM-Cu8GeSe6), cd (δ-solid solutions of CuAsSe2), and df (β-solid solutions of As2Se3). The nonvariant process HTM-Cu8GeSe6 ↔ LTM-Cu8GeSe6 + γ + LU3 at 970 K (697 °C) is caused by the polymorphous transition of Cu8GeSe6 at 983 K (710 °C) in the Cu2Se-GeSe2 system. The section then crosses another plane of the nonvariant peritectic process LU2 + LTM-Cu8GeSe6 ↔ η + δ at 650 K (377 °C). The volume of the monovariant eutectic process L ↔ δ + LTM-Cu8GeSe6 converges to this plane. The alloy 25 mol.% Cu8GeSe6-75 mol.% As2Se3 is two-phase according to XRD (Fig. 5) because the nonvariant process LU2 + LTM-Cu8GeSe6 ↔ η + δ at point g ends with the exhaustion of both liquid and the crystals of LTM-Cu8GeSe6, so the field 11 is two-phase. This field separates two three-phase regions of the co-existence of LTM-Cu8GeSe6, δ, η phases (field 10) and of the monovariant eutectic process L ↔ δ +η (field 12). The latter it ends at the plane of nonvariant eutectic process LE1↔ δ + η + β at 565 K (292 °C). The field of another monovariant eutectic process L ↔ δ + β (field 15) also converges to this plane. Below this plane the alloys are three-phase, δ + η + β (field 18).
The vertical section Cu8GeSe6-As2Se3: 1-L, 2-L + γ, 3-HTM-Cu8GeSe6 + γ, 4-L + γ + HTM-Cu8GeSe6, 5-HTM-Cu8GeSe6 + LTM-Cu8GeSe6 + γ, 6-γ + LTM-Cu8GeSe6, 7-L + γ + LTM-Cu8GeSe6, 8-L + LTM-Cu8GeSe6, 9-L + δ + LTM-Cu8GeSe6, 10-LTM-Cu8GeSe6 + η + δ, 11-η + δ, 12-L + η + δ, 13-L + δ, 14-L + β, 15-L + δ + β, 16-δ + β, 17-LTM-Cu8GeSe6 + δ, 18-δ + η + β, 19-β (where β-solid solutions of As2Se3, γ-solid solutions of Cu2Se, δ-solid solutions of CuAsSe2)
3.6 The Vertical Section A–B (A: 85 mol.% GeSe2-15 mol.% Cu2Se; B: 85 mol.% As2Se3-15 mol.% Cu2Se)
The section A–B was investigated by DTA and XRD methods (Fig. 6). The section liquidus consists of the curves ab of the primary crystallization of α-solid solutions of GeSe2, bc (η-solid solutions of Cu2GeSe3), cd (δ-solid solutions of CuAsSe2). The vertical section crosses two subsystems of the quasi-ternary system Cu2Se-GeSe2-As2Se3. In the GeSe2-Cu2GeSe3-As2Se3 subsystem, the section crosses the plane of nonvariant eutectic process LE2↔ α + η + β at 540 K (267 °C). The nonvariant eutectic process LE1↔ η + δ + β takes place in the Cu2GeSe3-As2Se3-Cu2Se subsystem at 565 K (292 °C). The samples below the planes of these processes are three-phase (fields 11, 12). These three-phase regions are separated by two-phase field 6, the existence of which is caused by the intersection with the quasi-binary system Cu2GeSe3-As2Se3. Monovariant eutectic processes L ↔ α + η and L ↔ η + β converges onto the plane of the nonvariant process at 540 K (267 °C). Monovariant eutectic processes L ↔ η + β (field 5), L ↔ η + δ (field 7), L ↔ β + δ (field 8) converges to the plane of the nonvariant eutectic process at 565 K (292 °C). The phase composition at the annealing temperature of 513 K (240) was determined by x-ray phase analysis (Fig. 6).
The vertical section A–B (A: 85 mol.% GeSe2-15 mol.% Cu2Se; B: 85 mol.% GeSe2-15 mol.% Cu2Se): 1-L, 2-L + α, 3-L + α + η, 4-L + η, 5-L + η + β, 6-η + β, 7-L + η + δ, 8-L + β + δ, 9-L + δ, 10-δ + β, 11-η + α + β, 12-η + δ + β (where α-solid solutions of GeSe2, β-solid solutions of As2Se3, γ-solid solutions of Cu2Se, δ-solid solutions of CuAsSe2)
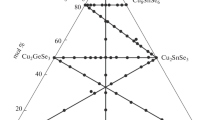
3.7 Isothermal Section of the Quasi-Ternary System Cu2Se-GeSe2-As2Se3 at 513 K (240 °C)
Based on the results of x-ray diffraction and MSA of the 137 samples (Fig. 7) the isothermal section of the system at 513 K (240 °C) was plotted (Fig. 8). It was investigated, that binary compounds crystallizes: Cu2Se in space group C2/c, a = 0.7135(2) nm, b = 1.2383(1) nm, c = 2.7387(4) nm, β = 94.307(2); GeSe2 in space group P21/c, a = 0.7007(2) nm, b = 1.6819(5) nm, c = 1.1806(3) nm, β = 90.74(2); As2Se3 in space group P21/c, a = 0.4267(2) nm, b = 0.9874(5) nm, c = 1.2794(7) nm, α = 109.96(4), what is in good agreement with literature data [19] for Cu2Se, [20] for GeSe2 and [21] for As2Se3. The existence of the three ternary compounds Cu8GeSe6, Cu2GeSe3 and CuAsSe2 was confirmed. Diffractograms of the ternary compounds were indexed: Cu2GeSe3 in space group Imm2, a = 1.1859(1) nm, b = 0.3951(4) nm, c = 0.54879(2) nm; Cu8GeSe6 in space group P63cm, a = 1.26421(2) nm, c = 1.17567(3) nm; CuAsSe2 in space group R3 (Cu7As6Se13 structure type) with the cell parameters a = 1.4014(2) nm, c = 0.9583(3) nm, what is in good agreement with literature data.[8,9,14]
The quasi-ternary system is divided into 4 subsystems: Cu2Se-Cu8GeSe6-CuAsSe2; Cu8GeSe6-CuAsSe2-Cu2GeSe3; CuAsSe2-Cu2GeSe3-As2Se3, and Cu2GeSe3-GeSe2-As2Se3. No extensive solid solutions were found, and the solid solubility based on binary and ternary compounds is less than 2 mol.%.
3.8 Liquidus Surface Projection of the Quasi-Ternary System Cu2Se-GeSe2-As2Se3
Liquidus surface projection of the Cu2Se-GeSe2-As2Se3 system (Fig. 9 and Table 1) was plotted using the literature data,[7,10] the experimental results of the study of four vertical sections, two phase diagrams and additional samples to find out the nonvariant points (Fig. 9). The liquidus surface consists of the fields of the primary crystallization of γ-solid solutions of Cu2Se, δ-solid solutions of CuAsSe2, HTM-Cu8GeSe6, LTM-Cu8GeSe6, η-solid solutions of Cu2GeSe3 (the largest field), α-solid solutions of GeSe2, and β-solid solutions of As2Se3. The fields are separated by 11 monovariant curves and 13 nonvariant points. The Cu2GeSe3-As2Se3 section is quasi-binary and divides the studied system into two subsystems, Cu2Se-Cu2GeSe3-As2Se3 and As2Se3-Cu2GeSe3-GeSe2. Point U1 lies on the plane of the nonvariant peritectic process LU1 + γ ↔ δ + LTM-Cu8GeSe6 that takes place at 700 K (427 °C). Point U2 belongs to the plane of the nonvariant peritectic process LU2 + LTM-Cu8GeSe6 ↔ η + δ at 650 K (377 °C). The lines of monovariant eutectic equilibria LU2-E1 ↔ η + δ, Le5-E1↔ δ + β, Le4-E1↔ η + β converge to the point E1 of the nonvariant eutectic process LE1↔ η + δ + β at 565 K (292 °C). The points U3 and U4 lie on the planes of the isothermal processes HTM-Cu8GeSe6 ↔ LTM-Cu8GeSe6 + γ + LU3 and HTM-Cu8GeSe6 ↔ LTM-Cu8GeSe6 + η + LU4 at 970 K which exist due to polymorphic transformation of HTM-Cu8GeSe6 into LTM-Cu8GeSe6 in the Cu2Se-GeSe2 quasi-binary system. In the As2Se3-Cu2GeSe3-GeSe2 subsystem one nonvariant eutectic process LE2↔ α + η + β takes place at 540 K (267 °C). The curves of monovariant eutectic processes Le2-E2↔ η + α, Le3-E2 ↔ α + β, Le4-E2 ↔ η + β converge to the point E2.
4 Conclusions and Future Work
The component interaction in the Cu2Se-GeSe2-As2Se3 system has been investigated by direct synthesis, x-ray phase and differential thermal analysis methods. For the first time phase equilibria in the quasi-ternary system have been investigated, and the triangulation of the system has been performed at 513 K (240 °C). At this temperature the existence of six single-phase fields has been identified based on the components GeSe2, As2Se3, Cu2Se of the system and the ternary compounds Cu2GeSe3, Cu8GeSe6, CuAsSe2. The liquidus surface projection of the Cu2Se-GeSe2-As2Se3 quasi-ternary system has been built based on the literary and obtained results of the investigations of the vertical sections Cu2GeSe3-CuAsSe2, Cu8GeSe6-CuAsSe2, Cu8GeSe6-As2Se3, A–B (A = 85 mol.% GeSe2-15 mol.% Cu2Se; B = 85 mol.% As2Se3-15 mol.% Cu2Se) and two phase diagrams GeSe2-As2Se3 and Cu2GeSe3-As2Se3. The liquidus surface projection consists of fields of primary crystallization of γ-solid solutions of Cu2Se, δ-solid solutions of CuAsSe2, η-solid solutions of Cu2GeSe3, α-solid solutions of GeSe2 and β-solid solutions of As2Se3, and HTM-Cu8GeSe6, LTM-Cu8GeSe6. They are separated by 11 monovariant curves and 12 nonvariant points. Six nonvariant processes take place in the Cu2Se-GeSe2-As2Se3 system: LU1 + γ ↔ δ + LTM-Cu8GeSe6 at 700 K (427 °C), LU2 + LTM-Cu8GeSe6 ↔ η + δ at 650 K (377 °C), LE1↔ η + δ + β at 565 K (292 °C), LE2↔ α + η + β at 540 K (267 °C), the points U3 and U4 lie on the planes of the isothermal processes HTM-Cu8GeSe6 ↔ LTM-Cu8GeSe6 + γ + LU3 and HTM-Cu8GeSe6 ↔ LTM-Cu8GeSe6 + η + LU4 at 970 K which exist due to polymorphic transformation of HTM-Cu8GeSe6 into LTM-Cu8GeSe6 in the Cu2Se-GeSe2 quasi-binary system.
This work was performed in order to gather more data for a thermodynamic assessment of the Cu2Se-BIVSe2-As2Se3 systems, where BIV-Si, Ge, Sn, which is currently in progress.
References
T.B. Massalsky, Binary Alloy Phase Diagrams, American Society for Metals, Metals Park, 1986
G.Z. Vinogradova, Glass Formation and Phase Equilibriain Chalcogenide Systems. Binary and Ternary Systems, Nauka, Moscow, 1984
D. Chalyy and M. Shpotyuk, Chalcogenide Glasses for High-Reliable Temperature Sensors, Lviv Polytech. Natl. Univ. Bull. Electron., 2012, 734, p 17-20
C. Carcaly, N. Chezean, J. Rivet, and J. Flahaut, Description of the GeSe2-Cu2Se System Phase Transition of the Cu8GeSe6 Compound, Bull. Soc. Chim. Franc, 1973, 4, p 1192-1195
T.V. Zolotova and Y.A. Karagodin, Investigation of phase equilibria in the Cu-Ge(Sn)-Se systems at the Cu2Se-Ge(Sn)Se2 sections, Sb. nauch. tr. po probl. mikroelektron., vol. XXI. MIET, Moscow, 1975, p 59-61
E.P. Rogachova, A.N. Melikhova, and N.M. Panasenko, Investigation of the GeSe2-Cu2Se System, Izv. Akad. Nauk SSSR. Neorgan. Mater., 1975, 11(5), p 839-843
Ya.E. Romanyuk and O.V. Parasyuk, Phase Eguilibria in the Quasi-Ternary Cu2Se-ZnSe-GeSe2 System, J. Alloys Compd., 2003, 348, p 195-202
E. Parthe and J. Garin, Sphalerite and Wurzit Structures for Ternary Chalcogenides with the Composition 12463, Monatsh. Chem., 1971, 102(5), p 1197-1208
S. Jualmes, M. Julien-Pouzol, P. Laruelle, and J. Rivet, High- and Low-Temperature Modifications of Double Selenide of Copper and Germanium, Acta Cryst., 1991, 47, p 1799-1803
S.A. Dembovskiy, V.V. Kyrylenko, and A.S. Khvorostenko, Phase Equilibria and Glass Formation in the As2Se3-Cu2Se and As2Se3-SnSe(PbSe) Systems, Neorgan. Mater., 1971, 7(10), p 1859-1861
A.S. Khvorostenko, V.V. Kyrylenko, B.I. Popov, S.A. Dembovskiy, V.K. Nikitina, and N.P. Lushnaya, Phase Diagram of the As2Se3-Cu2Se System, Neorgan. Mater., 1972, 8(1), p 73-79
R. Blachnik and G. Kurz, Compounds in the System Cu2Se-As2Se3, J. Solid State Chem., 1984, 55, p 218-224
K. Cohen, J. Rivet, and J. Dugue, Description of the Cu-As-Se Ternary System, J. Alloys Compd., 1995, 224, p 316-329
Y. Takéuchi and H. Horiuchi, The Application of the Partial Patterson Method and the Thirteen Fold Hexagonal Superstructure of Cu7As6Se13, Z. Kristallogr., 1972, 135, p 93-119
N.A. Goryunova, B.T. Kolomiyetz, and V.P. Shylo, Glassy Seminconductors, Zhurn. Techn. Fiziki, 1958, 28(5), p 981
L.G. Ayo and V.F. Kokorina, Glass Formation and Properties of Glasses of the As-Ge-Se System, Optico-mech. Prom., 1963, 2, p 36-43
G.M. Orlova, N.A. Alimbarashvili, I.I. Kozhyna, and A.P. Dorogokuntseva, The Character of the Structural-Chemical Interaction of the Components in the Glassy System As-Se-Cu, Zhurn. Neorg. Khimii, 1972, 45(11), p 128
V.Ya. Anosov, M.I. Ozerova, and Yu.Ya. Fialkov, Fundamentals of Physico-Chemical Analysis, Nauka, Moscow, 1976
L. Gulay, M. Daszkiewicz, O. Strok, and A. Pietraszko, Crystal Structure of Cu2Se, Chem. Met. Alloys, 2011, 4, p 200-205
G. Dittmar and H. Schäfer, The Crystal Structure of Germanium Dieselenide, Acta Cryst., 1976, 32, p 2726-2728
A. Renninger and B. Averbach, Crystalline Structures of As2Se3 and As4Se4, Acta Cryst., 1973, 29, p 1583-1589
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Klymovych, O., Ivashchenko, I., Olekseyuk, I. et al. Quasi-Ternary System Cu2Se-GeSe2-As2Se3. J. Phase Equilib. Diffus. 41, 157–163 (2020). https://doi.org/10.1007/s11669-020-00796-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00796-3