Abstract
Recently, new duplex surface treatments involving the use of pre-electroplating and pulsed plasma electrolytic nitrocarburizing (PPEN/C) have led to the possibility of using this material for applications usually typical for tool steels. In this work, we present results concerning the duplex treatment of pre-electroplating of Cr and PPEN/C of AISI 1020 mild steel. The samples were electroplated in a plating bath. After electroplating, the samples were nitrocarburized. The effects of time and applied voltage of PPEN/C process were discussed. The samples were characterized using scanning electron microscopy (SEM), atomic force microscopy (AFM), and microhardness testing. Electroplated layers of up to 100 μm were obtained. The subsequent PPEN/C increased the surface hardness to 1200 HV0.2 due to the formation of nitrides and carbides. Decreasing the size of nanocrystalline carbonitrides will lead to improvement of their wear resistance significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several processes are currently used to provide wear protection to metal surfaces in contact, and to extend the life of critical components used in bearings, cutting tools, and aerospace industry. Chromium carbide/nitride, such as CrC and CrN, films have high hardness, low friction coefficient, and good corrosion resistance (Ref 1). They can improve the load-bearing capacity and wear resistance of steel components that are widely used in engineering and industrial applications. Till now, various technologies have been developed to deposit or form these carbide/nitride, such as ion beam-assisted deposition (Ref 2), and plasma carbiding/nitriding (Ref 1). Hard coatings, which can withstand high stress without plastic deformation and fracture, are more effective in reducing friction and wear than soft layers. Despite its attractive engineering properties, mild steel appears to lack good wear resistance. However, the carbide/nitride films on steel produced by the aforementioned deposition methods are thin, otherwise the adhesion of the films is insufficient due to the increased internal stress in the films, and thereby delamination of the films has been observed under wear condition. Although plasma carbiding/nitriding treatment at 700-900 °C leads to firmly adhered carbide/nitride layer, the formation of the compound layer with the thickness more than 10 μm requires much higher temperature and longer treatment times (Ref 2), which results in worse fatigue behavior and earlier fracture due to the aging of the substrate material. It has been proved that the improvement efficacy of the wear resistance and load-bearing capacity strongly depend on the thickness and adhesion of the hard films (Ref 1), thus alternative methods to produce thick and firmly adhered carbide/nitride films on mild steel substrate at low temperature are still worth exploring. Plasma electrolysis is one of the most successful processes in which an adherent carbonitride layer on the surface of the part is obtained by subjecting the part to a saturated electrolyte from nitrogen and carbon at a sufficient temperature for ions diffusion. Plasma electrolytic carbonitriding has the advantage of providing close dimensional tolerance and low cost. It could be successfully used for different metals (Ref 3, 4). The present investigation was aimed at producing stable carbonitride coatings on pre-electroplated mild steel substrate. In this article, a relatively new method, pulsed plasma electrolytic carbonitriding (PPEC/N), was used to apply penetrative coatings on AISI 1020 substrate. Tribological properties of samples were studied after carbonitriding, using wear rig using normalized ASTM G105 standard. Temperature, as the main effective factor on carbonitriding process and process duration were discussed. Discussion about surface temperature was done by its relative factor of process, i.e., applied voltage during the coating process. Scanning electron microscope was employed to determine the layers on the surface.
Technical Work Preparation
Materials and Methods
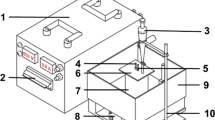
Chemical composition of AISI 1020 mild steel is given in Table 1 that resulted from quantometric test. In quantometric tests materials will transform to gas phase by an arc discharge on their surface and the elements in gas phase will be detected by analyzing with a laser beam. The beam can detect elements as well as their weight percentages. Most of the samples used were of 20 mm in diameter and 5 mm in thickness. The surfaces of the substrates were mechanically polished sequentially by 120, 400, 600, 800, 1200, and 3000 grit wet SiC emery paper, followed by fine polishing with alumina slurry to yield a mirror finish and chromium electroplating before being placed in the electrolyte. Finally, they were cleaned with acetone prior to carbonitriding. A schematic diagram of the reactor assembly used in this study is shown in Fig. 1. This unit consists of three main parts, an external cooling unit to maintain a constant temperature of electrolyte, an external power supply to strike and maintain plasma, and a bath for holding different parts and relevant electrolyte.
Scheme of the plasma reactor. 1: Cathode support; 2: Pulsed DC power supply; 3: Jacketed stainless vessel and also anode of the system; 4: Water inlet; 5: Cathode fixture; 6: Solution addition; 7: Thermocouple for measuring the temperature of electrolyte; 8: Water outlet; 9: Gas exhaust; 10: Treated cathode
The pulsed DC voltage necessary to obtain good carbonitriding is between 400 and 600 V. The work pieces to be carbonitrided are placed as the cathode of the system. The carbonitriding temperature was measured using a Type K thermocouple embedded exactly 500 μm from the surface of the sample as shown in Fig. 2. The temperature was measured by an analog voltmeter for fast response. Carbonitriding was carried out in the temperature range between 700 and 900 °C as measured by the thermocouple. Energy for diffusion of nitrogen and carbon into the work piece is provided by the kinetic energy transfer generated by the ion bombardment. The actual temperature of the sample may therefore be slightly different from the surface of it due to plasma heating.
Opened system used for measuring surface temperature of the sample. 1: CP-Ti Sample; 2: A hole between sample for thermocouple that machined up to 500 μm near the surface of other side of the sample; 3: Pure alumina mini crucible for insulating head of the thermocouple; 4: Head of type K thermocouple; 5: Alumina shield for insulating thermocouple between stainless steel rod; 6: Stainless steel rod; 7: Polymeric heat resistance and insulating coating for stainless steel rod
The cross-sectional samples of carbonitrided pieces were mounted in reinforced bakelite to ensure edge retention. Specimens were ground through successive SiC papers automatically, grades 240, 320, 400, 600, 800, 1000, 1200, 1500, 2000, 2500, 3000, and 4000 and finally polished with a 6 micron diamond paste and finished with a 0.1 micron alumina cloth.
Polishing times were increased by increasing the SiC abrasive paper number to avoid the onset of unacceptable beveling of the specimen edges. Rotation during grinding was found to be too harmful for the thin carbonitrided layers, resulting in heavy damage and fractures of the layers, and fixed grinding on SiC papers by a specific load automatically, without rotation of specimen between paper grades, was found to be effective.
The carbonitrided specimens were etched in Kroll’s reagent (1–3 mL HF, 2–6 mL HNO3, 100 mL water) (Ref 5). Thickness of various layers formed during carbonitriding was measured using photomicrographs taken at different magnifications using standard optical microscopes and a scanning electron microscope. The micro hardness indentations were made by means of a Buhler-Micromet1 micro hardness tester. A 200 g load with 20 s as loading time was used to provide optimum indentation depths across various layers.
Wear resistance was measured using a rig using ASTM G105 standard. The test conditions are detailed in Table 2. The test block is loaded against a ring that rotates at a given speed. The block and the ring were cleaned in acetone using an ultrasonic cleaner before and after the test, dried and weighed by means of an analytical balance to the nearest 0.1 mg. The scar width on the block is measured using an optical microscope. The friction force required to keep the block in place is continuously measured during the test with a load cell. The wear rate was evaluated by the weight loss of both the block and the ring.
Results and Discussion
Conditions of PEC/N
All the parts, with the exception of the thermocouple in the electrolyte shown in Fig. 1, were made of stainless steel. The chemicals used in electrolyte were pure carbamide and Natrium carbonate that were diluted in distilled water prior to entering into the plasma reactor. The work pieces were being ultrasonically cleaned in acetone before carbonitriding process. Following these procedures, small parts of pre-electroplated AISI 1020 steel were successfully carbonitrided. Incorporation of oxygen in the system develops a band on the surface and carbon contaminates with an unsightly black surface. A striking result was the successful carbonitriding of all surfaces of the mild steel samples including the lower surface around the contact with the stainless steel rod holder. This result indicates either uniform plasma penetration around the sample or more likely rapid transport by surface diffusion of nitrogen and carbon atoms along the carbonitrided surfaces. A drawback is that this phenomenon has so far prevented the development of any suitable masking technique. It might be noted that substrate at the carbonitriding temperature diffusionally bonds to most possible masking agents.
Microstructural Features
Plasma carbonitrided sample at 900 °C for 90 min resulted in the formation of several distinct phases on the surface as shown in the SEM micrograph of the cross-sectional sample in Fig. 3. Nanocrystalline microstructure was obtained in compound layer and surface morphology of the compound layer shows that this layer may exhibit good friction coefficient as these can be seen in Fig. 4 and 5. Compound layers grew parallel to the surface of the work pieces. The over layers consisted of oxide and nitride/carbide that formed due to increasing temperature of the substrate. A thick layer of solid solution of nitrogen and carbon was formed in the substrate. These results are compatible with our previous research (Ref 6). Figure 6 is an example of XRD pattern of a treated sample. The growth of various carbonitride layers in the substrate under various carbonitriding conditions are illustrated in Fig. 7. Different SEM micrographs of the cross sections of the substrate proved a gradual increase in the thickness of various layers with increasing carbonitriding voltage and as a result of that carbonitriding temperature and also treatment time. It must be mentioned that in this process after a period of time the compound layer thickness will not grow significantly.
Growth Kinetics
Kinetics of carbonitride layer growth on samples was studied. The overall growth of all layers (compound and diffusive layer) obeyed a simple t 1/2 relationship as shown in Fig. 7 for the substrate.
Hardness Measurements
To evaluate the effects of carbonitriding temperature on the extent of nitrogen and carbon dissolution, micro hardness traverses were carried out on samples which has been heat treated at 700, 800, and 900 °C for 21 min of exposure times. The effect of carbonitriding temperature on the micro hardness is illustrated in Fig. 8. A significant increase in the Knoop hardness up to 1100 is observed at the surface when compared with about 250 at the core in mild steel with increasing temperature (see Fig. 9). Carbonitriding at higher temperature also resulted in a higher hardness which may be due merely to the increased thickness of the hardened layers. The gradual decrease in the hardness toward the core indicates the decreasing amount of nitrogen and carbon in the carbonitride layers. All of the contours tend to be steep near the interface, indicating increasing concentration of nitrogen and carbon in such regions. The results in the present work are in agreement with most of the studies which indicated that the rate-controlling mechanism in the growth of carbonitride layers is diffusion of nitrogen and carbon through the product layers under high electrical field.
Wear Test
Although a lot of work has been carried out on the wear resistance of carbonitrided steels there is very little data published on the wear resistance of plasma electrolytic carbonitrided samples. It was observed in some investigations that rough nitrided surfaces showed better wear resistance than lapped nitrided surfaces or lapped implanted surfaces. The authors attributed this improvement in the wear resistance to the reduction in friction induced by chemical modification of the surface as a result of compound layer. In the present investigation, wear test according to normalized ASTM G105 was used to evaluate the wear resistance of the carbonitrided samples.
This is one of the most widely used industrial technique for evaluating the adhesive wear resistance of components. The weight loss of the uncarbonitrided and plasma electrolytic carbonitrided wear blocks is compared in Fig. 10 which indicates that the plasma electrolytic carbonitride block is an order of magnitude or more superior than the uncarbonitrided block. A slight reduction of the coefficient of dynamic friction was also observed in the carbonitrided sample (0.14) when compared with uncarbonitrided sample (0.23). Although a brief carbonitriding gives a major benefit, the results show that with longer time and thicker carbonitrided layer, wear resistance can be further improved. The scar widths of the uncarbonitrided and plasma electrolytic carbonitrided blocks were compared and it illustrated a major improvement in the wear resistance of the carbonitrided samples. In the carbonitrided blocks, major wear groves are seen while after carbonitriding only a very narrow scar is seen.
Finally, it has been revealed that the wear resistance of carbonitrided layers has a direct relationship with the average size of nanocrystalline carbonitrides. Figure 11 shows that by lowering the size of nanocrystalline carbonitrides, their wear resistance will improve significantly.
Conclusions
-
1.
AISI 1020 steel was successfully treated using plasma electrolytic carbonitriding and the process parameters were established for small components. Multiphase coating was performed based on CrN and CrC and also complex phases.
-
2.
Plasma electrolytic carbonitriding offers a hard case of as high as 1100 Knoop hardness as compared with 250 of the core.
-
3.
Nanocrystalline compound layer and a solid solution of nitrogen and carbon in the substrate in sequence from the surface were identified.
-
4.
SEM and micro hardness measurements indicate that the growth rate of carbonitrided layers is controlled essentially by the diffusion of nitrogen and carbon through the carbonitrided layers under high electrical field.
-
5.
Wear tests indicated that the carbonitrided parts exhibited excellent wear resistance when compared with uncarbonitrided part. Four to seventeen times less weight loss was found for the carbonitrided samples.
-
6.
Decreasing the size of nanocrystalline carbonitrides will lead to improvement of their wear resistance significantly.
References
Sh. Ahangarani, F. Mahboubi, A.R. Sabour, Effects of Various Nitriding Parameters on Active Screen Plasma Nitriding Behavior of a Low-Alloy Steel, Vacuum, 2006, 80, 9, pp. 1032–1037
C. Palacio, A. Arranz, Synthesis of Ti–Cr–N Thin Films by Reactive Ion-Beam Mixing of Ti/Cr Interfaces, Surf. Sci., 2006, 600, 11, Pp. 2385–2391
M. Aliofkhazraei, A.S. Rouhaghdam, P. Taheri, Ch. Dehghanian, Systematic Study of Nanocrystalline Plasma Electrolytic Nitrocarburising of 316L Austenitic Stainless Steel for Corrosion Protection, J. Mater. Sci. Technol., 2007, 23, 5, 665–671
M. Aliofkhazraei, A.S. Rouhaghdam, and T. Shahrabi, Pulsed Nanocrystalline Plasma Electrolytic Carburising for Corrosion Protection of a γ-TiAl Alloy (Part 1: Effect of Frequency and Duty Cycle), J. Alloy. Compds., 2008, 460(1-2), p 614–618
G.F. Vander Voort, Metallography: Principles and Practice, McGraw-Hill, 1984, ISBN 0-07-066970-8
M. Aliofkhazraei, A.S. Rouhaghdam, M. Sabouri, Effect of Frequency and Duty Cycle on Corrosion Behavior of Pulsed Nanocrystalline Plasma Electrolytic Carbonitrided CP-Ti, J. Mater. Sci., 2008, 43, 1624–1629
Acknowledgment
This work was funded by Arvandan Oil and Gas Production Company (TMU 85-09-66) and National Association of Nanoscience and Nanotechnology of Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliofkhazraei, M., Mofidi, S., Sabour Rouhaghdam, A. et al. Duplex Surface Treatment of Pre-Electroplating and Pulsed Nanocrystalline Plasma Electrolytic Carbonitriding of Mild Steel. J Therm Spray Tech 17, 323–328 (2008). https://doi.org/10.1007/s11666-008-9179-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-008-9179-z