Abstract
This study presents the dielectric properties of Ba2Zr2Si3O12 ceramics, synthesized using the solid state method. The cubic crystal structure of Ba2Zr2Si3O12 ceramics was conclusively verified through the analysis of XRD patterns and subsequent structural refinement. Refined lattice parameters of a = b = c = 10.23178 Å were found, with a corresponding unit cell volume of V = 1071.159026 Å3. The grain microstructure of the ceramics was characterized using SEM, which showed density of 97.6% for the Ba2Zr2Si3O12 ceramic. The Si-O bond valences (VSi-O) were found to be closely related to the τf of the Ba2Zr2Si3O12 ceramics. The microwave dielectric properties of the Ba2Zr2Si3O12 ceramics sintered at 1490°C were ascertained to be εr = 15.08, Q × f = 14885 GHz (f = 9.9 GHz), and τf = −78.6 ppm/°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microwave dielectric ceramics play a crucial role in communication technology as essential components in microwave electronics. These ceramics are commonly employed as resonators, filters, and substrates in integrated circuits.1,2 They possess three key parameters: an appropriate dielectric constant, a high quality factor, and a stable temperature coefficient of resonant frequency.3,4,5 The demand for dielectric materials with reduced size, high-frequency capability, and robust thermal stability has become increasingly urgent. Hence, there is a pressing need to investigate and produce dielectric substrates with enhanced performance. A high dielectric constant can effectively reduce the device's volume, but it simultaneously generates more heat within the integrated circuit. Conversely, a low dielectric constant can decrease the signal transmission delay, with the delay time being proportional to the dielectric constant.6,7 Moreover, it can increase the operating rate, thereby minimizing system energy losses. A higher quality factor can significantly improve the frequency selectivity, which is particularly critical in filters requiring precise frequency discrimination.8,9,10 In summary, materials featuring a low dielectric constant, high quality factor, and excellent thermal stability are the optimal choice for high-frequency electronic components.
Microwave ceramics with low dielectric constant have attracted the attention of many scholars because low dielectric constant can reduce signal delay. Li et al.11 studied a Li4WO5 ceramic with rock salt structure, and the crystal phase changed from cubic to orthorhombic at 700°C. Ba2MgWO6 ceramics with a double perovskite structure were discovered by Wang et al.12 The relative dielectric constant calculated based on the Fourier transform infrared (FTIR) spectrum is close to the measured value, which is about 16. Wu et al.13 found a spinel structure of MgGa2O4 ceramics, which not only has a low dielectric constant, but also has a τf value near zero. Jiang et al.14 studied a rock salt structure Li5ZnSnNbO8 ceramic with space group Fm-3 m, which has high density and good microwave properties (εr = 17.17, Q × f = 51519 GHz and τf = −28.2 ppm/°C). In recent years, microwave ceramics based on Si and Ge have garnered significant attention from researchers owing to their exceptional microwave dielectric properties. For instance, Zou et al. reported the discovery of an anti-reductive ceramic, Ba2ZnSi2O7 (Q × f = 27200 GHz), 15 exhibiting low dielectric loss. Du et al. investigated an orthorhombic microwave ceramic, Li4SrCaSi2O8 (Q × f = 90094 GHz),16 which demonstrated a high quality factor. Additionally, cubic microwave ceramics generally exhibit low dielectric loss, exemplified by Li2ZnGe3O8 (Q × f = 47400 GHz),17 Li4Mg2NbO6F (Q × f = 93300 GHz),18 Li4NbO4F (Q × f = 61,111 GHz),19 and Li5GaO8 (Q × f = 127040 GHz).20
In this study, we focus on the investigation of a novel microwave ceramic, Ba2Zr2Si3O12, synthesized by the traditional solid-state method. The microwave dielectric properties of Ba2Zr2Si3O12 ceramics are thoroughly explored. The crystal structure of Ba2Zr2Si3O12 ceramics is characterized using x-ray diffraction (XRD) analysis and subsequent refinement. Overall, Ba2Zr2Si3O12 ceramics with reduced dielectric loss exhibit promising potential as dielectric materials.
Experimental Procedure
Ba2Zr2Si3O12 ceramics were synthesized via the traditional solid-state method. High-purity raw materials, including BaCO3 (99%, Aladdin), ZrO2 (99%, Aladdin), and SiO2 (99%, Aladdin), were precisely weighed according to the chemical formula ratio. Table I shows the quantity of raw materials used. The resulting powder mixture was subjected to ball milling for 8 h, utilizing alcohol as the milling medium. Subsequently, the obtained slurry was dried in an electric furnace and calcined at 1300°C for 4 h. The same process was used to produce the dried powder. The dried powder was mixed with an appropriate amount of poly(vinyl alcohol) (PVA) binder and pressed under pressure of 20 MPa to form several white cylindrical specimens. These specimens were sintered in air at temperatures ranging from 1430°C to 1510°C for 4 h. The sintered sample was thermally etched for 30 min at 50°C below the sintering temperature after polishing.
The density of the Ba2Zr2Si3O12 samples was measured using the Archimedes principle. The sample was placed into the upper tray to obtain the mass of the sample in air. Then the sample was put into the lower container, and the mass of the sample in water was obtained after complete invasion of water. Finally, the density of the sample was calculated. To investigate the phase structure of the Ba2Zr2Si3O12 ceramics, XRD analysis was performed. The XRD data of the samples were refined using GSAS software. The microstructure of the Ba2Zr2Si3O12 ceramics was investigated using scanning electron microscopy (SEM) with a Hitachi SU1510 microscope (Hitachi, Japan). The key microwave dielectric properties, namely the relative dielectric constant (εr), quality factor (Q × f), and temperature coefficient of resonant frequency (τf), were ascertained using the Hakki–Coleman dielectric resonator technique. When taking τf measurements, a high- and low-temperature test chamber was employed, with a temperature range of 25°C through 85°C. The τf value was computed using the following equation:
Results and Discussion
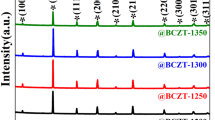
The XRD pattern of the Ba2Zr2Si3O12 samples sintered at temperatures of 1430–1510°C is presented in Fig. 1. The XRD results demonstrate that the samples calcined within this temperature range exhibit consistent patterns and align well with the standard PDF card (ICDD #00-025-1466), confirming the crystal phase to be Ba2Zr2Si3O12 without any impurity phases. To further analyze the crystal structure, the XRD data of the sample were refined using the K2Mg2S3O12 compound, which shares the same structure as Ba2Zr2Si3O12. Figure 2a exhibits the refinement of the XRD data for Ba2Zr2Si3O12 sintered at 1490°C, demonstrating good agreement between the simulated and experimental data. The refinement results of the Ba2Zr2Si3O12 ceramics are summarized in Table II. The Rwp and Rp values at various temperatures are both less than 15%, with Rp < Rwp.21 This finding further confirms the cubic structure of Ba2Zr2Si3O12, belonging to the P123 (198) space group. The atomic coordinates and occupation fractions of the Ba2Zr2Si3O12 ceramics are listed in Table III. Figure 2b shows the crystal structure of the Ba2Zr2Si3O12 ceramics, where a unit cell consists of four Ba2Zr2Si3O12 chemical formulas, eight BaO6 octahedra, eight ZrO6 octahedra, and 12 SiO4 tetrahedra, with each atom occupying a specific position. The Ba and Zr atoms are situated at 4a sites, while the Si and O atoms are located at 12b positions.
SEM images of the polished and thermally etched cross-sections and grain size distribution of the samples sintered at 1430–1510°C are presented in Figs. 3 and 4, respectively. As the sintering temperature increases, the average grain size of the sample continues to increase. At a calcination temperature of 1430°C, the sample exhibits a higher presence of pores between the grains, with an average grain size of 2.70 μm. Despite the presence of some pores, the sample sintered at the optimal temperature of 1490°C achieves a relative density of 97.6%. In addition, the average grain size of the sample is 4.03 μm. However, at 1510°C, the grain size of the sample increases dramatically, reaching an average size of 4.37 μm.22 Additionally, the sample exhibits a greater number of pores and poorer morphological quality due to the higher temperature employed.
Figure 5a illustrates the variation in relative density as a function of temperature for the Ba2Zr2Si3O12 samples. The relative density increases from 90.4% to 96.1% as the temperature increases from 1430°C to 1470°C, reaches its maximum value at 1490°C (97.6%), and subsequently exhibits a slight decrease. The temperature-dependent curve of the relative dielectric constant is shown in Fig. 5b. Ba2Zr2Si3O12 ceramics exhibit a peak value of 15.08 at 1490°C and a minimum value of 13.72 at 1430°C. The observed trend indicates an initial increase followed by a decrease in the εr value with temperature. εr is influenced by various factors, including density, ionic polarizability, porosity, molecular volume, and the presence of secondary phases. The lower εr value of Ba2Zr2Si3O12 ceramics can be attributed to the low ionic polarizability of Zr4+ (α = 3.25 Å3) and Si4+ (α = 0.87 Å3) ions.23 XRD and SEM analyses confirm the absence of apparent secondary phases in Ba2Zr2Si3O12 ceramics, indicating that secondary phases do not contribute significantly to the observed dielectric behavior. The relationship between the porosity-corrected dielectric constant (εcorr) and εr can be expressed as:24
where p represents porosity. The εcorr value of Ba2Zr2Si3O12 ceramics sintered at 1490°C is measured to be 15.62, slightly higher than the experimental value. The molecular volume is expected to vary inversely with the εr value;25 however, the observed changes in the crystal volume of Ba2Zr2Si3O12 ceramics reported in Table II do not conform to this relationship. For instance, the sample sintered at 1470°C exhibits a larger unit cell volume than that sintered at 1450°C, while displaying an increasing trend in εr. This behavior can be attributed to the corresponding increase in the relative density of the sample. Similarly, the sample sintered at 1510°C shows a slightly smaller cell volume than that sintered at 1490°C, but the εr value decreases slightly, likely due to a decrease in relative density.
The temperature dependence of Q × f and packing fraction of Ba2Zr2Si3O12 ceramics is presented in Fig. 5c. Q × f is influenced by various factors including densification, impurities, secondary phases, crystal structure, and packing fraction. The Q × f value achieves a maximum of 14,885 GHz at 1490°C. Prior to 1490°C, the Q × f value presents an increasing trend with temperature, followed by a decrease at 1510°C (14,195 GHz). The packing fraction of Ba2Zr2Si3O12 ceramics can be calculated using the following equation:26
where Z represents the number of Ba2Zr2Si3O12 chemical formula units in the unit cell, with a value of 4 for Ba2Zr2Si3O12 ceramics. The packing fraction of Ba2Zr2Si3O12 ceramics calcined at 1490°C is calculated to be 62.54%. A higher packing fraction corresponds to weaker lattice vibration, resulting in lower dielectric loss, thus showing a positive correlation with Q × f. However, it is observed that the packing fraction is inversely proportional to the Q × f value when the sample is calcined between 1450 and 1470°C. Importantly, the Q × f value displays a similar trend as the relative density, suggesting a significant influence of density on the Q × f of Ba2Zr2Si3O12 ceramics. The decrease in relative density at 1510°C is identified as a major cause for the deterioration of Q × f.
Figure 5d displays the relationship between τf and temperature for Ba2Zr2Si3O12 ceramics. The τf value of Ba2Zr2Si3O12 ceramics reaches a maximum of −78.6 ppm/°C at 1490°C, gradually increasing from 1430°C to 1470°C, and then decreasing to −79.5 ppm/°C at 1510°C. The τf is closely connected to the bond valence of chemical bonds. The bond valence of Ba2Zr2Si3O12 ceramics can be calculated using the following formulas:27
where Vij represents the sum of all bond valences, dij represents the bond length between atoms i and j, Rij represents the bond valence parameter, and b is a constant with a value of 0.37. Figure 6 illustrates the variation in Si-O bond valence and τf for Ba2Zr2Si3O12 ceramics. Previous studies have demonstrated an inverse relationship between bond valence and |τf| in crystal structures.28 The Si-O bond valences (VSi-O) for Ba2Zr2Si3O12 ceramics sintered at 1450°C, 1470°C, and 1490°C are calculated as 4.82, 5.05, and 5.21, respectively. Therefore, the decrease in |τf| at 1490°C is likely attributed to the increase in bond valence. Similarly, the increase in |τf| at 1510°C can be attributed to a decrease in bond valence. As depicted in the figure, the change in τf is not significant, which may be attributed to the absence of secondary phases and the insensitivity of Ba2Zr2Si3O12 ceramic material to temperature variations.29 Figure 7 shows the variation curves of εr and tan δ of the samples sintered at 1430–1510°C. εr and tan δ have the opposite trend, and tan δ first decreases and then increases. When the sintering temperature is 1430–1490°C, tan δ falls from 8.55 to the lowest point 6.65, and εr of the sample has a maximum value of 15.08. The tan δ of the sample sintered at 1510°C rises slightly, and εr declines to 14.93, which may be attributed to the decrease in the relative density of the sample.
Conclusion
In this study, we successfully synthesized a Ba2Zr2Si3O12 ceramic by the traditional solid-state reaction method and considered its microwave properties. Through XRD and Rietveld refinement analysis, we determined that the optimal sintering temperature range for achieving a pure cubic Ba2Zr2Si3O12 ceramic is 1430–1510°C. SEM images confirmed the uniformity and denseness of the sample grains when sintered at 1490°C. The microwave dielectric properties of the Ba2Zr2Si3O12 ceramics calcined at 1490°C for 4 h were as follows: εr = 15.08, Q × f = 14,885 GHz (f = 9.9 GHz), and τf = −78.6 ppm/°C. These results highlight the low loss and low dielectric constant characteristics of the Ba2Zr2Si3O12 ceramic, positioning it as a highly promising material for microwave dielectric ceramics applications.
References
J. Zhang, R. Zuo, and Y. Cheng, Relationship of the structural phase transition and microwave dielectric properties in MgZrNb2O8–TiO2 ceramics. Ceram. Int. 42, 7681 (2016).
F. Li, Y. Li, Y. Li, X. Feng, J. Zhang, X. Liu, Y. Lu, S. Wang, Y. Liao, T. Tang, and Q. Wen, Enhanced Na+-substituted Li2Mg2Mo3O12 ceramic substrate based on ultra-low temperature co-fired ceramic technology for microwave and terahertz polarization-selective functions. J. Eur. Ceram. Soc. 43, 384 (2023).
C. Dong, H. Wang, T. Yan, J. Zhao, J. Xu, and D. Wang, The influence of CaF2 doping on the sintering behavior and microwave dielectric properties of CaO-B2O3-SiO2 glass-ceramics for LTCC applications. Crystals 13(5), 748 (2023).
S. Li, C. Li, M. Mao, K. Song, Y. Iqbal, A. Khesro, S.S. Faouri, Z. Lu, B. Liu, S. Sun, and D. Wang, High Q×f values of Zn-Ni co-modified LiMg0.9Zn0.1-xNixPO4 microwave dielectric ceramics for 5G/6G LTCC modules. J. Eur. Ceram. Soc. 42(13), 5684 (2022).
T. Yan, C. Dong, J. Zhao, A. Khesro, Z. Liu, S. Sun, J. Li, R. Sun, and D. Wang, Lead-free borosilicate glass/fused quartz composites for LTCC applications. J. Mater. Sci. Mater. Electron. 33(18), 15033 (2022).
W. Bian, G. Zhou, Y. Dong, X. Lu, H. Zhu, S. Ta, L. Wang, and Q. Zhang, Structural analysis and microwave dielectric properties of a novel Li2Mg2Mo3O12 ceramic with ultra-low sintering temperature. Ceram. Int. 47, 7081 (2021).
Y. Chen, H. Li, X. Xu, Y. Zhang, J. Du, H. Wu, L. Liu, and G. Duan, Effects of (Ba1/3Sb2/3)4+ on the structure and dielectric properties of Ce2Zr3(MoO4)9 ceramic at microwave frequency. J. Alloy. Compd. 939, 168804 (2023).
Z.A. Mikhaylovskaya, E.S. Buyanova, A.I. Malkin, A.N. Korotkov, N.S. Knyazev, and S.A. Petrova, Morphological and microwave dielectric properties of Bi: SrMoO4 ceramic. J. Solid State Chem. 316, 123555 (2022).
L. Huang, S. Ding, X. Yan, T. Song, and Y. Zhang, Structure and microwave dielectric properties of BaAl2Si2O8 ceramic with Li2O–B2O3 sintering additive. J. Alloy. Compd. 820, 153100 (2020).
R. Gupta, E.Y. Kim, H.S. Shin, G.Y. Lee, and D.H. Yeo, Structural, microstructural, and microwave dielectric properties of (Al1-xBx)2Mo3O12 ceramics with low dielectric constant and low dielectric loss for LTCC applications. Ceram. Int. 49, 22690 (2023).
J. Li, L. Fang, H. Luo, J. Khaliq, Y. Tang, and C. Li, Li4WO5: a temperature stable low-firing microwave dielectric ceramic with rock salt structure. J. Eur. Ceram. Soc. 36, 243 (2016).
Y. Wang, J. Lv, J. Wang, F. Shi, and Z. Qi, Lattice vibrational characteristics, crystal structure and dielectric properties of Ba2MgWO6 microwave dielectric ceramic. Ceram. Int. 47, 17784 (2021).
S. Wu, J. Xue, R. Wang, and J. Li, Synthesis, characterization and microwave dielectric properties of spinel MgGa2O4 ceramic materials. J. Alloy. Compd. 585, 542 (2014).
Y. Jiang, Y. Shen, J. Yang, Z. Fang, X. Zhang, P. Zhao, and B. Tang, A novel ultra-low loss ceramic Li5ZnSnNbO8 with a rock salt structure. Mater. Chem. Phys. 277, 125457 (2022).
Z.Y. Zou, K. Du, X.K. Lan, W.Z. Lu, X.C. Wang, X.H. Wang, and W. Lei, Anti-reductive characteristics and dielectric loss mechanisms of Ba2ZnSi2O7 microwave dielectric ceramic. Ceram. Int. 45, 19415 (2019).
Q. Du, Q. Wen, L. Jiang, S. Liu, L. Ma, and H. Li, A novel low-temperature sintering microwave dielectric ceramic Li4SrCaSi2O8 with low-ϵr and low loss. Ceram. Int. 49, 22617 (2023).
H. Xiang, L. Fang, W. Fang, Y. Tang, and C. Li, A novel low-firing microwave dielectric ceramic Li2ZnGe3O8 with cubic spinel structure. J. Eur. Ceram. Soc. 37, 625 (2017).
S. Zhai, P. Liu, and S. Zhang, A novel high-Q oxyfluoride Li4Mg2NbO6F microwave dielectric ceramic with low sintering temperature. J. Eur. Ceram. Soc. 41, 4478 (2021).
X. Chu, J. Jiang, J. Wang, Y. Wu, L. Gan, and T. Zhang, A new high-Q×f Li4NbO4F microwave dielectric ceramic for LTCC applications. Ceram. Int. 47, 4344 (2021).
L. Ao, Y. Tang, J. Li, W. Fang, L. Duan, C. Su, Y. Sun, L. Liu, and L. Fang, Structure characterization and microwave dielectric properties of LiGa5O8 ceramic with low-εr and low loss. J. Eur. Ceram. Soc. 40, 5498 (2020).
G. Yao, J. Yan, J. Tan, C. Pei, P. Liu, H. Zhang, and D. Wang, Structure, chemical bond and microwave dielectric characteristics of novel Li3Mg4NbO8 ceramics. J. Eur. Ceram. Soc. 41, 6490 (2021).
C.F. Xing, J. Bao, Y.F. Sun, J.J. Sun, and H.T. Wu, Ba2BiSbO6: A novel microwave dielectric ceramic with monoclinic structure. J. Alloy. Compd. 782, 754 (2019).
R.D. Shannon, Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 73, 348 (1993).
K. Du, C.Z. Yin, Y.B. Guo, C. Zhang, X.C. Wang, W.Z. Lu, and W. Lei, Phase transition, infrared spectra, and microwave dielectric properties of temperature-stable CaSnSi1-xGexO5 ceramics. Ceram. Int. 47, 24781 (2021).
E.S. Kim, C.J. Jeon, and P.G. Clem, Effects of crystal structure on the microwave dielectric properties of ABO4 (A = Ni, Mg, Zn and B = Mo, W) ceramics. J. Am. Ceram. Soc. 95, 2934 (2012).
X. Zhang, Z. Fang, Y. Jiang, M. Wang, S. Gee, L. Zhou, H. Yang, F. Si, P. Zhao, Z. Xiong, S. Zhang, and B. Tang, Microwave dielectric properties of a low firing and temperature stable lithium magnesium tungstate (Li4MgWO6) ceramic with a rock-salt variant structure. J. Eur. Ceram. Soc. 41, 171 (2021).
J. Li, L. Fang, H. Luo, Y. Tang, and C. Li, Structure and Microwave dielectric properties of a novel temperature stable low-firing Ba2LaV3O11 ceramic. J. Eur. Ceram. Soc. 36, 2143 (2016).
W.S. Xia, L.X. Li, L.J. Ji, P. Zhang, P.F. Ning, and Q.W. Liao, Phase evolution, bond valence and microwave characterization of (Zn1−xNix)Ta2O6 ceramics. Mater. Lett. 66, 296 (2012).
J. Li, Y. Tang, Z. Zhang, W. Fang, L. Ao, A. Yang, L. Liu, and L. Fang, Two novel garnet Sr3B2Ge3O12 (B = Yb, Ho) microwave dielectric ceramics with low permittivity and high Q. J. Eur. Ceram. Soc. 41, 1317 (2021).
Acknowledgments
This work was supported by the graduate scientific research innovation project of Shaoyang University (Grant No. CX2022SY035) and the National Natural Science Foundation of China (Grant No. 52102123).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liang, D., Zhang, X. et al. Crystal Structure, Sintering Behavior, and Microwave Dielectric Properties of Low-Permittivity Ba2Zr2Si3O12 Ceramics. J. Electron. Mater. 52, 7164–7170 (2023). https://doi.org/10.1007/s11664-023-10651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10651-z