Abstract
Dielectric properties of a magnesium silicate (MgSiO3) linear low-density polyethylene nanocomposite are studied in the X-band frequencies to realize its application as a substrate for high-frequency devices. MgSiO3 ceramics are synthesized by conventional solid state technique. Structural and morphological characteristics of MgSiO3 nanoparticles are confirmed by X-ray diffraction, Fourier transform infrared spectroscopy and transmission electron microscopy. Transmission electron microscopy images reveal the average particle size to be ~100 nm. Composites are prepared by uniform dispersion of the nano-inclusions MgSiO3 in a linear low-density polyethylene matrix in three different wt.%, viz. 2%, 4% and 6%. The fractured lateral side of the composite is examined under a scanning electron microscope to ensure the uniform dispersion of the inclusions in the polymer. Water absorption measurement is carried out based on ASTM D570-98. Densities of the samples are measured by hydrostatic weighing by using Archimedes principle. Thermal conductivity of the composites is measured by modified hot plat method. Nicholson-Ross approach is used to investigate the dielectric characteristics of composites. The permittivity and dielectric loss tangent of the nano-composite in the X-band are found to be ~2.2–2.5 and 10−2–10−4 for all the inclusion concentration respectively. To see the applicability of the nanocomposite in the field of antenna, return losses are calculated from complex permittivity and complex permittivity data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main concern for electronic devices with the development of modern electronic industries is substrates with specific properties for high-frequency operation.1 The key factors that determine the capability of a material to be used in high-frequency microwave devices are its complex permittivity and complex permeability. Since a single material may not always be able to meet all the requirements for a specific application, composites such as a polymer matrix with inclusions of various types (nano, ceramic, magnetic etc.) could be a good choice.2 Dielectric ceramics have good dielectric strength, low dielectric constant and low loss tangent,3 which ensures that composites of polymer-dielectric ceramic nanomaterials can be potential substrate materials for different types of microwave applications.
Many ceramic nanomaterials such as Mg2SiO4, Ba(Mg1/3Ta2/3)O3, Mg4Nb2O9, and ZnAl2O4 have been reported so far because of their suitable microwave dielectric properties.4 Nano-ceramic-PTFE composite and fiberglass polymer resin composites are used for high-frequency microwave substrate applications.5 In Ref. 6 Silicon rubber-BaBiLiTeO6 ceramic polymer composite substrate is reported for 5G applications. The composites reported the complex permittivity (εʹ) and loss tangent (tan δe) are 6.86 and 4.7 ×10−2 , respectively, at 10 GHz. In Ref. 7 dielectric properties of TiO2-polyolefin-based composite are investigated. For 70 wt% filler loading, the reported values of εʹ and tan δe are 7.03 and 4.2×10−3, respectively. In Ref. 8 Dielectric properties of PTFE loaded with varying %VF of MgTiO3 are studied. The measured value of εʹ is 5.5 and tan δe = 2.7× 10−4 (at 10 GHz), making the composite a promising material for electronic packaging. Dielectric properties of CaTiO3 -loaded polypropylene composite fabricated through compression molding are studied using X-band cavity perturbation for microwave substrate application.9 The composite has an effective εʹ and tan δe is 11.74 and 7×10−3 , respectively, for the highest filler concentration. Dielectric properties of PTFE composites filled with perovskite (Ca,Li,Sm)TiO3 (CLST) dielectric ceramic with volume fractions up to 60% are prepared and studied at 10 GHz.10 The obtained εʹ and tan δe is 5.5 and 2.7 × 10−4 for optimum filler loading makes it a promising material in the electronic packaging field. In Ref. 11 Si3N4 -filled PTFE composites is prepared for composites for substrates at microwave frequencies. At 62 %VF of Si3N4 ceramic filler, the prepared Si3N4/PTFE composite exhibits excellent low εʹ and tan δe , and the values are 4.03 and 1.4 × 10−3 , respectively. A low-density polyethylene (LDPE)-nano-titania (TiO2) composite with εʹ = 2.4 and tan δ = 4×10−3 has been reported as substrate for X-band devices.12 It is also reported in Ref. 12 that the bandwidth and S11 of the device can be further enhanced by the use of a graded composite as substrate material. Moreover, a frequency-independent behaviour of complex permittivity is reported in a comparative study of dielectric behaviour for different composites at the X-band.13 The composites used are nano-barium titanate (BaTiO3) inclusions in two different polymer matrices, namely, polyaniline (PANI) powder and maleic resin. In Ref. 14 microstrip patch antennas are fabricated over composites of polyvinyl chloride with nano-barium titanate (εʹ = 37) and nano-neodymium titanate (εʹ = 85) inclusions. A modified W-shaped Ka band antenna on an Al2O3 ceramic substrate with εʹ = 7 is presented in Ref. 15. In Ref. 16 a circular patch monopole antenna on a high permittivity ceramic polytetrafluoroethylene (PTFE) composite with εʹ = 6.3 is reported with enhanced radiation characteristics. In Ref. 17 a rectangular microstrip patch antenna is designed on a (Zn0.7Mg0.3)TiO3 ceramic dielectric substrate with εʹ = 21.3. In Ref. 18 (Bi13Co11)Co2O40-Co3O4 ceramic nanocomposite is synthesized and widely researched for application as a microstrip patch antenna (MPA) substrate. The reported εʹ and tan δ of the material are 6.58 and 2×10−4 , respectively.
In this paper, nano-MgSiO3 is synthesized and used as inclusions in a linear low-density polyethylene (LLDPE) polymer matrix. LLDPE is a polymer with linear characteristics and acceptable electrical properties, good tensile properties significant resistance to chemicals, low water absorbance and high thermal stability. The decomposition temperature of LLDPE is 438.85°C with weight loss of 0.01%. The major weight loss occurs between the temperatures 441.98° and 513.15°C due to growth of volatiles.19,20,21 The softening temperature of LLDPE is ~85°C.22 Measurements of complex permittivity and permeability of the composites (LLDPE-nano-MgSiO3) with 2, 4, and 6 wt.% of MgSiO3 with in LLDPE polymer matrix is performed in the X-band.
Experimental Work
Synthesis of Nano-MgSiO3 and LLDPE-Nano-MgSiO3 Composite Substrates
MgSiO3 nanoparticles are synthesized by conventional solid-state method23 by using high-purity (>99%) oxides MgO and SiO2. Firstly, MgO and SiO2 (Sigma Aldrich) powders are ball milled for 5 h. After drying, the MgO and SiO2 powders are mixed uniformly and calcined at 1200°C for 8 h. The powders are then ball milled again for another 2 h before drying to obtain the nanoparticles. To fabricate the composite for complex permittivity and permeability characterization, LLDPE is first dissolved in toluene and stirred using a mechanical stirrer at 100°C. After dissolving, the desired wt.% (2, 4 and 6) of the synthesized MgSiO3 nanoparticles are added to make a homogenous mixture of the inclusions in the polymer. A viscous solution is obtained when toluene completely evaporates. The solution is then poured into a die with dimensions 10 × 22 × 2 mm3, and left to congeal for 2 h at room temperature, and a composite substrate of LLDPE-nano-MgSiO3 is thus obtained.24 Further, it is observed that beyond 6 wt.%, the ductility of the substrate decreases.
Structural and Morphological Characterization
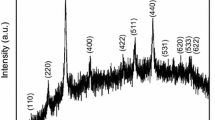
X-ray diffraction (XRD) patterns of the synthesized MgSiO3 nanoparticles are shown in Fig. 1 recorded by using a Bruker D8 (Make: BRUKER AXS, GERMANY, Model: D8 FOCUS) X-ray powder diffractometer using Cu Kα radiation (λ = 1.5406 Å). The peaks at 2θ = 36.81°, 42.80°, 62.16°, 74.56° and 78.47° corresponding to the (h k l) value (1 1 2), (3 3 0), (0 6 0), (2 1 4), (4 6 4) are the diffraction peaks of MgSiO3 (ICDD 01-074-2017). A thorough examination of the pattern reveals that the sample has an orthorhombic structure having lattice parameters: a = 4.7450 Å, b = 4.9070 Å, c = 6.8530 Å.
Fourier transform infrared (FTIR) spectra of nano-MgSiO3 is recorded using a NICOLET FTIR spectrometer in 400–4000 cm−1 range (Make: NICOLET, model: IMPACT 410). FTIR spectra are obtained by mixing the sample with KBr and grinding to a very fine powder. The FTIR spectrum of the synthesized MgSiO3 is shown in Fig. 2. The -OH stretching vibration caused by physical adsorption of water and bound water is responsible for the broad characteristic band ranging from 3770 cm−1 to 2480 cm−1 in Fig. 2. The stretching vibration of zeolite water causes the distinctive band at 1641 cm−1. The distinctive bands at 1186–1047 cm−1 belong to Si-O bending vibrations, whereas the band at 480 cm−1 correlates to Mg-O stretching vibrations.
To confirm the average particle size of the synthesized nanomaterial, a transmission electron microscope (TEM) (Model: TECNAI G2 20 S-TWIN Make: FEI COMPANY, USA) is used. The TEM image shown in Fig. 3 reveals that the average particle size of synthesized nano-MgSiO3 is ~100 nm. To confirm the uniform distribution of MgSiO3 in LLDPE composite substrates, the lateral side of fractured samples of 6 wt% is scanned via a scanning electron microscope (SEM) (Model: JSM- 35CF,Make: JEOL). The SEM images of LLDPE-nano-MgSiO3 composite substrates are shown in Fig. 4. It is observed that clustering of inclusions formed in the composite (shown in the inset of Fig. 4), which is due to the high viscosity of LLDPE. It is confirmed from the SEM images that MgSiO3 nanoparticles are uniformly distributed in the LLDPE matrix.
Material properties may change under the influence of adverse environmental conditions such as high moisture or humidity. So properties such as water absorbance, density, thermal conductivity, coefficient of thermal expansion (CTE) must be explored. Water absorption measurement is carried out based on ASTM D570-98 and as shown in Fig. 5. For this increase in wt% of pure LLDPE and the composites (2, 4 and 6%), a sample of size 10 × 22 × 2 mm3 is measured after immersing in distilled water. At first, weights of the dry composite is measured. Then, at room temperature, identical pieces of pure LLDPE and composites are dipped in distilled water. After soaking for 24 h, the samples are removed from water and the weight is recorded. This procedure is repeated until saturation is achieved.25 The percentage of water absorption is calculated by using the expression given below
where wa = percentage of absorbing water. wf = final weight of the pieces. wi = initial weight of the pieces.
From the water absorption graph it is seen that the saturation for all the samples are obtained after 168 h of immersion. The saturated water absorption values is given in Table I.
Densities of the samples are measured by hydrostatic weighing by using the Archimedes principle. For this, a beaker that is partially filled with ethanol is placed on balance and the combined mass of the beaker is measured (w1). Then the composite sample is suspended in the ethanol and the new reading is taken (w2). Finally, the sample is allowed to settle on the bottom of the beaker and a reading is taken (w3).26 By using the value of w1 w2 w3 and d (density of ethanol), the density is measured by using the following expression
The obtained value of density is listed in Table II.
The thermal conductivity of pure LLDPE and the composites are measured by a modified heat flow meter. The experimental setup consists of a sample holder with insulated chamber for heat application, heat sensors and an interfacing computer for data acquisition. The schematic is shown in Fig. 6a. For measurement, a sample of dimensions 3 × 3 × 0.02 cm2 is inserted between a hot plate and a cold plate, and the thermal conductivity is determined once the steady-state is achieved by applying Fourier’s law27 expressed as
where Q/A is heat flow per unit area, k is the thermal conductivity, x is the thickness of the sample, Th is the hot surface temperature, Tc is the cold surface temperature
The calculated value of thermal conductivity of pure LLDPE and the composites are shown in the Table III. The thermal conductivity of LLDPE is very low because of the strong C-H bond and absence of free electrons, although heat transfer is observed due to vibrations of the bond. As the percentage of inclusion increases, the value of thermal conductivity increases due to the relatively higher thermal conductivity of MgSiO3 than LLDPE. The increase in thermal conductivity as a function of filler wt.% is shown in Fig. 6b. The increasing trend in thermal conductivity due to filler loading is minimal due to the low filler increase.
The coefficient of thermal expansion (CTE) is a crucial feature that must be investigated further. CTE is determined using the mixing rule, which states that the CTE of a two-phase composite is directly proportional to the wt.% of the filler.28 The mixing rule is stated as follows:
where αf, αm and αc are the filler, matrix and composite, and CTEs, respectively, and f is the filler volume percent. Table IV shows the computed CTE values.
Dielectric Characterization
The Nicholson–Ross approach29 is used to calculate complex permittivity and permeability at the X-band. An Agilent WR-90 X11644A rectangular waveguide line, an Agilent E8362C vector network analyzer, sample container of λ/4 thickness, and an interfacing computer are used in the measurement setup. Prior to measurements, the system was calibrated using the through–reflect–line (TRL) approach to reduce any inaccuracies.30 Following TRL calibration, LLDPE-nano-MgSiO3 composite substrates of 10 ×22 × 2 mm3 are placed into the sample holder to obtain complex permittivity and permeability.
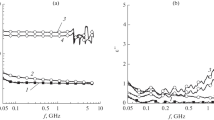
The real part of complex permittivity (εʹ) and dielectric loss tangent (tan δe) of LLDPE-nano-MgSiO3 for the considered wt.% in the X-band microwave frequency at room temperature is shown in Fig. 7a and b. Pure LLDPE and MgSiO3 have a εʹ of 2.2 and 6.4.17 The standard error of εʹ and tan δe from its mean is depicted as the error bars in the plots of Fig. 7a and b. The deviations observed are due to various reasons such as casting pressure and formation of agglomerated of the inclusions in the polymer matrix. The real part of complex permittivity (εʹ) is found to be almost constant over the X-band frequency range. The average values of real permittivity of the composite with 2, 4 and 6 wt.% inclusion content are 2.31, 2.42 and 2.56, respectively. Since the wt.% variances of inclusions in the host matrix are minimal, the change in εʹ is also minor. Because of intrinsic electric dipole polarization and interfacial polarization, permittivity of the composite substrate increases as the inclusion content is increased. The polarization in MgSiO3 is because of the presence of Mg2+ ions. As Mg2+ ions are strongly polarizable, increase in Mg2+ ions as inclusion concentration increases, enhances the total permittivity of the composite.31 A slight non-linear behaviour is observed in the complex permittivity measurements due to space charge accumulation at the multiple interfaces formed as a result of heterogeneous composite system of dispersant MgSiO3 in the LLDPE matrix.32
Figure 7b depicts the dielectric loss tangent, tan δe, of the composite LLDPE-nano-MgSiO3. Dielectric loss essentially relates to two forms of energy dissipation, namely conduction loss and dielectric loss. Prospective dielectric substance has low energy loss as heat. The tan δe values of the composite LLDPE-nano-MgSiO3 appears to range between 10−2 and 10−4 with frequency. Because of the coupling effect of the host matrix and inclusion in the Fabry–Perot resonance (FPR) region, negative values of tan δe in the X-band are observed.33 Negative loss tangent values are not necessarily an inherent property of the material, as they also depend on the thickness of the substrate.34 Corresponding FPR can readily alter the linked electromagnetic field if the substrate thickness is λ/2. In this investigation, a sample holder thickness of λ/4 is considered. In their studies, Axelrod et al. explain this unusual behaviour. According to the study, when more energy is emitted than absorbed at a specific frequency, a negative loss phenomenon is seen. The total energy of the sample must be conserved according to the principle of conservation of energy. The non-compensated matrix-anchored charges inside the polymer matrix and on the interface is responsible for the negative loss phenomenon. Non-bonding orbitals, full or empty for positive and negative ions, may generate charge anchoring, which facilitates charge separation. As a result, the molecules achieve a metastable state in which charge separation causes energy accumulation. Conditions such as frequency and temperature can remove this metastable state. Charge avalanche recombination may occur at the required temperature and frequency, resulting in energy release.35
The real part of complex permeability (μʹ) and loss tangent (tan δm) for 2, 4 and 6 wt.% of LLDPE-nano-MgSiO3 composite is shown in Fig. 8a and b. The figure shows the standard deviation of μʹ and tan δm from its mean value. Increasing inclusion content in the host matrix reduces the average value of μʹ from 1.07 to 1.04. This might have been caused by the presence of non-magnetic MgSiO3 in the non-magnetic polymer host matrix, which lowers intergranular magnetic interaction. The value of tan δm varies within the range of 10−1–10−5.
The comparison of dielectric properties of LLDPE-MgSiO3 composite with a few of the recently reported polymer-ceramic composites is listed in Table V.
Return Loss
By using complex permittivity and permeability data obtained from measurements conducted, return loss (RL) of the fabricated sample is calculated. The RL is evaluated by using Eq. 5, which is expressed as
where
where Zn is the input impedence w.r.t. the free space, fp is the frequency of operation and t = 2 mm is the thickness of the substrate and c = 3×108m/s is the speed of light.36 Calculated RL of the composite substrate over the X-band is shown in Fig. 9. The figure suggests a low value of RL as expected with a minimum value of −1 dB. At lower frequencies, a small notch is noticed in RL, but at higher frequencies, it tends towards 0 dB. From the literature, it is observed that nanocomposites with an RL value −45 dB is suitable for absorber application.37 The obtained values of RL suggests that LLDPE- nano-MgSiO3 composite can be used as a substrate for high frequency microstrip patch antenna applications.
Conclusion
MgSiO3 nanoparticles are synthesized by conventional solid state technique. The diffraction peaks at 2θ = 36.81°, 42.80°, 62.16°, 74.56° and 78.47° corresponding to the (h k l) value (1 1 2), (3 3 0), (0 6 0), (2 1 4), (4 6 4) in the XRD, confirms the presence of magnesium silicate. In the FTIR studies, the characteristic bands at 1186–1047 cm−1 and at 480 cm−1 obtained from FTIR studies correspond to the Si-O bending vibrations and Mg-O stretching vibration, respectively. The TEM images confirm the average sizes of the nano-MgSiO3 as ~100 nm. Composites of LLDPE and nano-MgSiO3 are prepared with 2 wt.%, 4 wt.% and 6 wt.% of MgSiO3. The SEM images indicate that inclusions are uniformly dispersed in LLDPE. The complex permittivity shows a linear trend with minimal variation, which is due to the accumulation of space charge at the inclusion-polymer interfaces. As per the investigation, the composite can be utilized as microstrip patch antennas substrate, which is further confirmed by the calculated RL values. The complex permittivity and dielectric loss tangent results established in this work also fulfil the International Technology Roadmap for Semiconductors (ITRS) criteria for substrate materials.38
References
M.T. Sebastian, R. Ubic, and H. Jantunen, Low-loss Dielectric Ceramic Materials and Their Properties. Int. Mater. Rev. 60, 392 (2015).
S. Fu, Z. Sun, P. Huang, Y. Li, and N. Hu, Some Basic Aspects of Polymer Nanocomposites: A Critical Review. Nano Mater. Sci. 1, 2 (2019).
C. Zhang, R. Zuo, J. Zhang, and Y. Wang, Structure-Dependent Microwave Dielectric Properties and Middle Temperature Sintering of Forsterite (Mg1–xNix)2SiO4. J. Am. Ceram. Soc. 98, 3 (2015).
P.S. Anjana, M.T. Sebastian, M.N. Suma, and P. Mohanan, Low Dielectric Loss PTFE/CeO2 Ceramic Composites for Microwave Substrate Applications. Int. J. Appl. Ceram. Technol. 5, 325 (2008).
M.H. Ullah, M.T. Islam, M.R. Ahsan, W.N.L. Mahadi, T.A. Latef, and M.J. Uddin, A Low-cost Fiberglass Polymer Resin Dielectric Material-Based Microstrip Patch Antenna for Multiband Applications. Sci. Eng. Compos. Mater. 23, 447 (2016).
V.L. Vilesh and S. Ganesanpotti, Silicone Rubber-BaBiLiTeO6 Composites: Flexible Microwave Substrates for 5G Applications. J. Electron. Mater. 1, 11 (2022).
C. Wang, B. Wu, X. Mao, T. Deng, R. Li, Y. Xu, and X. Tang, The Effects of Concentration and Particle Size of TiO2 on the Dielectric Properties of Polyolefin-Based Microwave Substrates. Chem. Select 5, 1464 (2020).
H. Peng, H. Ren, M. Dang, Y. Zhang, Z. Gu, X. Yao, and H. Lin, The dimensional effect of MgTiO3 ceramic filler on the microwave dielectric properties of PTFE/MgTiO3 composite with ultra-low dielectric loss. J. Mater. Sci. Mater. Electron. 30, 6680 (2019).
V. Drishya, A.N. Unnimaya, R. Naveenraj, E.K. Suresh, and R. Ratheesh, Preparation, Characterization, and Dielectric Properties of PP/CaTiO3 Composites for Microwave Substrate Applications. J. Appl. Ceram. Technol. 1, 810 (2016).
L. Zheng, J. Zhou, J. Shen, W. Chen, Y. Qi, S. Shen, and S. Li, The Dielectric Properties and Dielectric Mechanism of Perovskite Ceramic CLST/PTFE Composites. J. Mater. Sci. Mater. Electron. 28, 11665 (2017).
Y. Yuan, Z. Li, L. Cao, B. Tang, and S. Zhang, Modification of Si3N4 Ceramic Powders and Fabrication of Si3N4/PTFE Composite Substrate with High Thermal Conductivity. Ceram. Int. 45, 16569 (2019).
D. Sarmah, N.S. Bhattacharyya, and S. Bhattacharyya, Study of Graded Composite (LDPE/TIO2) Materials as Substrate for Microstrip Patch Antennas in X-band. IEEE Trans. Dielectr. Electr. Insul. 20, 1845 (2013).
H.C. Pant, M.K. Patra, A. Verma, S.R. Vadera, and N. Kumar, Study of the Dielectric Properties of Barium Titanate-Polymer Composites. Acta Mater. 54, 3163 (2006).
A. Hoorfar and A. Perrotta, An Experimental Study of Microstrip Antennas on Very High Permittivity Ceramic Substrates and Very Small Ground Planes. IEEE Trans. Antennas Propag. 49, 838 (2001).
M.H. Ullah and M.T. Islam, Design of a Modified W-shaped Patch Antenna on Al2O3 Ceramic Material Substrate for Ka-Band. Chalcogenide Lett. 9, 61 (2012).
M.H. Ullah and M.T. Islam, Miniaturized Modified Circular Patch Monopole Antenna on Ceramic-Polytetrafluroethylene Composite Material Substrate. J. Comput. Electron. 13, 211 (2014).
D.D. Sandu, O. Avadanei, A. Ioachima, G. Banciua, and P. Gasner, Microstrip Patch Antenna With Dielectric Substrate. J. Optoelectron. Adv. Mater. 5, 1381 (2003).
J.A.L. Matias, I.B.T. Silva, M.E.T. Sousa, J.B.L. Oliveira, M.A. Morales, and D.R. da Silva, (Bi13Co11)Co2O40-Co3O4 Nanocomposites: Synthesis, Characterization and Application as Substrate for Microstrip Patch Antenna. Ceram. Int. 47, 21530 (2021).
P.J. Gogoi, S. Bhattacharyya, and N.S. Bhattacharyya, Linear Low Density Polyethylene (LLDPE) as Flexible Substrate for Wrist and Arm Antennas in C-Band. J. Electron. Mater. 44, 1071 (2015).
K.S. Chun, S. Husseinsyah, and N.F. Syazwani, Properties of Kapok Husk-Filled Linear Low-Density Polyethylene Eco Composites: Effect of Polyethylene-Grafted Acrylic Acid. J. Thermoplast. Compos. Mater. 29, 1641 (2016).
J. He, H. Ye, S. Liu, and J. Zhao, Preparation and Properties of LLDPE/PMVS Blends. Polym. Polym. Compos. 14, 611 (2006).
M. Shafiq, T. Yasin, and S. Saeed, Synthesis and Characterization of Linear Low-Density Polyethylene/Sepiolite Nanocomposites. J. Appl. Polym. Sci. 123, 1718 (2012).
M.E. Song, J.S. Kim, M.R. Joung, and S. Nahm, Synthesis and Microwave Dielectric Properties of MgSiO3 Ceramics. J. Am. Ceram. Soc. 91, 2747 (2008).
P. Dutta, B. Saikia, P.R. Alapati, and K. Borah, Linear Low-Density Polyethylene-Thermotropic Liquid Crystal Composite Substrate for High Frequency Devices: Dielectric Characterization. J. Electron. Mater. 50, 1434 (2021).
N.S.B.M. Hafidz, M.S.B.M. Rehan, and H.B. Mokhtar, Effect of Alkaline Treatment on Water Absorption and Thickness Swelling of Natural Fibre Reinforced Unsaturated Polyester Composites. Mater. Today Proc. 48, 720 (2022).
J.J. Bensch and H.J. Brynard, New Approach to Density Measurements Using Archimedes’s Principle. Nat. Phys. Sci. 239, 96 (1972).
M.G. Gomes, I. Flores-Colen, F. Da Silva, and M. Pedroso, Thermal Conductivity Measurement of Thermal Insulating Mortars with EPS and Silica Aerogel by Steady-State and Transient Methods. Constr. Build. Mater. 172, 696 (2018).
S. Thomas, V. Deepu, S. Uma, P. Mohanan, J. Philip, and M.T. Sebastian, Preparation, Characterization and Properties of Sm2Si2O7 Loaded Polymer Composites for Microelectronic Applications. Mater. Sci. Eng. B 163, 67 (2009).
A.M. Nicolson and G.F. Ross, Measurement of the Intrinsic Properties of Materials by Time Domain Techniques. IEEE Trans. Instrum. Meas. 19, 377 (1970).
G.F. Engen and C.A. Hoer, Thru-Reflect-Line: An Improved Technique for Calibrating the Dual Six-Port Automatic Network Analyzer. IEEE Trans. Microw. Theory Technol. 27, 987 (1979).
S. Ozah and N.S. Bhattacharyya, Nanosized Barium Hexaferrite in Novolac Phenolic Resin as Microwave Absorber for X-band Application. J. Magn. Magn. Mater. 342, 92 (2013).
A.F. Ahmad, Z. Abbas, S.J. Obaiys, and D.M. Abdalhadi, Improvement of Dielectric, Magnetic and Thermal Properties of OPEFB Fibre-Polycaprolactone Composite by Adding Ni-Zn Ferrite Polymers. Polymers 9, 12 (2017).
T. Karpisz, B. Salski, P. Kopyt, and J. Krupka, Measurement of Dielectrics from 20 to 50 GHz with a Fabry-Pérot Open Resonator. IEEE Trans. Microw. Theory Tech. 67, 1901 (2019).
K.L. Zhang, Z.L. Hou, L.B. Kong, H.M. Fang, and K.T. Zhan, Origin of Negative Imaginary Part of Effective Permittivity of Passive Materials Chin. Phys. Lett. 34, 097701 (2017).
E. Axelrod, A. Puzenko, Y. Haruvy, R. Reisfeld, and Y. Feldman, Negative Dielectric Loss Phenomenon in Porous Sol-Gel Glasses. J. Non-Cryst. Solids 352, 4166 (2006).
R. Kumar, H.K. Choudhary, S.P. Pawar, S. Bose, and B. Sahoo, Carbon Encapsulated Nanoscale Iron/Iron-Carbide/Graphite Particles for EMI Shielding and Microwave Absorption. Phys. Chem. Chem. Phys. 19, 23268 (2017).
H.K. Choudhary and B. Sahoo, Microwave Absorption Property of Hydrothermal Synthesized RGO/PbFe12O19 Nano Composite. J. Converg. Technol. 3, 918 (2017).
X. Huang and C. Zhi, Polymer Nano Composite: Electrical and Thermal Properties (Berlin: Spinger, 2016), pp. 17–18.
Acknowledgments
The authors would like to acknowledge Prof. Nidhi S Bhattacharya, Department of Physics, Tezpur University (Central), Assam, for the support and guidance provided for carrying out some of the experimental works reported in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dutta, P., Borah, K. Dielectric Studies of Nano-Magnesium Silicate and Linear Low-Density Polyethylene Composite As a Substrate for High-Frequency Applications. J. Electron. Mater. 51, 5368–5375 (2022). https://doi.org/10.1007/s11664-022-09775-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09775-5