Abstract
A conducting polymer, polyaniline (PANI)/Ni0.5Zn0.5Fe2O4 composites with high dielectric absorbing properties and electromagnetic shielding effectiveness at low frequencies were successfully synthesized through a simple in situ emulsion polymerization. PANI was doped with hydrochloric acid to improve its electrical properties and interactions with ferrite particles. PANI/Ni0.5Zn0.5Fe2O4 composites were characterized by X-ray diffraction analysis, scanning electron microscopy, transmission electron microscopy, Fourier transform infrared spectroscopy and thermal gravimetric analysis. Frequency dependence of dielectric and ac conductivity (σac) studies have been undertaken on the PANI/Ni0.5Zn0.5Fe2O4 composites in the frequency range 50 Hz–5 MHz. The electrical conduction mechanism in the PANI/Ni0.5Zn0.5Fe2O4 is found to be in accordance with the electron hopping model. Further, frequency dependence of electromagnetic interference (EMI) shielding effectiveness (SE) is studied. The EMI shielding effectiveness is found to decrease with an increase in the frequency. The maximum value 55.14 dB of SE at 50 Hz was obtained at room temperature for PANI/Ni0.5Zn0.5Fe2O4 composites in the 50 Hz–5 MHz frequency range. PANI/Ni0.5Zn0.5Fe2O4 composites were demonstrated as a promising functional material for the absorbing of electromagnetic waves at low frequencies because of a large amount of dipole polarizations in the polymer backbone and at the interfaces of the Ni–Zn ferrite particles and PANI matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electromagnetic radiation impact on human beings and other living beings due to radiations emitted from mobile and other communication devices is a major problem faced recently. Further, electromagnetic interference with other electric and magnetic devices is also a major problem leading to decrease in the efficiency of the devices. Electromagnetic interference (EMI) shielding is needed to protect electronics instruments from electromagnetic interference which is emitted by computer circuit, radio transmitters, cellular phones, electric motors and overhead power lines etc. [1–4]. In order to shield the electromagnetic microwave interference, a considerable amount of research has been focused on the development of the composites of polymers and inorganic materials that have enhanced microwave absorption and electromagnetic shielding properties. Polymer based magnetic composites have been widely used in the area of EMI shielding, because of their unique collective properties of electrical conduction, corrosion resistance, low density and flexibility, which are better than those of metallic materials [1–8].
In particular, conductive polymer based ferrite composite materials are promising candidates for EMI shielding application due to their favorable properties. Ferrite materials have attracted special attention in the field of electronics and telecommunication industry because of their novel dielectric and electrical properties. Ferrite particles possess important characteristics, such as low permittivity, high resonance frequency, high resistivity, low density and good chemical stability [9]. The properties of ferrites are sensitive to their composition and microstructure, which in turn are sensitive to their processing conditions [10–14]. Conducting polymers are attractive class of materials similar to metals while retaining flexibility and processability of conventional polymers [15, 16]. Among the various conducting polymers polyaniline (PANI) occupies a foremost position, because of its distinctive characteristics like inexpensiveness of the monomer, ease of processing and excellent stability. Nowadays, many interesting studies focus on the conductive polymer/ferrite composites to get the functional materials with the characteristics of both conductive polymer and ferrite particles. Further, these composites have been widely used because of their lower density as well as their good environmental stability.
In the current work, the Ni0.5Zn0.5Fe2O4 particles were synthesized by solution combustion method and PANI/Ni0.5Zn0.5Fe2O4 composites were synthesized by in situ polymerization method. Structural, magnetic, dielectric and electromagnetic shielding studies have been undertaken on the synthesized composites.
2 Experimental
2.1 Materials
All chemicals and reagents were analytical (AR) grade and used as received without further purification. All solutions were freshly prepared.
2.2 Synthesis of Ni0.5Zn0.5Fe2O4 ferrites
The combustion method has been considered to be fast, simple and inexpensive, allowing the production of fine, homogeneous crystalline powders without the risk of contamination. The Ni0.5Zn0.5Fe2O4 has been prepared by solution combustion method using stoichiometric composition of Nickel nitrate, Zinc nitrate and Ferric nitrate as oxidizers and Urea as a fuel. The aqueous solution containing redox mixture was taken in a Pyrex dish and heated in a muffle furnace maintained at 450 ± 10 °C. The mixture finally yields porous and voluminous powder.
2.3 Preparation of PANI/Ni0.5Zn0.5Fe2O4 composites
PANI/Ni0.5Zn0.5Fe2O4 composites having Aniline monomer to ferrite weight ratio of 1:4.8 were synthesized by in situ emulsion polymerization method [7]. Solution of Aniline (0.5 mL) was dissolved in 50 mL (1 M) HCl. Ammonium persulfate (APS) (0.58 g), which acted as an oxidant was dissolved in 50 mL (1 M) HCl. About 2.4 g of Ni0.5Zn0.5Fe2O4 particles were added to the oxidant solution. Ferrite particles dispersed in APS was added drop-wise in the aniline emulsion. Temperature of the reaction mixture was controlled below 5 °C by using an ice bath. Over the course of reaction, the colour of the mixture had transformed from milky white to dark green. Polyaniline/Ferrite composites were obtained by centrifuging the green mixture for 15 min, then washed with de-ionized water and filtered by using Whatman filter paper No. 3 and then dried to get the PANI/Ni0.5Zn0.5Fe2O4 composites in powder form.
2.4 Characterization
The structural characterization was carried out using the Philips X-ray diffractometer. The morphology of the PANI was examined by scanning electron microscopy (SEM) (JEOL Model JSM-6390LV). Morphology of PANI/Ni0.5Zn0.5Fe2O4 composite was examined by transmission electron microscopy (TEM) (JEOL/JEM 2100). The Fourier transform infrared (FT-IR) spectrum (400–4000 cm−1) of the sample was recorded on a Bruker Alpha spectrophotometer in KBr pellets. Magnetic studies were conducted using vibrating sample magnetometer (Lakeshore VSM 7410). Dielectric, ac conductivity and electromagnetic shielding studies on the PANI/Ni0.5Zn0.5Fe2O4 composites have been undertaken using impedance analyzer model HIOKI 3532-50 LCR HiTESTER Version 2.3 (frequency range 42 Hz–5 MHz). The measurements were carried out on the samples in the form of compressed pellet at room temperature in the frequency range 50 Hz–5 MHz.
3 Results and discussion
Figure 1 shows the X-ray diffraction pattern of PANI/Ni0.5Zn0.5Fe2O4 composites. Analysis of X-ray diffraction pattern revealed the formation of Ni–Zn ferrite with single spinel phase [17]. The average crystallite size of the Ni0.5Zn0.5Fe2O4 sample was determined by the Debye–Scherrer formula, \(D = \frac{{{\text{k}}\uplambda }}{{\beta \text{cos} \theta }}\), where D is the crystallite size, k = 0.9 is a correction factor to account for the particle shapes, β is the full width at a half maximum of the most intense diffraction peak (311) plane, λ is the wavelength of a Cu Kα radiation (1.5418 Å) and θ is the Bragg angle. The average crystallite size of the prepared sample is around 46 nm. Observed peak at 2θ = 25° indicates the semi crystalline nature of PANI present within the composite [18].
The morphology of polyaniline and PANI/Ni0.5Zn0.5Fe2O4 composites were analyzed by SEM and TEM respectively. The SEM micrograph of pure PANI is shown in Fig. 2. The network structure of pure PANI can be clearly observed in Fig. 2. Polyaniline is tubular in shape and tends to form an entangled network that is beneficial for effective transport of electrons [7]. The TEM image of PANI/Ni0.5Zn0.5Fe2O4 composites is displayed in Fig. 3. The TEM image (Fig. 3) shows that the ferrite particles are embedded in the PANI matrix.
Figure 4 shows the FTIR spectrum of the as-prepared PANI/Ni0.5Zn0.5Fe2O4 composites in the wave number range 400–4000 cm−1 in KBr medium. The FTIR spectrum of PANI/Ni0.5Zn0.5Fe2O4 composites shows a strong absorption band around 481–800 cm−1. The vibrating band at around 481–800 cm−1 corresponds to the metal-oxide stretching vibrations present in the PANI/Ni0.5Zn0.5Fe2O4 composite sample. The band at around 588 cm−1 is attributed to the tetrahedral stretching vibration in the crystal lattices of the ferrite [7, 19]. Observed peaks at 1640, 1555 and 1493 cm−1 are the characteristic peaks of polyaniline. The band at 1640 cm−1 is attributed to the C=N vibration of the quinoid ring. The bands with absorption at 1555 and 1493 cm−1 in the spectrum are attributed to the C=C stretching vibrations of the quinoid ring and benzenoid ring respectively. The bands at 1400–1625 cm−1 and 1128 cm−1 of the composites indicate the coupling effect of ferrite and polyaniline [7, 20].
In order to see the effect of temperature on the thermal behavior of the PANI/Ni0.5Zn0.5Fe2O4 composites, thermo-gravimetric analysis of PANI/Ni0.5Zn0.5Fe2O4 composites has been carried out from 25 to 700 °C in the nitrogen atmosphere. The thermogram of PANI/Ni0.5Zn0.5Fe2O4 composites (Fig. 5) reveals that composite is thermally stable up to 371 °C with a weight loss of less than 6 %. A weight loss of 1.62 % has been observed up to 100 °C, which is attributed to the evaporation of free water molecules from the composite [7]. The weight loss of 3.91 % observed between 100 and 371 °C is ascribed to the removal of water and dopant molecules adsorbed on polyaniline. A weight loss of 10.98 % has been observed from 371 to 700 °C, which is attributed to the decomposition of molecular chains of polyaniline [21].
Magnetic measurements on PANI/Ni0.5Zn0.5Fe2O4 composites were carried out using vibrating sample magnetometer at room temperature. M–H curve for PANI/Ni0.5Zn0.5Fe2O4 composites is as shown in above Fig. 6. It can be seen that the magnetization of PANI/Ni0.5Zn0.5Fe2O4 composites exhibits hardly hysteretic behavior under the applied magnetic field. Similar behavior has been observed for polyacrylamide/Zn0.4Ni0.5Cu0.1Fe2O4 composites [22]. The values of coercivity (H c ) and saturation magnetization (M s ) of PANI/Ni0.5Zn0.5Fe2O4 composites are found to be 46.33 G and 0.88342 emu respectively. Observed lower values of H c and M s for the present PANI/Ni0.5Zn0.5Fe2O4 composites may be attributed to the decreased interactions among the Ni–Zn ferrite particles within the PANI polymer matrix. Further, various anisotropy mechanisms such as magnetocrystalline anisotropy, surface anisotropy and interparticles interactions also contribute to the magnetic properties of the composites [23, 24].
Figure 7 shows the frequency dependence of the dielectric constant (ε′) at room temperature for PANI/Ni0.5Zn0.5Fe2O4 composites. The dielectric constant is found to decrease with the increase in the frequency. The dielectric constant of PANI/Ni0.5Zn0.5Fe2O4 composites depends mainly on the polarization in the polymer backbone and the interfacial polarizations among the Ni–Zn ferrite particles and PANI matrix. The variation of dielectric constant with frequency reveals the dispersion due to Maxwell–Wagner type interfacial polarization which is in agreement with the Koop’s phenomenological theory [25, 26]. According to this model, a dielectric medium has been assumed to be made of well conducting grains, which are separated by poorly conducting grain boundaries. In such case, the applied field on the sample drops primarily across the grain boundaries and a space charge polarization is built up at the grain boundaries. The space charge polarization is regulated by the existing free charges on the grain boundary and the conductivity of the sample. The effect of grain boundaries is predominant at lower frequencies. Ferrite ions can be considered as dipolar as they possess Fe3+ and Fe2+ ions [17, 27, 28]. It is well known that the exchange of electrons between Fe3+ and Fe2+ ions results in the local displacement of charge carriers in the direction of electric field leads to electric polarization [26]. At lower frequencies exchange of electrons between Fe3+ and Fe2+ ions is in coordination with external applied alternating field so that dipole alignment is well in-phase with alteration in field thus contributing maximum polarization. As the frequency of applied alternating field increases polarization decreases and attains certain value, this is because that beyond certain frequency of external field there is lagging in exchange of electrons between Fe3+ and Fe2+ ions with external applied alternating field, thus the dipole alignment cannot keep up with external applied alternating field thus unable to contribute to total polarization [29, 30]. In polyaniline, strong polarization occurs due to presence of polaron/bipolaron and other bound charges (dipoles), which lead to the high value of ε′. Observed higher value of dielectric constant for PANI/Ni0.5Zn0.5Fe2O4 composites at lower frequency is due to the difference in the relative dielectric constant of Ni-Zn ferrite and polyaniline which results in the accumulation of more space charge and strong orientation polarization that consequently leads to the improved electromagnetic wave absorption. Thus, the decrease of the dielectric constant with increasing frequency in the present PANI/Ni0.5Zn0.5Fe2O4 composites can be ascribed to the fact that the electron exchange between Fe2+ and Fe3+ ions within the PANI matrix cannot follow the change of the external applied field beyond a certain frequency.
The power of the dielectric loss of the absorbing material is characterized by the dielectric loss angle tangent tanδ = ϵ″/ϵ′. Good dielectric loss material should possess a larger dielectric loss tangent independent of frequency. Frequency dependence of dielectric loss tangent (tanδ) at room temperature for PANI/Ni0.5Zn0.5Fe2O4 composites is as shown in Fig. 8. At lower frequencies the rate of decrease in dielectric loss is found to be much higher than at higher frequencies. The dielectric loss decreases with the increasing frequency, which is a normal behavior of any ferrite material [31]. At lower frequencies the exchange of electron between Fe2+ and Fe3+ ions within the PANI matrix is high energy consuming process since it is the region of high resistivity, thus the loss is high. Electron exchange process with much lower energy consumption is possible at higher frequencies since it is low resistivity region compared to lower frequencies, thus the loss is low.
The variation of ac conductivity with frequency at room temperature is as shown in the Fig. 9. The ac conductivity is found to increase with an increase in the frequency. The electrical conductivity in ferrites is mainly due to the hopping of electrons between ions of the same element present in more than one valence state and distributed randomly over crystallographic equivalent lattice sites [31]. Values of ac conductivity is less at low frequency region because of less hopping of electrons between Fe2+ and Fe3+ ions due to activeness of grain boundaries, as frequency increases the conductive grains become more active, this enhances the hopping of electrons between Fe2+ and Fe3+ ions, thereby increases the ac conductivity. At low frequency, the ac conductivity is found to be weakly frequency dependent due to the nonequilibrium occupancy of the trap charges in the PANI matrix. A further amplification of frequency reduces the occupancy of the trap centers by making them available for conduction [32]. It enables the conductive state to become more active by promoting the hopping of electrons and holes.
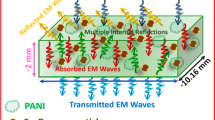
An electromagnetic shield is a conductive material which attenuates (through reflection and absorption) electromagnetic energy [1–8]. The electromagnetic interference (EMI) shielding effectiveness (SE) is defined as the ratio of the incident to the transmitted power and is usually expressed in decibels (dB) as follows;
where, Pi is the incident power and Pt is the transmitted power.
The total EMI shielding effectiveness of material of thickness t is given by SE = SE R + SE A , where SE R and SE A are due to reflection and absorption, respectively. i.e.
The reflection part of SE is given by [1, 33],
and the absorption part of SE is,
where t is the thickness of the shield, μr is the magnetic permeability, δ is the skin depth, \(\delta = \left[ {\frac{2}{{\omega_{H} \mu \sigma_{ac} }} } \right] ^{1/2}\) defined as the depth to which the radiation penetrates into the material, while its intensity decreases to \(e^{ - 1}\) of its original strength and ω H is angular frequency (ω H = 2πf H ) and εo is the permittivity of the free space. From the above equations, it is clear that the SE depends mainly on the conductivity of the material. In conducting polymers, SE results both from reflection and the absorption [34].
Using the above two Eqs. (3) and (4), the value of SER and SEA were calculated. The total SE of the composites was calculated by adding SER and SEA. The SE of PANI/Ni0.5Zn0.5Fe2O4 composites as a function of frequency at room temperature is shown in the Fig. 10. The maximum value 55.14 dB of SE at 50 Hz was obtained for PANI/Ni0.5Zn0.5Fe2O4 composites at room temperature. According to Guan et al. [35] and Li et al. [36], the material can be used for electromagnetic shielding purpose in the low frequency range if the SE is higher than 5 and 15 dB respectively. Our PANI/Ni0.5Zn0.5Fe2O4 composites possess acceptable shielding effectiveness (~8.41 to 55.14 dB) in the studied frequency range. The SE of the present PANI/Ni0.5Zn0.5Fe2O4 composites were found to decrease with increase of frequency, suggesting that the present samples may only be effective for the electromagnetic interference shielding at low frequencies. The SE values of PANI/Ni0.5Zn0.5Fe2O4 composites are high at low frequency region due to dipolar and interfacial polarizations, which plays an important role in lower frequency region [8]. From SE variations, it is clear that at the lower frequency region the PANI/Ni0.5Zn0.5Fe2O4 composite is a good shielding material with a high SE value of 55.14 dB. Present PANI/Ni0.5Zn0.5Fe2O4 composite possess combined advantages of both Ni–Zn ferrite and conducting polymer PANI. In the case of PANI polymer, the electromagnetic waves are reduced due to the intrinsic dipole polarization, whereas for ferrites, the attenuation of the electromagnetic waves is due to the existence of free electrons and material defects [7].
4 Conclusions
A conducting polymer, PANI/Ni0.5Zn0.5Fe2O4 composites were synthesized by inexpensive one-step in situ polymerization of Aniline monomer with Ni0.5Zn0.5Fe2O4 particles, where Ni0.5Zn0.5Fe2O4 particles were prepared via simple solution combustion method using stoichiometric composition of nickel nitrate, zinc nitrate and ferric nitrates as oxidizers and urea as a fuel. PANI/Ni0.5Zn0.5Fe2O4 composites were characterized by XRD, SEM, TEM, FTIR spectroscopy and TG analysis. Frequency dependence of dielectric and ac conductivity (σac) studies have been undertaken on the PANI/Ni0.5Zn0.5Fe2O4 composites in the frequency range 50 Hz-5 MHz. The electrical conduction mechanism in the PANI/Ni0.5Zn0.5Fe2O4 is found to be in accordance with the electron hopping model. Further, frequency dependence of EMI shielding effectiveness is studied. The EMI shielding effectiveness is found to decrease with an increase in the frequency. The maximum value 55.14 dB of SE at 50 Hz was obtained at room temperature for PANI/Ni0.5Zn0.5Fe2O4 composites in the 50 Hz–5 MHz frequency range. Finally, present PANI/Ni0.5Zn0.5Fe2O4 composites also exhibited a high dielectric constant in the low frequency range, so these composites can be utilized in the charge storage devices, decoupling capacitors and electromagnetic interference (EMI) shielding applications.
References
N.F. Colaneri, L.W. Shacklette, IEEE Trans. Instrum. Meas. 41, 291 (1992)
D.D.L. Chung, J. Mater. Eng. Perform. 9(3), 350 (2000)
S. Geetha, K.K. Satheesh Kumar, C.R. Rao, M. Vijayan, D.C. Trivedi, J. Appl. Polym. Sci. 112, 2073 (2009)
J. Ma, K. Wang, M. Zhan, RSC Adv. 5, 65283 (2015)
F.X. Qin, H.X. Peng, N. Pankratov, M.H. Phan, L.V. Panina, M. Ipatov, V. Zhukova, A. Zhukov, J. Gonzalez, J. Appl. Phys. 108, 044510 (2010)
V. Panwar, J.-O. Park, S.-H. Park, S. Kumar, R.M. Mehra, J. Appl. Polym. Sci. 115, 1306 (2010)
W. Wang, S.P. Gumfekar, Q. Jiao, B. Zhao, J. Mater. Chem. C 1, 2851 (2013)
B.J. Madhu, S.T. Ashwini, B. Shruthi, B.S. Divyashree, A. Manjunath, H.S. Jayanna, Mater. Sci. Eng., B 186, 1 (2014)
Z.W. Li, Z.H. Yang, L.B. Kong, Procedia Eng. 75, 19 (2014)
B.J. Madhu, K. Bindu, S. Hamsa, C.P. Sowmya, B. Shruthi, A. Manjunath, G.H. Virupakshappa, in IEEE conference Publications, International Conference on Nano Science, Engineering and Technology (ICONSET), 373 (2011). doi:10.1109/ICONSET.2011.6167984
B.J. Madhu, S. Razikha Banu, M. Kavya, B. Shruthi, M.S. Vasanthkumar, T.V. Sannamma, M. Surendra, in IEEE Conference Publications, International Conference on Nano Science, Technology and Societal Implications (NSTSI11), 1 (2011). doi:10.1109/NSTSI.2011.6111787
B.J. Madhu, M. Kavya, S. Razika Banu, B. Shruthi, C.P. Sowmya, H.S. Jayanna, Adv. Mater. Res. 584, 295 (2012)
B.J. Madhu, V. Jagadeesha Angadi, H. Mallikarjuna, S.O. Manjunatha, B. Shruthi, R. Madhu Kumar, Adv. Mater. Res. 584, 299 (2012)
B.J. Madhu, B.N. Rashmi, A. Banu, G.A. Seema, B. Shruthi, H.S. Jayanna, AIP Conf. Proc. 1512, 1008 (2013)
M.A. Rahman, P. Kumar, D. Park, Y. Shim, Sensors 8, 118 (2008)
N. Gandhi, K. Singh, A. Ohlan, D.P. Singh, S.K. Dhawan, Compos. Sci. Technol. 71, 1754 (2011)
J. Azadmanjiri, Mater. Chem. Phys. 109, 109 (2008)
S.B. Kondawar, A.I. Nandapure, B.I. Nandapure, Adv. Math. Lett. 5(6), 341 (2014)
H.I. Hsiang, C.C. Chen, J.Y. Tsai, Appl. Surf. Sci. 245, 252 (2005)
A.A. Farghali, M. Moussa, M.H. Khedr, J. Alloys Compd. 499, 98 (2010)
M. Guy, Y. Jin, S. Woen, S. Soon, Synth. Met. 124, 342 (2001)
H. Qiu, X. Feng, L. Li, C. Xiang, H. Qian, Mater. Chem. Phys. 124, 1039 (2010)
J. Jiang, L. Li, F. Xu, J. Phys. Chem. Solids 68, 1656 (2007)
K.W. Wagner, Am. Phys. 40, 317 (1973)
C.G. Koops, Phys. Rev. 83, 121 (1951)
N. Rezlescu, E. Rezlescu, Phys. Status Solidi A 23, 575 (1974)
L.I. Rabinkin, Z.I. Novika, Ferrites, Minsk (1960), p. 146
K. Iwauchi, Jpn. J. Appl. Phys. 10, 1520 (1971)
S.N. Dolia, P.K. Sharma, M.S. Dhawana, S. Kumar, A.S. Prasad, A. Samariya, Appl. Surf. Sci. 258, 4207 (2012)
M. George, S.S. Nair, A.M. John, P.A. Joy, M.R. Anantharaman, J. Phys. D Appl. Phys. 39, 900 (2006)
K.M. Batoo, M.S. Ansari, Nanoscale Res. Lett. 7, 112 (2012)
M. Younas, M. Nadeem, M. Atif, R. Grossinger, J. Appl. Phys. 109, 093704 (2011)
M.J. Paligova, V.P. Saha, V. Kresaleka, J. Stejskal, O. Quadrat, Phys. A 335, 421 (2004)
A. Ohlan, K. Singh, A. Chandra, V.N. Singh, S.K. Dhawan, J. Appl. Phys. 106, 044305 (2009)
H.T. Guan, S.H. Liu, Y.P. Duan, J. Cheng, Cem. Concr. Compos. 28, 467 (2006)
N. Li, Y. Huang, F. Du, X.B. He, X. Lin, H.J. GaO, Y.F. Ma, F.F. Li, Y.S. Chen, P.C. Eklund, Nano Lett. 6, 1141 (2006)
Acknowledgments
Authors wish to acknowledge the STIC, CUSAT, Cochin for XRD, SEM, TEM and TG analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhu, B.J., Gurusiddesh, M., Kiran, T. et al. Structural, dielectric, ac conductivity and electromagnetic shielding properties of polyaniline/Ni0.5Zn0.5Fe2O4 composites. J Mater Sci: Mater Electron 27, 7760–7766 (2016). https://doi.org/10.1007/s10854-016-4764-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4764-2