Abstract
The high-temperature interactions between vanadium-titanium magnetite carbon composite hot briquettes (VTM-CCBs) and pellets were systematically investigated under simulated blast furnace conditions with respect to the reduction behavior, softening–melting–dripping characteristics, gas permeability, and Ti(C, N) precipitation mechanisms. The results showed that VTM-CCB charging can promote the reduction of the pellet in the packed bed and decrease the compressive strength of the pellet after reduction. The compressive strength of the VTM-CCB after reduction decreased with increasing temperature when the FC/O ratio (the ratio of the fixed carbon mol(C) in coal to the reducible oxygen mol(O) in iron oxides) was higher than 1.0. With an FC/O ratio lower than 1.0, the compressive strength of the VTM-CCB initially decreased and then increased. The FC/O ratio has a significant influence on the softening–melting interaction mechanism between the VTM-CCB and the pellet. With an FC/O ratio of 0.8, the bonding layer at the interface between the pellet and the VTM-CCB (consisting of molten fayalitic slag) can promote the softening process, thereby decreasing the softening start and end temperatures. By increasing the FC/O ratio to 1.4, a dense metallic iron shell with relatively high strength formed at the interface and restricted the collapse of the packed bed, thereby increasing the softening start and end temperatures and ensuring the transport of the reduction gas through the packed bed. The melting point of the primary slag phase increased with increasing FC/O ratio due to a decrease in the FeO content, which resulted in an increase in the melting start temperature from 1273 °C to 1294 °C (1546 K to 1567 K). The gas permeability in the cohesive zone increased with an increasing FC/O ratio of the VTM-CCB due to a combination of the skeletal role performed by the residual VTM-CCB and the decrease in the liquid slag proportion. In addition, as the FC/O ratio increased to 1.4, unconsumed carbon promoted the precipitation of Ti(C, N) at the slag–carbon and slag–metal interfaces, which resulted in a substantial increase in the dripping temperature and deterioration of the dripping behavior of the packed bed. Therefore, to suppress the precipitation of Ti(C, N) and improve the dripping behavior of the packed bed, the FC/O ratio of the charged VTM-CCB should be controlled within an appropriate range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a type of complex iron ore containing iron, vanadium, titanium, etc., vanadium-titanium magnetite (VTM) is widely distributed in China, the Americas, Russia, and South Africa. In China, there are approximately 18 billion tons of VTM deposits in the Panxi and Chengde Districts.[1,2,3] Presently, VTM concentrate, following sintering and pelletizing, is conventionally used as the raw material for the blast furnace (BF) smelting process. However, according to on-site production practices, some problems remain in the BF smelting VTM process, such as high coking rates, low gas utilizations, and low vanadium yields.[4,5] These problems are mainly attributed to the poor reducibility of the VTM sinter and the VTM pellet, poor gas permeability of the packed bed, and precipitation of Ti(C, N).
Carbon composite iron ore hot briquettes (CCB) have been proposed as a new type of BF burden with the aim of achieving low-carbon and low-temperature iron production.[6,7,8,9] CCB exhibits great reducibility because the coal and iron ore in CCB closely adjoin, which accelerates carbon gasification and iron oxide reduction.[10] Based on the advantages of CCB, we propose the preparation and application of VTM-CCB to improve the BF smelting VTM. In our previous studies,[11,12,13] the preparation parameters, compressive strength, reduction mechanisms and kinetics, and volumetric shrinking characteristics of VTM-CCB were systematically investigated. All the research results showed that the VTM-CCB exhibited excellent metallurgical properties and met the basic requirements for BF smelting. To further evaluate the feasibility of BF smelting VTM-CCB, the effect of VTM-CCB charging on the reduction and the melting/dripping behaviors of the packed bed should be investigated and explained.
In the BF smelting process, the interaction between different iron-bearing materials significantly affects the softening–melting property of mixed burden, thereby influencing the cohesive zone. Wu et al.[14,15] found that the interaction between the sinter of high basicity and lump ores would improve the formation temperature interval, the viscosity, and the fluidity of the primary slag. She et al.[16] investigated the softening–melting–dripping properties of mixed burden with charging metallized pellets and demonstrated that the softening–melting properties of the lump ores, or oxidized pellets, were dramatically improved by interaction between the lump ores (or oxidized pellets) and the metallized pellets, while there was no obvious interaction between the sinter and the metallized pellets. Kaushik and Fruehan[17] studied the interaction between acid and olivine fluxed pellets and illustrated that the interaction of material occurs in four stages, namely, sintering of solid phases at the interface, incipient melt formation, interaction of liquid at the interface, and interaction of the cores of the material. Nogueira and Fruehan[18,19,20] examined the interaction between different types of pellets at high temperatures under load and demonstrated that the meltdown of the pellets reduced to 80 pct seems to be controlled by the metallic iron shell, while the meltdown of the pellets reduced to 60 pct appears to be determined by both the metallic iron and the liquid slag. These previous studies were mainly focused on the interactions among fluxed pellets, acid pellets, sinters, lump ore, and metallized pellets. For carbon-bearing feed materials, the interaction mechanism has not been reported and should be emphasized and examined. As a new type of carbon-bearing burden, the carbon content of VTM-CCB greatly affects the reduction and softening–melting behaviors of the packed bed, especially the precipitation of Ti(C, N). Therefore, the softening–melting–dripping mechanism, the optimization of the carbon content of the charged VTM-CCB, and the high-temperature interaction between VTM-CCB and traditional ferrous burden should be systematically explored.
The high-temperature properties and the interaction between different ferrous materials have a notable influence on BF operation. Presently, reduction and softening–melting–dripping tests are most commonly used to evaluate the properties of ferrous materials, and although various laboratory setups and experimental conditions have been developed for this purpose, with similar apparatuses and procedures being employed, none have yet been standardized. One reason is that in these types of simulations, it is sometimes difficult to define the experimental conditions due to a lack of knowledge concerning the conditions in the high-temperature zones of the BF. Furthermore, when the experimental conditions are chosen according to the operation of one BF, those conditions may not be applicable to another furnace. To simulate the reduction conditions in the industrial BF, it is necessary to know the conditions that exist in such a furnace. The most important parameters with respect to the behavior of iron ore during the reduction process include the charging method of mixed burden and the temperature, gas (gas composition and flow rate), and loading profiles.
In this study, all reduction and softening–melting–dripping tests were conducted with equivalent amounts of ferrous material (500 g) and coke (110 g). Notably, the mass of the charged coke was calculated based on a coke rate of 370 kg/tHM utilized in an industrial BF at Cheng Steel in China. The charged coke was placed above and below the ferrous material layer. During the SMD tests, the applied load was 0.175 MPa at temperatures below 900 °C and increased to 0.35 MPa when the temperature was 900 °C or greater. It should be noted that the loading profile corresponds roughly to the weights of the overlying burden of the lumpy and cohesive zones, respectively. Differences have been observed in the temperature profiles and reduction gases for different temperature ranges during SMD tests. In our study, the selected temperature profiles were 10 °C/min for temperatures below 900 °C, 3 °C/min in the range from 900 °C to 1020 °C, and 5 °C/min in the range from 1020 °C to the end of the experiment. Pure (100 pct) N2 gas was used for temperatures below 400 °C; 60 pct N2, 26 pct CO, and 14 pct CO2 for temperatures between 400 °C and 900 °C; and 70 pct N2 and 30 pct CO for temperatures between 900 °C and experimental end temperature. In current studies, the aforementioned temperature profiles and gas profiles are widely applied to investigate the reduction and softening–melting–dripping behaviors of various iron-bearing burdens and to simulate industrial BF conditions in China.[21]
In this study, the high-temperature interactions between the VTM-CCB and pellet, including the reduction behavior and compressive strength of the pellet and VTM-CCB, and the softening–melting characteristics, gas permeability, and the precipitation mechanism of Ti(C, N), were systematically investigated under BF conditions. First, the effect of the charged VTM-CCB with different FC/O ratios on the reduction and the compressive strengths of the pellet and VTM-CCB were explored by isothermal reduction in the temperature range from 900 °C to 1100 °C (1173 K to 1373 K). Next, the interaction between the VTM-CCB and pellet during the softening–melting–dripping process was explored. Finally, the influence of the VTM-CCB charging on the precipitation of Ti(C, N) was discussed with the aid of thermodynamic calculations and SEM–EDS analysis. It is anticipated that the data derived from the aforementioned investigations should provide a theoretical basis for, and technically support, the application of VTM-CCB with appropriate FC/O ratios in actual BF smelting.
Experimental
Raw Materials
The main raw materials utilized in this study included the VTM-CCB, pellet, and coke. The pellet and coke were obtained from the Iron and Steel Company in China, and the VTM-CCB samples were prepared under laboratory conditions. The chemical compositions of the VTM concentrate, and the proximate and ash analyses of the coal used to prepare VTM-CCB, are listed in Tables I and II, respectively. The proximate analyses and chemical compositions of the coke used in the softening–melting experiments are listed in Table III. It should be noted that the total iron content (TFe) and FeO content were determined by the titration method. The pct Fe2O3 was obtained by subtracting the FeO content from the TFe content.
The VTM-CCB was prepared by the hot briquetting and heat treatment processes. First, the VTM concentrate and coal were ground to a particle size of less than 75 µm. Second, the fine VTM and coal were mixed homogeneously. Third, the mixtures were charged into an ellipsoid die and heated to 200 °C (473 K) and briquetted under a pressure of 40 MPa. Thereafter, the briquettes were charged into a heat furnace at 500 °C (773 K) for 3 hours. After the heat treatment, the ellipsoidal VTM-CCB sample size was 21 mm × 19 mm × 13 mm, and the weight of each VTM-CCB sample was approximately 9 g. Pellets with diameters in the range of 10 to 12.5 mm were used in this study; the weight of each pellet was approximately 4.5 g. The chemical compositions of the pellets and the VTM-CCB with different FC/O ratios are listed in Table IV. In this study, the FC/O ratio of VTM-CCB, calculated by the ratio of the fixed carbon mol(C) to the reducible oxygen mol(O) in iron oxides, was set in the range from 0.8 to 1.4 (FC/O = 0.8, 1.0, 1.2, 14). It should be noted that the reducible oxygen is the total oxygen in the iron oxides.
Experimental Methods
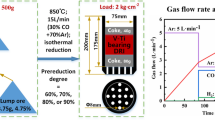
The experiments in the present study included three parts. First, isothermal reduction tests were conducted to explore the effect of VTM-CCB charging on the reduction behavior of the pellet. The isothermal reduction temperature ranged from 800 °C to 1100 °C (1073 K to 1373 K), and the reduction duration was 40 minutes in every test. The reduction samples (100 g) consisted of 20 wt pct VTM-CCB and 80 pct (mass) pellet. During the isothermal reduction experiments, the furnace was initially heated to the preset temperature. Then, the samples were charged into the furnace and reduced under the preset conditions associated with temperature and reduction gases. The FC/O ratio of the charged VTM-CCB ranged from 0.8 to 1.4. The detailed scheme of the isothermal reduction experiments is listed in Table V. The isothermal reduction furnace schematic diagram is shown in Figure 1(a). During reduction, the reduction gases contained 30 pct CO and 70 pct N2 and had a total flow rate of 4 L/min.
Second, the influence of the charged VTM-CCB (with different FC/O ratios) on the softening–melting performance of the packed bed was investigated by conducting softening–melting experiments. To guarantee transport of the reduction gas through the packed bed consisting of molten iron and slag, dried coke with a diameter of 10 to 12.5 mm and a layer thickness of 30 and 17 mm was placed above and below the packed bed, as shown in Figure 1(b). Notably, the total mass of the charged coke was 110 g, which was calculated based on a coke rate of 370 kg/tHM in an industrial BF at Cheng Steel in China. The mixed iron-bearing burden of approximately 500 g, containing 20 pct (mass) VTM-CCB and 80 pct (mass) pellet, was charged into a graphite crucible with a diameter of 75 mm. The five different mixed burden cases were consistent with the isothermal reduction, as listed in Table V. During the softening–melting–dripping experiment (SMD experiment), the temperature, contraction, and pressure drop of the packed bed were recorded automatically. The softening–melting results of the packed bed were represented by an average of three measurements. The integrated burden charged in the graphite crucible was heated and reduced by simulating the aforementioned BF conditions.
Third, to investigate the high-temperature interaction mechanism between the pellet and VTM-CCB, interaction reduction experiments were conducted. The interaction couple between the pellet and VTM-CCB was prepared as follows; the pellet powder was first placed into a die with a diameter of 8 mm and pressed into a compact substrate (at 20 MPa pressure for 1 minute) by using a uniaxial hydraulic press. Then, the VTM-CCB powder was placed into the same die (on top of the compacted pellet powder) and pressed together with the pellet substrate to form an interaction couple, again by applying 20 MPa of pressure for 1 minute. The dimensions of the pellet and VTM-CCB substrates before reduction are shown in Figure 2(a). The reduction furnace used in this part of the experiment is shown in Figure 1(c); it comprises an electrical furnace, a gas control system, a computer control system, and a digital camera. The reduction furnace was heated to the preset temperature and was purged with high-purity nitrogen at 4 NL/min for 1 hour before conducting the isothermal reduction. The preset isothermal reduction temperatures and reduction duration are listed in Figure 2(c). Next, the interaction couple was quickly charged into the reduction furnace. The reduction gas consisted of 30 pct CO and 70 pct N2, and its total flow rate was 4 NL/min. After the predetermined reduction duration, the interaction couple was removed from the tube and quickly cooled to room temperature under the protection of high-purity nitrogen. Finally, the liquid resin was injected into the cooled sample to fix the interaction couple and maintain its internal structure. After solidification, the interaction couple was sectioned perpendicular to the pellet and VTM-CCB interface, as shown in Figure 2(b), and then analyzed via SEM–EDS.
Results and Discussion
Reduction Behavior
In this study, the reduction degree (R) of the pellet is calculated by the following formula:
where m0 is the initial mass of the pellets (g), mt is the terminal mass of the pellets after reduction (g), wFeO is the FeO content of the pellets before reduction (wt pct), and \( w_{{{\text{Fe}}_{2} {\text{O}}_{3} }} \) is the Fe2O3 content of the pellets before reduction (wt pct). It should be noted that the mixtures containing pellet and VTM-CCB samples were separated by hand after reduction, and then, the pellet weight was recorded.
Figure 3 presents the terminated reduction degree of pellets for the different cases. The terminated reduction degree of the pellets discernibly increased with increasing temperature. This is because higher reduction temperatures result in higher gas mass transfer coefficients. A higher gas mass transfer coefficient is attributed to an intensification of gas molecule motion and a mitigation of the diffusion resistance.[22] Notably, the terminated reduction degree of pellets increased with an increasing FC/O ratio of the charged VTM-CCB, especially at temperatures higher than 900 °C (1173 K). At 1100 °C (1373 K), the terminated reduction degree of pellets was 34.8 pct in case 1 and 36.9 pct in case 5. This indicates that VTM-CCB charging could effectively reduce pellets.
Following the isothermal reduction experiments, the pellet–(VTM-CCB) reaction interface was investigated by SEM of the interaction couple cross section. Figure 4 shows the BSE images and EDS analyses of the interaction couple cross section after reduction under different conditions. An obvious reduction transition region can be observed in the oxygen and iron mapping images, which indicate that the pellet region contacted with the VTM-CCB was preferentially reduced. At 900 °C (1173 K), the thickness of the reduction transition region is relatively narrow and increased slightly by increasing the FC/O ratio of the charged VTM-CCB from 0.8 to 1.4, as shown in Figures 4(a) and (b); this indicates that the FC/O ratio of VTM-CCB has little effect on the reduction strength of the pellet. However, at 1100 °C (1373 K), the thickness of the reduction transition region obviously increased as the FC/O ratio increased from 0.8 to 1.4 and is also much wider than transition region thickness observed at 900 °C (1173 K), as shown in Figures 4(c) and (d). The difference in the reduction transition region thickness at different temperatures and FC/O ratios is mainly caused by carbon gasification. As a type of carbon-bearing composite agglomerate, carbon gasification predominantly relies on the temperature and carbon content of the VTM-CCB. At 900 °C (1173 K), large amounts of unconsumed carbon remain at the interface of the VTM-CCB due to the relatively slow carbon gasification reaction rate, as shown in the carbon elemental mapping images in Figures 4(a) and (b); this results in insufficient CO gas (provided by carbon gasification) for the reduction of the pellet interface. However, by increasing the temperature to 1100 °C (1373 K), carbon gasification occurred more vigorously, and the carbon at the interface of the VTM-CCB was nearly all consumed, as shown in the carbon elemental mapping images in Figures 4(c) and (d). As a result, the reduction of the pellet interface was efficiently promoted, especially with a higher FC/O ratio for the charged VTM-CCB. As shown in Figures 4(e) and (f), the EDS points 1 to 5 indicate high oxygen contents, which suggests that after reduction at 900 °C (1173 K), the pellet was predominantly composed of iron oxides and gangue. By increasing the temperature to 1100 °C (1373 K), the metallic iron phase appeared at the interface of the pellet, as shown in Figure 4(g) and EDS points 6, when the FC/O ratio of the VTM-CCB was 0.8. When the FC/O ratio was further increased to 1.4, the metallic iron phase clearly increased, as shown in Figure 4(f) and EDS point 10. Therefore, it can be concluded that the reduction of the pellet could be promoted by increasing the FC/O ratio of the charged VTM-CCB, especially at a higher reduction temperature.
BSE images and mapping elemental scanning analyses of the interaction couple after reduction at different temperatures: (a) reduced at 900 °C (1173 K) with a FC/O of 0.8, (b) reduced at 900 °C (1173 K) with a FC/O of 1.4, (c) reduced at 1100 °C (1373 K) with a FC/O of 0.8, (d) reduced at 1100 °C (1373 K) with a FC/O of 1.4, (e) higher magnification BSE images of (a) and EDS analyses, (f) higher magnification BSE images of (b) and EDS analyses, (g) higher magnification BSE images of (c) and EDS analyses, and (h) higher magnification BSE images of (d) and EDS analyses
The compressive strength of the pellets after reduction is correlated with the FC/O ratio of the charged VTM-CCB and the reduction temperature. Figure 5 shows the compressive strengths of the pellets and VTM-CCB before and after reduction in different cases, as listed in Table V. It should also be noted that the compressive strength of the pellets is represented by an average of 20 measurements performed with an electronic universal compressive strength testing machine. As shown in Figure 5(a), the compressive strength of the pellet after reduction indicates a sharp decrease when compared with the compressive strength before reduction. At the same temperature, the compressive strength decreased gradually with increasing FC/O ratio of the charged VTM-CCB. As shown in Figure 5(b), the compressive strength of VTM-CCB after reduction notably decreased with increasing temperature when the FC/O ratio was higher than 1.0. With an FC/O ratio lower than 1.0, the compressive strength of VTM-CCB samples initially decreased and then increased. In particular, when the reduction temperature was 1100 °C (1373 K) and the FC/O ratio was 0.8, the compressive strength of the VTM-CCB increased to 937 N.
The compressive strength of the pellet and VTM-CCB is related to the porosity and structure. Figure 6 presents the microstructure of the pellet and VTM-CCB before and after reduction in different cases. As shown in Figure 6(a), the pellet before reduction has a dense microstructure compared with the pellet after reduction. In addition, the pellet with VTM-CCB charging exhibited a porous microstructure compared with the pellet without VTM-CCB charging. There are also more pores and cracks generated in the pellet after reduction with VTM-CCB charging compared with the pellet after reduction without VTM-CCB charging. In other words, VTM-CCB charging could strengthen the reduction of the pellet and promote the formation of pores and cracks, which results in a decrease in the compressive strength of the pellet after reduction.
Microstructure of the pellet and VTM-CCB before and after reduction in different cases: (a) pellet before reduction, (b) pellet reduced at 800 °C in case 1, (c) pellet reduced at 1000 °C in case 1, (d) pellet reduced at 800 °C in case 5, (e) pellet reduced at 1000 °C in case 5, (f) VTM-CCB (FC/O = 0.8) before reduction, (g) VTM-CCB reduced at 900 °C in case 2, (h) VTM-CCB reduced at 1100 °C in case 2, (i) VTM-CCB (FC/O = 1.4) before reduction, and (j) VTM-CCB reduced at 900 °C in case 5, (k) VTM-CCB reduced at 1100 °C in case 5
The compressive strength of VTM-CCB is primarily dependent on the FC/O ratio and the reduction temperature. With an FC/O ratio higher than 1.0, the promotion of reduction at higher temperature results in the consumption of the blending coal and an increase in void and crack formation, as shown in Figures 6(j) and (k). However, when the FC/O ratio was lower than 1.0, the blending coal was almost completely consumed, and the formation of an iron-coupled crystal skeleton was also promoted, as shown in Figure 6(h), which resulted in a remarkable increase in compressive strength.
Softening–Melting–Dripping Characteristics
To evaluate the interaction between the pellet and the VTM-CCB during softening and melting, some characteristic temperatures were defined in this research. T4, the temperature at which the contraction of the packed bed reaches 4 pct, is defined as the softening start temperature because the contraction of the packed bed notably rises when the temperature reaches T4. When the contraction of mixed burden reaches approximately 40 pct, the iron-bearing particles are surface cohesive and gradually bond to form a denser structure, which results in a gradual increase in the pressure drop across the packed bed.[14,23] Therefore, T40, the temperature at which the contraction of the packed bed reaches 40 pct, is defined as the softening end temperature. Figure 7(a) shows the effect of the FC/O ratio of charged VTM-CCB on the softening behavior of the packed bed. It can be seen that the softening start temperature T4 initially decreased from 1139 °C to 1115 °C (1412 K to 1385 K) and then increased to 1126 °C (1399 K), and the softening end temperature T40 initially decreased from 1234 °C to 1217 °C (1507 K to 1490 K) and then increased to 1240 °C (1513 K). The softening temperature interval (T40–T4) gradually widened with increasing FC/O ratio of the VTM-CCB, which is favorable for the gas–solid reduction of the packed bed.[21]
The values for the softening start temperature T4 and the softening end temperature T40 are correlated to the softening and shrinking characteristics of VTM-CCB and the interaction between the pellet and the VTM-CCB during reduction. The molten phase generation and aggregation growth have a positive effect on the softening and shrinking behavior of VTM-CCB. To evaluate the phase generation of VTM-CCB during the reduction, the full equilibrium calculation was conducted on 100 g of the VTM-CCB by FactSage 7.0. For the thermodynamic equilibrium calculation, the FToxide and FSstel databases were used. The species in the starting materials are dependent on the chemical compositions of the VTM-CCB produced at different FC/O ratios, as listed in Table I. A closed system was selected for the calculation process, and potential products were identified for an N2 atmosphere at a total pressure of 1.013 × 105 Pa in the temperature range from 1000 °C to 1400 °C. Figure 7(b) presents the calculated results for liquid slag, molten iron, residual carbon, and [C]pct in the molten iron. The equilibrium calculations from FactSage 7.0 predict that the molten slag percentage discernibly decreases with increasing FC/O ratio of the VTM-CCB from 0.8 to 1.4. Molten iron is only formed with FC/O ratios higher than 1.0 due to the presence of enough carbon to promote carburization of the metallic iron, which decreases the melting temperature of reduced iron. However, residual carbon existed when the FC/O ratio of the VTM-CCB was 1.2 and 1.4, which seriously deteriorated the aggregation growth of liquid iron and inhibited the softening and shrinking of the VTM-CCB. Our previous study illustrated[13] that liquid slag is the key factor that influences the softening and shrinking of VTM-CCB. Therefore, a higher FC/O ratio would prevent the shrinking of the charged VTM-CCB samples, thereby increasing the softening start temperature T4 and softening end temperature T40.
Figure 8 presents the BSE images and elemental mapping distribution at the interface between the pellet and VTM-CCB (FC/O = 0.8). Interaction couple consisting of the VTM-CCB and pellet was reduced at 1200 °C (1473 K) for 5 and 10 minutes. When the reduction duration was 5 minutes, many pores and cracks formed at the interface area, as shown in Figures 8(a) and (c). In the pellet and VTM-CCB areas away from the interface, the occurrence of pores and cracks clearly decreased, as shown in Figures 8(b) and (d). In addition, due to insufficient carbon, wustite (gray phase) could be observed in the VTM-CCB samples. By increasing the reduction duration to 10 minutes, the pores and cracks at the interface area decreased, and a bonding layer could be observed, as shown in Figures 8(e) and (g).
BSE images and EDS analyses at the interface between the pellet and the VTM-CCB (FC/O 0.8) after reduction at 1200 °C: (a) BSE image of interface reduced for 5 min, (b) BSE image of the pellet area away from the interface reduced for 5 min, (c) BSE image of pellet area near the interface reduced for 5 min, (d) BSE image of VTM-CCB area near the interface reduced for 5 min, (e) BSE image of interface reduced for 10 min, (f) BSE image of pellet area away from the interface reduced for 10 min, (g) higher magnification BSE images of (e), and (h) higher magnification BSE images of the interface
As shown in Figure 8(h), the bonding interface between the pellet and VTM-CCB mainly consists of a bright phase (point 1), a light gray phase (point 3), a gray phase (point 2) and a dark gray phase (points 4 and 5), and a dark gray phase that encompasses particles of the other phases. According to the EDS results, the bright phase, the light gray phase, and the gray phase are metallic iron, iron oxide, and Ti-bearing iron oxide, respectively. The average atomic ratio of Si:Fe:O (1.0:2.2:4.1), at EDS points 4 and 5, closely matches the stoichiometry of fayalite (Si:Fe:O = 1.0:2.0:4.0), which suggests that the dark gray phase is a fayalite slag phase. Therefore, it can be concluded that the wustite in the VTM-CCB reacts with SiO2 in pellet to form fayalitic slag at the interface. As a result, the fayalitic slag, with a relatively low melting point, formed at the interface would accelerate the softening process of the packed bed, thereby decreasing the softening start temperature T4 and the softening end temperature T40 compared with the packed bed without VTM-CCB charging.
By increasing the FC/O ratio of the VTM-CCB to 1.4, the softening start temperature T4 and softening end temperature T40 gradually increased, which is mainly caused by the formation of the dense metallic iron shell at the interface between the pellet and the VTM-CCB. Figure 9 presents the BSE images and elemental mapping distribution of the interaction couple consisting of the VTM-CCB (FC/O = 1.4) and pellet after reduction at 1200 °C (1473 K) for 5 and 10 minutes. With a reduction duration of 5 minutes, a narrow bonding layer started to form, and there are some pores and cracks at the interface, as shown in Figures 9(a) through (c). In the pellet area away from the interface, the pores and cracks clearly decrease, as shown in Figure 8(b).
BSE images and EDS analyses at the interface between the pellet and the VTM-CCB (FC/O = 1.4) after reduction at 1200 °C: (a) BSE image of interface reduced for 5 min, (b) BSE image of the pellet area away from the interface reduced for 5 min, (c) BSE image of pellet area near the interface reduced for 5 min, (d) BSE image of VTM-CCB area near the interface for reduced 5 min, (e) BSE image of interface reduced for 10 min, (f) BSE image of pellet area away from the interface reduced for 10 min, (g) higher magnification BSE images of (e), (h) higher magnification BSE images of the interface, (i) higher magnification BSE images of fayalitic slag in (g), and (j) higher magnification BSE images of metallic iron shell in (g)
According to the elemental mapping distribution, it can be deduced that the bonding layer mainly consists of metallic iron due to the amount of carbon in the VTM-CCB (FC/O = 1.4). Increasing the reduction duration can widen the metallic iron shell and decrease the pores at the interface, as shown in Figures 9(e) through (g). In addition, it can be deduced from the EDS analyses of points 4 to 6 in Figure 9(j)- that the background gray phases intergrown with the metallic phases in the bonding metallic layer mainly consist of fayalite and iron oxide. As shown in Figures 9(g) and (i), the molten fayalitic slag layer and the iron oxide layer formed at the edge of the pellet near the interface. According to the EDS analyses for point 2, the atomic ratio of Si:Fe:O (1.0:1.9:3.5) closely matches the fayalite stoichiometry (Si:Fe:O = 1.0:2.0:4.0), which indicates the dark phase shown in Figure 9(i) is fayalitic slag. In addition, the fayalitic slag layer also contains some metallic iron (bright phase), iron oxide (point 1), and Ti-bearing iron oxide (point 3). Although the molten fayalitic slag layer promotes some area softening inside the pellet, the dense metallic iron shell at the interface exhibits a relatively high strength and restricts the softening and shrinking of the pellet during reduction. In other words, the dense metallic iron shell at the interface could prevent the collapse of the packed bed and promote the transport of the reduction gas through the packed bed, thereby strengthening the reduction process. Therefore, the softening start temperature T4 and the softening end temperature T40 gradually increased with increasing FC/O ratio of the charged VTM-CCB.
With further increases in temperature, the iron-bearing packed bed starts melting, and the contraction continues rising steeply. As the melting of the iron-bearing packed bed occurs, the gas permeability would noticeably deteriorate. Therefore, the temperature associated with a substantial increase in pressure drop is defined as the melting start temperature Ts in this study.[21,23] The dripping temperature TD is the temperature at which the pig iron starts to drip from the graphite crucible. Figure 10 shows the effect of the FC/O ratio of the charged VTM-CCB on the melting behavior of the packed bed. The melting start temperature Ts increased gradually from 1273 °C to 1294 °C (1546 K to 1567 K) with increasing FC/O ratio of the charged VTM-CCB. The dripping temperature TD changed slightly from cases 1 to 4 and then increased substantially to 1485 °C (1758 K) in case 5. The position of the melting temperature interval (TD–Ts), namely, the cohesive zone, shifted down gradually, and its size first narrowed and then widened. In general, minimizing the size and lowering the position of the cohesive zone in the BF would increase the gas permeability of the packed bed and enhance productivity.[24] Therefore, with respect to the cohesive zone of the BF, the appropriate FC/O ratio for the charged VTM-CCB is 1.2.
Increases in Ts are correlated with the primary slag melting point. To obtain the primary slag, interrupted softening–melting–dripping tests were conducted at 1300 °C. During the interrupted tests, the gas supply and power supply were stopped immediately when the temperature of the packed bed reached 1300 °C, and nitrogen gas was introduced into the reaction tube at a flow rate of 5 NL/min to cool down the integrated burden and discharge the reduction gas as quickly as possible. After cooling to room temperature, the FeO concentration in the primary slag was detected by titration with potassium dichromate solution. Note that the titration process of FeO was conducted in HCl solution under a CO2 gas atmosphere. The other oxides were calculated theoretically, as listed in Table VI. The FeO content in the primary slag decreased gradually because the reduction of the packed bed was promoted by increasing the FC/O ratio of the charged VTM-CCB. According to the slag compositions, the slag liquidus temperature was calculated by FactSage 7.0; the calculated slag liquidus temperatures vs pct FeO are presented in Figure 11. The melting point of the primary slag increased gradually from cases 1 to 5, which resulted in an increase in the melting start temperature Ts of the packed bed.
The substantial increase in the dripping temperature TD in case 5 could be attributed to the residual VTM-CCB and precipitation of Ti(C, N). Figure 12 presents the SEM–EDS analyses of the residual slag and iron mixture after softening–melting-dripping tests in different cases. In case 1, the mixture mainly contains a silicate slag phase (point 1), Ti oxide (point 2), metallic iron (point 3), and magnesia-alumina spinel (point 4). In case 3, the main phases include Ti oxide (point 5), metallic iron (point 6), and magnesia-alumina spinel (point 7). Notably, compared with the residual mixture in case 1, the assemblage of the iron and slag in case 3 was obviously promoted due to the increase in gas permeability and enhanced iron carburization by the charged VTM-CCB. By further increasing the FC/O ratio to 1.4, residual VTM-CCB containing large amounts of unconsumed carbon (point 8), Ti(C, N) (point 9), and silicate slag phase (point 10) could be observed in the slag and iron mixture, as shown in Figure 12(c). The unconsumed solid carbon particles can increase the passing and dripping resistance of the slag and iron mixture and increase the dripping temperature, TD. In addition, the excessive carbon in the VTM-CCB can promote the reduction of Ti oxide to Ti carbide. Generally, as a solid dispersed phase in slag, Ti(C, N) substantially increases the slag viscosity,[25,26] which results in a substantial increase in the dripping temperature, TD. The detailed precipitation mechanism of Ti(C, N) is discussed in the following part of this paper.
Gas Permeability
In this study, the permeability index (S value) was introduced to quantify the permeability in the cohesive zone of the packed bed. The S value can be calculated by Eq. [2].
where Ts and TD are the melting start temperature and dripping temperature, respectively. Pm and ΔPS are the pressure drop at certain temperatures and the pressure drop at the melting start temperature, respectively. In general, a smaller S value indicates a greater permeability of the packed bed.
As shown in Figure 13, the maximum pressure drop and the S value gradually decreased with increasing FC/O ratio of the VTM-CCB, which indicates that the permeability in the cohesive zone could be increased by increasing the FC/O ratio of the charged VTM-CCB. The gas permeability in the cohesive zone mainly relies on the porosity and macrostructure of the packed bed. In general, a higher contraction indicates a denser packed bed structure, which would impede gas transport through the packed bed. In this study, the porosity was characterized by the contraction of the packed bed during the softening–melting-dripping tests. As shown in Figure 14(a), in the temperature range from 1300 °C to 1400 °C (1573 K to 1673 K), the contraction in case 1 is obviously higher than in other cases. Notably, at the same temperature, the contraction decreased when increasing the FC/O ratio of the charged VTM-CCB. Figure 14(b) shows the cross section of the packed bed after softening–melting-dripping tests. It can be clearly seen that the mixture of slag and iron in case 1 indicates a denser microstructure. By increasing the FC/O ratio, the mixture of slag and iron gradually becomes porous. In particular, when the FC/O ratio is higher than 1.2, the residual VTM-CCB acts as skeleton in the molten mixture, which serves to decrease the contraction and ensures transport of the reduction gas through the packed bed.
In addition, the pressure drop in the cohesive zone inside the BF correlates to the loss of permeability of the coke bed due to the liquid exudation from the ferrous materials. As the liquid slag volume fraction increases, it will tend to expand to fill in the space of the packed bed and decrease the void space in the burden layer, thereby impeding gas transport. Therefore, the isothermal liquidus projections in the CaO-SiO2-MgO-Al2O3-TiO2-FeO pseudoternary diagrams were drawn by FactSage 7.0 to evaluate the liquid slag proportion in different cases. Considering the effect of FeO and the other oxides on the pseudoternary diagram, the chemical compositions of the slag in different cases were obtained by chemical analysis, as shown in Figure 15(a).
Many studies[25,26,27] discovered that Ti3+ is unstable and tends to be oxidized by Fe2+ according to the reaction: Ti2O3 + FeO = Fe + TiO2, thereby decreasing the Ti3+ concentration in the Ti-bearing slag effectively during solidification. In this study, the FeO content is approximately 30 pct in the primary slag, which indicates that the existence of Ti3+ is unlikely. Therefore, in our present study, the titanium oxide is assumed to be TiO2 with respect to evaluating the effect of titanium oxide on the melting point of the slag. For the phase diagram calculations, the selected database was FToxide. The input chemical compositions of the slag are listed in Figure 15(a). The possible products were identified under a total pressure of 1.01325 × 105 Pa at a temperature of 1400 °C (1673 K). As shown in Figure 15(b), the liquid slag proportion decreased for the charged VTM-CCB. As shown in the superimposed five pseudoternary diagrams, the liquid slag generation zone was notably lower for charged VTM-CCB. However, the liquid slag zone changed slightly with an increase in the FC/O ratio of the VTM-CCB, which matches the slag morphologies shown in Figure 15(b).
The phase components of the liquid slag in different cases (shown in Figure 15(b)) were investigated by XRD analyses. As shown in Figure 16, the residual slag mainly consists of (Mg, Fe)Ti2O5, Fe2SiO4, Ca3Al2(SiO3)4 and FeO. To clearly observe the changes in the FeSi2O4 and FeO peaks, the XRD patterns were enlarged, as shown in Figures 16(b) and (c). The peaks of Fe2SiO4 and FeO decreased slightly from cases 1 to 5, which matches well with the detected FeO content in liquid slag, as shown in Figure 11(a). It is well known that the melting point of Fe2SiO4 is approximated at 1150 °C (1423 K), and the decrease in Fe2SiO4 could decrease the liquid slag proportion and increase the gas permeability in the cohesive zone. Therefore, considering the skeleton of the residual VTM-CCB and the liquid slag proportion, a higher FC/O ratio is favorable for increasing the gas permeability of the packed bed.
Precipitation of Ti(C, N)
Previous studies have illustrated that the precipitation of Ti(C, N) significantly influences the BF smelting VTM.[28,29,30] Therefore, as a type of carbon-bearing material, the influence of VTM-CCB charging on the precipitation of Ti(C, N) should be considered. First, the relationships between ΔG and the temperature of the potential reactions during the precipitation of TiC and TiN were calculated by FactSage 7.0. Generally, in VTM, the Ti-bearing phases mainly exist as ulvöspinel (2FeO·TiO2) and ilmenite (FeO·TiO2). From the perspective of gradual deoxidation, the reduction sequence of titanium oxide is TiO2 → Ti3O5 → Ti2O3 → TiO → Ti.[31] To simplify the thermodynamic calculation, TiO2, not the intermediate reduction products, such as Ti2O3, Ti3O5, and TiO, was selected as the titanium oxide during the thermodynamic calculations in this study. During the calculations, the molten iron was assumed to be carbon-saturated, and the activity level of [C] was 1.0. Ignoring other trace elements in the molten iron, the activity level of [Ti] ranges from 0.02 to 0.07, and the titanium concentration ranges from 0.21 to 0.57. The following conclusions can be obtained from Figure 17. (1) TiO2 could be reduced to [Ti] by the dissolved carbon [C] in the molten iron and the solid carbon C in the residual VTM-CCB. (2) TiO2 could be reduced to TiC by the solid carbon C and the dissolved carbon [C] in the molten iron. (3) In the molten iron, [Ti] can react with [C] and N2 to form TiC and TiN. (4) In the presence of N2, TiO2 could be reduced to TiN. In addition, the occurrence of R7 indicates that TiC and TiN cannot exist alone and mainly precipitate as a solid solution Ti(C, N).
Next, to investigate the amount of TiC and TiN formed during the reduction of VTM-CCB, thermodynamic equilibrium analyses were carried out on 100 g of VTM-CCB by FactSage 7.0. During the thermodynamic equilibrium calculations, the selected databases included FactPs and FToxide. The gas atmosphere used for the calculation consisted of 30 pct CO and 70 pct N2. The input chemical compositions of the VTM-CCB produced at different FC/O ratios are listed in Table IV. Figure 18 shows the effect of temperature and FC/O ratio on the generation mass of TiC and TiN during reduction of the VTM-CCB. In the temperature range from 1300 °C to 1400 °C (1573 K to 1673 K), the mass of TiC and TiN substantially increased only when the FC/O ratio was higher than 1.2. Notably, the mass of TiC increased with increasing temperature when the FC/O ratio was 1.4, while the mass of TiN decreased with increasing temperature. The opposing mass changes for TiC and TiN are predominantly caused by the transformation reaction (R7) listed in Figure 17.
Figure 19 presents a BSE image and elemental mapping distribution of the nondripped substance of the packed bed with charged VTM-CCB (FC/O = 1.4). The nondripped mixture mainly consisted of residual VTM-CCB, metallic iron, and slag. It should be noted that the residual VTM-CCB contains large amounts of unconsumed carbon. In the carbon element distribution image, an obvious concentration gradient could be observed from the residual VTM-CCB layer to the metal and slag layers, which indicates that the carburization process could be intensified by the excessive carbon in VTM-CCB. Notably, the Ti(C, N) particles mainly precipitated at the slag–carbon and slag–metal interfaces. In particular, large amounts of Ti(C, N) particles precipitated at the slag–metal interface and even encompassed the iron particles. It should also be noted that the precipitated Ti(C, N) particles in the alloy interior area are less than those in the slag–metal interface. Previous literature illustrates that Ti(C, N) solid particles have a substantial impact on the viscosity of slag once they enter into the molten phases (slag and metal).[30,32,33,34] The viscosity of the slag increased sharply with the formation of Ti(C, N). In addition, as the solid dispersed phase in slag, Ti(C, N) would increase the internal friction of the suspension slag system, thus thickening the slag, which would obviously deteriorate the dripping behavior of the slag and iron mixture. Therefore, to suppress the precipitation of Ti(C, N) and improve the dripping behavior of the packed bed, the FC/O ratio of the charged VTM-CCB should be controlled in an appropriate range.
Combining the aforementioned thermodynamic calculations and SEM–EDS analysis results, the effect mechanism of VTM-CCB charging on the Ti(C, N) precipitation can be divided into two parts, as shown in Figure 20. At the slag–metal interface, the unconsumed carbon in residual VTM-CCB could intensify the carburization process and provide sufficient [C] as a reductant, thereby promoting the reduction of TiO2 to [Ti]. Generally, if the production rate of [Ti] is higher than the precipitation rate of TiC at the slag–metal interface, the residual [Ti] would be dissolved into the alloy interior area. Then, when [Ti pct] in the alloy and its activity level reach a certain value, [Ti] will react with the dissolved [C] to precipitate TiC. To compare the difference between the alloy interface and interior area, the enlarged BSE image of the alloy interior area in Figure 19 shows that the precipitated Ti(C, N) in the alloy interior area is significantly less than that in the slag–metal interface. Therefore, it can be concluded that at the slag–metal interface, TiO2 was reduced to [Ti] and TiC by the dissolved carbon [C] in molten iron, and then [Ti] in the molten iron reacted with [C] and N2 to form Ti(C, N), as shown in Figure 20(a). Previous research[31] illustrates that the precipitation of Ti(C, N) in the slag–carbon phase obeys the adsorption model. First, TiO2 in the slag moves to the carbon and is adsorbed at the carbon interface. Then, the adsorbed TiO2 is reduced by the carbon to form a Ti(C, N) solid solution. In addition, according to the Einstein–Roscoe equation, the addition of solid particles would increase the slag viscosity and surface tension substantially, and the carbon particles in the residual VTM-CCB are not easily dissolved in the molten slag. In this study, Ti(C, N) particles were not observed in the slag interior area. Therefore, it can be concluded that TiO2 was reduced to TiC and TiN directly by unconsumed carbon in the residual VTM-CCB and N2 gas at the slag–carbon interface, as shown in Figure 20(b).
Conclusions
The high-temperature interactions between the VTM-CCB and pellet, including the reduction behavior of the pellet, softening–melting characteristics, gas permeability, and the precipitation mechanism of Ti(C, N), were systematically investigated under BF conditions in this paper. The following conclusions can be obtained from this study.
(1) The reduction degree of the pellet increased gradually with increasing FC/O ratio of the charged VTM-CCB. Simultaneously, the promotion of the reduction process resulted in a decrease in the compressive strength of the pellet after reduction. The compressive strength of the VTM-CCB after reduction decreased with increasing temperature when the FC/O ratio was higher than 1.0. With an FC/O ratio lower than 1.0, the compressive strength of the VTM-CCB initially decreased and then increased.
(2) The FC/O ratio had a significant influence on the softening–melting interaction mechanism between the pellet and VTM-CCB. With an FC/O ratio of 0.8, the bonding layer (consisting of molten fayalitic slag) at the interface between the pellet and the VTM-CCB promoted the softening process, thereby decreasing the softening start temperature T4 and the softening end temperature T40. By increasing the FC/O ratio to 1.4, a dense metallic iron shell with a relatively high strength formed at the interface and restricted the collapse of the packed bed, thereby increasing the softening start temperature T4 and the softening end temperature T40 and guaranteeing the transport of reduction gas through the packed bed.
(3) The slag-phase melting point increased with increasing FC/O ratio of the charged VTM-CCB due to the decreasing FeO content in the slag, which resulted in the melting start temperature, Ts, increasing gradually from 1273 °C to 1294 °C (1546 K to 1567 K).
(4) In the cohesive zone, the gas permeability increased with increasing FC/O ratio of the charged VTM-CCB due to a combination of the skeleton role of residual VTM-CCB and the decrease of the liquid slag proportion.
(5) By increasing the FC/O ratio of the charged VTM-CCB to 1.4, unconsumed carbon promoted the precipitation of Ti(C, N) at the slag–carbon and slag–metal interfaces, which resulted in a substantial increase in the dripping temperature TD and deterioration of the dripping behavior of the iron and slag mixture. Therefore, to suppress the precipitation of Ti(C, N) and improve the dripping behavior of the packed bed, the FC/O ratio of the charged VTM-CCB should be controlled within an appropriate range.
References
[1] T. Hu, X.W. Lv, C.G. Bai, Z.G. Lun and G.B. Qiu: Metall. Mater. Trans. B, 2013, vol. 44, pp. 252-60.
Tang J, Chu MS, Feng C, Li F, Tang YT, Liu ZG (2017) ISIJ Int 57:1156-1165
[3] Z.G. Hao, H.G. Fei, L. Liu and T. Susan: Acta Geologica Sinica, 2012, vol. 87, pp. 286-87.
[4] H.G. Du: Principle of Blast Furnace Smelting Vanadium-Titanium Magnetite, Science Press, Beijing, 1996, pp. 1-10.
[5] S. Samanta, S. Mukherjee and R. Dey, JOM, 2015, vol. 67, pp. 467-76.
[6] T. Anyashiki, K. Fukada and H. Fujimoto: JFE Technical Report, 2009, vol. 13, pp. 1-6.
Kasai A, Toyota H, Nozawa K, Kitayama S (2011) ISIJ Int 51:1333-1335
[8] Y. Tanaka, T. Ueno, K. Okumura and S. Hayashi: ISIJ Int., 2011, vol. 51, pp. 1240-46.
[9] Y. Matsui, M. Sawayama, A. Kasai, Y. Yamagata and F. Noma: ISIJ Int., 2003, vol. 43, pp.1904-12.
[10] M. Naito, K. Takeda, Y. Matsui: ISIJ Int., 2015, vol. 55, pp. 7-35.
[11] W. Zhao, H.T. Wang, Z.G. Liu, M.S. Chu, Z.W. Ying and J. Tang: Steel Res. Int., 2017, vol. 88, pp. 1-9.
[12] W. Zhao, H.T. Wang, Z.G. Liu, M.S. Chu, Z.W. Ying, and J. Tang: JOM, 2017, vol. 69, pp. 1737-44.
[13] W. Zhao, M.S. Chu, H.T. Wang, Z.G. Liu, J. Tang, and Z.W. Ying: ISIJ Int., 2018, vol. 58, 823-32.
[14] X.L. Liu, S.L. Wu, W. Huang, K.F. Zhang, and K.P. Du: ISIJ Int., 2014, vol. 54, 2089-96.
[15] S.L. Wu, H.L. Han, H.F. Xu, H.W. Wang, and X.Q. Liu: ISIJ Int., 2010, vol. 50, 686-94.
[16] X.F. She, J.S. Wang, J.Z. Liu, X.X. Zhang, and Q.G. Xue: ISIJ Int., 2014, vol. 54, 2728-36.
[17] P. Kaushik and R. J. Fruehan: Ironmaking & Steelmaking, 2007, vol. 34, 10-22.
Nogueira PF, Fruehan RJ (2004) Metall Mater Trans B 35B:829-838
Nogueira PF, Fruehan RJ (2005) Metall Mater Trans B 36B:583-590
Nogueira PF, Fruehan RJ (2006) Metall Mater Trans B 37B:551-558
[23] G. J. Cheng, X. X. Xue, T. Jiang and P. N. Duan: Metall. Mater. Trans. B, 2016, vol. 47, pp. 1713-26.
[21] X. Z. Zhang, Principles of Transfer in Metallurgy, Metallurgy Industry Press, Beijing, 2005, pp. 386-90.
[22] S.L. Wu, B.Y. Tuo, L.H. Zhang, K.P. Du, and Y. Sun: Steel Res. Int., 2014, vol. 85, pp. 233-42.
[24] R.J. Fruehan: Metall. Mater. Trans. B, 2009, vol. 40, pp. 123-33.
Pesl J, Eriç RH (1999) Metall Mater Trans B 30B:695-705
[26] G. Eriksson, A.D. Pelton, E. Woermann, and A. Ender: Cheminform, 1997, vol. 100, pp. 1839-49.
[27] K. Hu, X.W. Lv, S.P. Li, W. Lv, B. Song, and K.X. Han: Metall. Mater. Trans. B, 2018, vol. 49, pp. 1963-73.
[28] G.H. Zhang, Y.L. Zhen, and K.C. Chou: ISIJ Int., 2015, vol. 55, 922-27.
[29] Y.L. Zhen, G.H. Zhang, and K.C. Chou: Metall. Mater. Trans. B, 2015, vol. 46, 155-61.
[30] H. Park, J.Y. Park, G.H. Kim, Il Sohn: Steel Res. Int., 2012, vol. 83, pp. 150-56.
[34] H.G. Du: Principle of Smelting Vanadium-Titanium Magnetite in the Blast Furnace, 1st ed., Science Press, Beijing, 1996, p. 58.
[31] N. Saito, N. Hori, K. Nakashima, and K. Mori: Metall. Mater. Trans. B, 2003, vol. 34, pp. 509-16.
Shankar A, Görnerup M, Lahiri AK, Seetharaman S (2007) Metall Mater Trans B 38B:911-915
Sohn I, Wang WL, Matsuura H, Tsukihashi F, Min DJ (2012) ISIJ Int 52:158-160
Acknowledgments
The authors are especially thankful to the National Natural Science Foundation of China (51574067), China Postdoctoral Science Foundation (2016M601321), Joint Funds of the National Natural Science Foundation of China (U1808212), and Fundamental Research Funds for the Central Universities (N172503016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted August 26, 2018.
Rights and permissions
About this article
Cite this article
Zhao, W., Chu, M., Liu, Z. et al. High-Temperature Interactions Between Vanadium-Titanium Magnetite Carbon Composite Hot Briquettes and Pellets Under Simulated Blast Furnace Conditions. Metall Mater Trans B 50, 1878–1895 (2019). https://doi.org/10.1007/s11663-019-01616-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01616-x