Abstract
The effect of TiO2 on the crushing strength for high-Cr V-Ti magnetite pellets was studied in this paper. On one hand, the crushing strength obviously decreased with the increasing TiO2 contents. On the other hand, the crushing strength had an obvious increase after grinding treatment for the high-Cr V-Ti magnetite and titanium concentrate. It is found that the crushing strength has great relations with the mineral phase and microstructure. The effect of TiO2 on the smelting mechanism for high-Cr V-Ti magnetite pellets was also studied in this paper. With the increasing TiO2 contents in the range of 2.47 to 12.14 pct, the softening start temperature and softening temperature gradually increased, and the softening zone gradually narrowed down; the melting start temperature and the dripping temperature increased, and the melting–dripping temperature zone also increased. The permeability index increased with the increasing TiO2 contents as a whole. In the process of slag–iron’s dripping and separating, it is proposed that amounts of Cr and V moving to the melted iron are obviously more than those moving to the slag, while amount of Ti moving to slag is much greater than that moving to the melted iron. It is demonstrated that Ti(C,N) generates increasingly with the increasing TiO2 contents and accumulates as especial regular rigid granules on the surface of coke. The size of melted iron decreased with the increasing TiO2 contents, and this is in accordance with the present investigations that the dripping difficulty increased with the increasing TiO2 contents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-chromium vanadium-titanium magnetite (hereinafter abbreviated as high-Cr V-Ti magnetite), rich in valuable elements including Fe, V, Ti, and Cr, is a kind of significant mineral resources with great developing value.[1–4] In Panxi-Hongge District in China, there is more than 3.5 billion tons of high-Cr V-Ti magnetite. Domestic high-chromium vanadium-titanium magnetite has not been exploited and made full use of on a large scale so far because of the immature utilization technology of the furnace slag and due to the fact that the utilization efficiency of valuable metals still urgently needs to be improved. Thus, it is significative to study the utilization of this special mineral resource.
During the last decade, considerable quantities of researches of ordinary V-Ti magnetite have been done.[5–15] However, study on high-Cr V-Ti magnetite has been scarce.[2,3,16] Cr2O3, a refractory oxide, affects the blast furnace reduction and slagging process; furthermore, chromium in molten iron significantly affects the follow-up converter vanadium extraction process.[4] Therefore, it is of great necessity to study the Cr-containing V-Ti magnetite.
As is known, the blast furnace is the major iron producing unit and will remain so for the foreseeable future. At present, the feasible and mainstream utilization process of high-Cr V-Ti magnetite should also be the route of agglomeration-blast furnace smelting.[1–3,16] Thus, pelleting production is the foundation for high-Cr V-Ti magnetite utilization. Xu studied the crushing strength change regulation and pellet microstructure of the oxidized pellets with the high-Cr V-Ti magnetite before and after being ground as the original materials.[17] Ou made researches on the effect of the MgO on the crushing strength of high-Cr V-Ti magnetite pellets.[18] However, till now there are no research documentations on the effect of TiO2 on the high-Cr V-Ti magnetite pelleting at home and abroad, let alone on the high-Cr V-Ti magnetite smelting mechanism in the blast furnace. Cheng et al.[2] studied the non-isothermal reduction mechanism and kinetics of high-chromium vanadium-titanium magnetite pellets under the simulated conditions of the lumpy zone of the blast furnace. Liu et al.[3] studied the reduction process of pellet containing high chromic vanadium-titanium magnetite in cohesive zone. Paananen et al.[19] researched the effect of TiO2 content on reduction of iron ore agglomerates, realized on the basis of adding rutile to magnetite and hematite concentrate. In addition, it is well known that TiO2 has great influence on the smelting of V-Ti magnetite, and it is supposed that TiO2 should have great effect on the blast furnace practice. Under the above background, this study focused on detailed researches of high-Cr V-Ti magnetite pelleting and blast furnace smelting mechanism with different TiO2 ratios realized by varying the titanium concentrate percent, and it is of great importance to carry out this work.
In the present work, the effect of TiO2 on the pellet crushing strength and microstructure was first studied. On the basis of preparing qualified oxidized high-Cr V-Ti magnetite pellets, softening-melting-dripping experiments of different TiO2-bearing pellets were carried out in the laboratory, and the effect of TiO2 on the smelting mechanism was further researched with different detecting means including XRF, ICP-AES, XRD, and SEM-EDS whose parameters are described in the experimental methods.
Experimental
Experimental Materials
In the present study, high-Cr V-Ti magnetite pellets are prepared from High-Cr V-Ti magnetite, domestic ordinary ore, titanium concentrate and mixed with 1 pct bentonite. All the original unground ores were sieved by the sieve of 0.5 mm in diameter. The mixing ratios of high-Cr V-Ti magnetite and domestic Oukong ore is 4:6, and 0, 5, 10, 20, and 30 mass pct titanium concentrate was added on the basis of high-Cr V-Ti magnetite and domestic Oukong ore, with the material proportions shown in Table I. High-Cr V-Ti green pellets were made by the balling disk with appropriate conditions including time, water, running and speed. Then the oxidized pellets were prepared from green pellets under simulated conditions of grate-kiln process, and the temperature regime is shown in Table II. The crushing strength of oxidized high-Cr V-Ti magnetite pellets whose sizes were 10 to 12.5 mm was measured according to the standard of GB/T 14201-1993 (Iron Ore Pellets—Determination of crushing strength). The number of experiments performed for each data point of crushing strength was sixty-six. The crushing strength of oxidized high-Cr V-Ti magnetite pellet samples adopted to carry out the latter softening-melting-dripping experiments were above 2000 N/a, and the size of pellet samples was also 10 to 12.5 mm. Table III describes the chemical composition of high-Cr V-Ti oxidized pellets with different mass percent of titanium concentrate, and detailed values of different TiO2 contents for the prepared pellet samples are 2.47, 4.44, 6.18, 9.22, and 12.14 pct for 0, 5, 10, 20, and 30 mass pct.
Experimental Methods
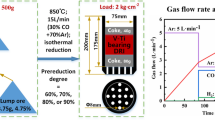
The softening-melting-dripping experiments of high-Cr V-Ti pellets were carried out in the furnace measuring the metallurgical properties made by northeastern University. The size of the softening-melting crucible is 75 mm of the internal diameter, 85 mm of the external diameter, and 180 mm of the height. There are 26 holes of 5 mm in diameter uniformly distributed at the bottom of the crucible. The holes are for the gas flowing through the furnace burden and for the dripping of the melted iron and slag.
In order to simulate the blast furnace charging conditions, the coke (size: 10–12.5 mm) of 20 mm height was put in the bottom of crucible and 500 g high-Cr V-Ti pellets were put on the nether coke, together with the coke of 40 mm height on the pellets. After charging raw materials in the crucible, the pressure lever and the charged crucible were put in the reduction tube and make sure the thermocouple was wired well. It is of great need to seal hermetically the reduction tube bottom well, preventing the gas leaking, as the accuracy of differential pressure should be guaranteed. Then the displacement transducer was adjusted to smoothly being pressed on the pressure lever by the external pressure. The pressure can be regulated during experiments by hands according to the loading extent of burden in the descending process. The loading values were 1.0 kg/cm2. With the preparation work done, the experimental conditions with definite temperature profile and reduction atmosphere in different temperature ranges shown in Table IV referring to previous studies[20] were adopted in the experimental process. The heating rates were 10 K/min (10 °C/min) below 1173 K (900 °C), 3 K/min (3 °C/min) at 1173 K (900 °C) to 1293 K (1020 °C), and 5 K/min (5 °C/min) from 1293 K (1020 °C) to the end. To prevent the dripped and not-dripped products from being oxidized again, argon gas was introduced immediately after the reduction finished.
The crushing strength of high-Cr V-Ti pellets was carried out by the electronic universal testing machine (WDW-1010; Chengde COTS Scientific Instruments co., Chengde, China). The chemical analysis of original pellet samples and different reduced products including the melted iron and slag was analyzed by means of X-ray fluorescent (XRF, ZSXPrimus II; Rigaku, Japan) and inductively coupled plasma atomic emission spectroscopy (ICP-AES, Optima 8300DV; PerkinElmer). The mineral phases of high-Cr V-Ti pellets and slag were analyzed by X-ray diffraction (XRD, X’ Pert Pro; PANalytical, Almelo, the Netherlands) with Cu Kα radiation (λ = 1.5406 Å), and the microstructure, element compositions and distributions of the pellets, and reduced products were examined by scanning electron microscopy–energy-disperse spectroscopy (SEM–EDS, Ultra Plus; Carl Zeiss GmbH, Jena, Germany). Before the XRF, ICP-AES, and XRD characterization, all the samples were ground to fine powders. Before the SEM-EDS characterization, the samples of the melted iron were ground to fine powders, and the samples of high-Cr V-Ti pellets and not-dripped products were bulks.
Results and Discussion
Effect of TiO2 on the Crushing Strength of High-Cr V-Ti Pellets
The crushing strength of high-Cr V-Ti pellets after oxidization with different TiO2 contents before and after grinding is shown in Figures 1(a) and (b). The grinding treatments were for the raw materials of high-chromium vanadium-titanium magnetite and titanium concentrate, hereinafter suitable. Before grinding, it is found that the crushing strength decreased from 2692 to 1346 N/a with the increasing TiO2 contents from 2.47 to 12.14 pct. The crushing strength of high-Cr V-Ti pellets with 6.18 pct TiO2 is just above 2000 N/a and lower than 2000 N/a with 9.22 and 12.14 pct TiO2. In order to meet the strength requirements of high-Cr V-Ti pellets smelting in the blast furnace, the original high-Cr V-Ti magnetite and titanium concentrate were ground for two minutes in the sampling machine. After grinding, the crushing strength of prepared high-chromium vanadium-titanium pellets is enhanced obviously and meets the needs of BF burden as all of the crushing strength values exceed 2000 N/a. It is of great requirements to study the mechanism of crushing strength difference before and after grinding with different TiO2 contents.
In Figures 2(a) through (c), the XRD pattern of high-Cr V-Ti pellets with 6.18, 9.22 and 12.14 pct TiO2 after grinding is presented, together with Figure 2(d) presenting the pellets with 12.14 pct TiO2 before grinding. The predominant Fe-bearing phase is Fe2O3, and there are Ti-bearing phases like Fe2TiO5 and Fe2Ti3O9; Cr-bearing phase like (Fe0.6Cr0.4)2O3, CrVO3, and Cr0.07V1.93O3; and V-bearing phase like V2O3, CrVO3, and Cr0.07V1.93O3. It is generally known that the chemical composition and production technology especially the roasting regime have great effects on the mineral phases of finished pellets.[21] Under the same roasting regime, with the increasing TiO2, it is found that the amount of pseudobrookite, namely Fe2TiO5, gradually increased while Fe2Ti3O9 decreased, and the amount of vanadium trioxide, namely V2O3, increased while CrVO3 decreased. Before and after grinding the titanium concentrate and high-Cr V-Ti magnetite, it is observed that the phase composition of the prepared oxidized pellets changes with the fact that the Fe2TiO5 decreased, and chromium-vanadium oxide named Cr0.07V1.93O3 transformed to CrVO3 with the appearance of (Fe0.6Cr0.4)2O3. In addition, appropriate amounts of SiO2, CaO, and MgO are necessary to guarantee adequate liquid phase amounts in the pellets which have relations with the improvement of microstructure beneficial to increasing the pellet strength.[21] Consequently, it is still required to examine the microstructure of high-Cr V-Ti pellets to further elucidate the reason of crushing strength difference.
Figures 3(a) and 4(a) exhibit the SEM microphotographs of high-Cr V-Ti pellets with 12.14 pct TiO2 before grinding at 100× and 300×, and Figures 3(b) and 4(b) exhibit the SEM microphotographs after grinding at 100× and 300×. Obvious hematite crystal stock was observed in the microstructure, and the contents of Ti in light gray area A are relatively lower than those in the dark gray area B. The gangue phase was also observed in the black area C. Figures 5(a) and (b) present the hematite phase with relatively more titanium of high-Cr V-Ti pellets with 12.14 pct TiO2 before and after grinding at 15000× and 10000×, and the EDS analysis of corresponding area D and E is shown in Figures 6(a) and 6(b), from which it is found that the Fe content in area D is much lower than that in area E, and there are titanium oxides distributed among the titanium hematite phase E. Comparing both kinds of microstructure, it is noticed that the crystalline grain was more regular distributed in the pellet structure with less irregular pores, and crystal stock was better improved by the grinding management. This is due to the fact that the grinding treatment is conducive to improving the specific surface areas of the ores and surface energy and activity of the particles, and that solid diffusion reactions are enhanced, resulting in the strengthening of pellet consolidation.[22] Also, the amount of ferrate (area F) and silicate (area G) shown in Figure 7 with corresponding EDS analysis in Figures 6(c) and (d) was observed relatively more in the SEM microphotographs of high-Cr V-Ti pellets with 12.14 pct TiO2 after grinding than that before grinding, beneficial to increase the liquid phase content and the bonding property of minerals which contribute to the increasing the crushing strength.
Figures 3(c) and 4(c) present the SEM microphotographs of high-Cr V-Ti pellets with 6.18 pct TiO2 after grinding at 100× and 300×. Figure 5(c) presents the hematite phase with relatively more titanium at 10000×, and Figure 7(c) presents the ferrate and silicate phase at 15000×. Comparing the microstructures of high-Cr V-Ti pellets after grinding with 6.18 and 12.14 pct TiO2, it is observed that the growing of titanium hematite in the pellet structure with 6.18 pct TiO2 was better than that with 12.14 pct TiO2, and the total amount of silicate and ferrate phases for the former was relatively more than the latter. With the increasing TiO2 contents, the FeTiO3 contents in the green pellets increased, which can bring about the decreasing oxidation velocity and extent and is less beneficial to the growing of the titanium hematite solid solution crystalline grain[23] during roasting consolidation process, so the crushing strength decreased. From above investigations, it is found that the crushing strength has great relations with the mineral phase and microstructure of high-Cr V-Ti pellets.
Softening-Melting-Dripping Behavior
The inner shape of the cohesive zone of the blast furnace is determined by the melt-down of ores, and the permeability and distribution of gas there is affected. Figure 8 shows the typical results of differential pressure and contraction of high-Cr V-Ti pellets with different mass pct TiO2 in the softening and melting-dripping process. The maximum values of differential pressure appeared with different burden structures. It is found that the contraction degree exceeds 100 pct due to the reality that the cardinal number of height was the pellet height in the crucible, and the coke layer was also pressed especially during the latter softening-dripping stage. It is also of great importance to elucidate the reduction swelling of high-Cr V-Ti pellets in the lumpy zone of the blast furnace shown in Figure 9. It is found that the pellet structure gradually swelled at a relatively lower velocity, and when shrinking, the shrinking rate was at a relatively higher rate. The reduction swelling and thermal swelling mainly cause to the expansion, and in this study, the maximum values of volumetric swelling degree increased from 1.45 to 4.15 pct correspondingly with the increasing TiO2 contents from 4.44 to 12.14 pct. Besides the factors including crystal lattice changes in the reduction process of hematite to magnetite, the formation of long iron whiskers during reduction[24] and the existence of CaO[25] which could cause the swelling and the factor of the existence of MgO and SiO2[25,26] which could reversely cause the swelling, TiO2 could also contribute to the swelling during reduction,[19] and structural changes probably increase with the increasing TiO2 contents.
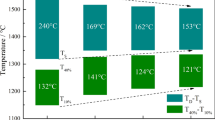
The typical results of softening start temperature (T 4), softening temperature (T 40), and softening zone (T 40 to T 4) of different furnace burdens shown in Figure 10 reveals the softening property change with the increasing TiO2 contents. T 4 and T 40 are the temperature that the shrinkage ratio of raw pellet layer reaches 4 and 40 pct, respectively. It is found that with the increasing TiO2 contents from 2.47 to 12.14 pct, the softening start temperature gradually increased from 1361 K to 1453 K (1088 °C to 1180 °C), and the softening temperature increased from 1474 K to 1523 K (1201 °C to 1250 °C), while the softening zone gradually narrows down from 113 K to 70 K (113 °C to 70 °C). This is increasingly beneficial to the stability of furnace practice and the progress of gas–solid reduction process. According to Yang et al.’s study[27] in which it has been stated that the softening start temperature selected as T 10 of ordinary V-Ti magnetite pellets was higher comparing with ordinary iron ore pellets. In this study, it is found that the difference in softening start temperature between the high-Cr V-Ti pellets and ordinary iron ore pellets was greater. On one hand, the rate of reduction of the phase (Fe,Cr)2O3 which is stable at the initial reduction stage is considerably lower than that for Fe2O3.[28] On the other hand, in Paananen et al.’s study it has been described that at the beginning of the reduction the samples containing 2 and 5 mass pct TiO2 were reduced more slowly than those containing 0 and 0.5 mass pct,[19] and in this study, it can be noticed that TiO2 with the content range of 2.47 to 12.14 pct has an obvious restraining effect on the reduction of high-Cr V-Ti pellets at the initial stage of reduction as the iron oxides combined with titanium oxides are more difficult to be reduced than separate iron oxides.[1]
In Figure 11, melting start temperature (T s), namely the temperature accompanying differential pressure with a massive jump, dripping temperature (T d), namely the temperature that the pig iron drips from the graphite crucible and melting-dripping zone (T d − T s) of different furnace burdens, are exhibited. It is found that the melting start temperature increased from 1496 K to 1576 K (1223 °C to 1303 °C) and the dripping temperature increased from 1611 K to above 1823 K (1338 °C to above 1550 °C), while the melting-dripping temperature zone also increased from 115 K to above 269 K (115 °C to above 269 °C). It is propitious to BF operation as the starting melt-down temperature progressively increases with the increasing TiO2 contents. However, the dripping temperature also rises, and the increasing rate of melting-dripping temperature zone is relatively higher, resulting in the property deterioration of melting-dripping temperature zone. It has been reported that T s, T d, and T d − T s of the pellet burden with 9.02 mass pct TiO2 were 1556 K, 1772 K, and 216 K (1283 °C, 1499 °C, and 216 °C)[27], and comparing with this previous study, it is found that Ts of the pellet burden with 9.22 pct TiO2 in this study is lower of 42 K (42 °C), T d is higher of 11 K (11 °C), and T d − T s is higher of 53 K (53 °C). Besides the TiO2 difference, lower V and Cr addition could also contribute to the result change, and it was further elucidated in the latter text. As can be seen clearly in Figure 12, with the increasing TiO2 contents, the melting start temperature increased and the softening-melting zone gradually shifted down, which is beneficial to the BF smelting. However, the softening-melting zone widened, and it is conversely beneficial to the BF practice.[20]
The factors influencing the melting-dripping characteristics, especially the phenomenon that the melted iron is distributed in slag and difficult to separate and the formation of foaming slag, should be greatly considered. Generally, this is due to the property changes in melted iron and slag for which the viscosity and surface tension need to be paid great attention. For the melted iron, the effect of [Ti] on the surface tension is lesser,[29] and the existence of [Ti] just decreases the surface tension slightly. However, the effect of [Ti] on the viscosity of the melted iron is obvious, and the viscosity increased with the increasing the titanium contents because the fact that the radius of Ti atom is bigger than that of Fe atom decreases the free space of iron melt.[30] In the Fe-Ti-C system, the effect of [Ti] on the viscosity has also relations with the solubility of [Ti] in the melted iron and the precipitation form of titanium, and when [Ti] exceeds the solubility, it will precipitate from melted iron which can result in the increasing viscosity of melted iron. For the slag, it is commonly indicated that TiO2 is the surface tension substance of slag and is beneficial to decreasing the surface tension of slag, but the effect of TiO2 on the surface tension is very small and has few influence on BF smelting.[31] Besides, it has been reported that TiO2 additions can decrease the slag viscosity and the corresponding activation energy of viscous flow.[32] However, considering the factor of titanium in this study, the existed form in the slag could be carbonitrides of titanium except for titanium oxides. The substances of TiC and TiN with high melting point are insoluble in slag and melted iron, but hang and diffuse in the slag, resulting in the slag being sticky. Furthermore, the slag and melted iron even can gather together seriously and are of great difficulty to separate. The increase in substances with high melting point causes to quicken of slag thickening, and this can affect the mass transfer condition of vanadium oxides between the slag and melted iron which can decrease the vanadium content in melted iron. Considering the effect of [V] on the viscosity of melted iron, generally [V] can lower the viscosity of melted iron, but the temperature that the viscosity increases suddenly is raised with the increasing [V] amount on account of the appearance of carbide with high melting point for [V] in the Fe-C-V system. However, the effect of [V] on the viscosity of melted iron is minor because the solubility of [V] in the melted iron is relatively large, and the precipitated vanadium amount decreases with the decreasing temperature. Furthermore, the effect of [V] on the melted iron is relatively much less than [Ti] and has some independence. Owing to the fact that the [V] amount in the melted iron mainly depends on the vanadium amount brought by the furnace burden and has just wee changes,[33] the effect of [V] could be neglected. For the slag, it has been described that the existence of vanadium oxides can restrain the reduction of titanium oxides in the titanium-bearing slag under the condition of above 2 mass pct vanadium content,[34] but the vanadium contents were much lower than 2 pct in this study, resulting in the fact that the restraining effect of vanadium oxides on titanium oxides could be neglected. However, considering the fact that vanadium oxides can be reduced to VC and then VC and Ti(C,N) can form solid solution, the viscosity increases correspondingly and the slag thickens which can increase the dripping difficulty. Furthermore, the surface tension between these two phases of VC and Ti(C,N) could also change because of the solid solution, resulting in the slag adhering to the coke surface.[1] In addition, with respect to Cr effect on the melted iron, the viscosity increases and the fluidity decreases with the increasing [Cr] in the melted iron—because of the decrease in the free space of iron melt as Cr radius is bigger than Fe radius and due to the fact that effect of [Cr] on the viscosity has also relations with the temperature of precipitated carbide for Cr in the melted iron and the form of chromium precipitation in the Fe-Cr-C system.[35] For Cr effect on the slag, as the viscosity significantly increases with the increasing Cr2O3 content from 0 to 4 pct[4] because Cr2O3 could easily react with MgO and Al2O3 to generate spinel phase (MgCr2O4, MgCrAlO4) with high melting points in the BF slag, the viscosity of slag formed from Cr-containing V-Ti magnetite could be higher than Cr-free V-Ti magnetite. In a word, the complicated factors contribute to the increasing dripping difficulty for the smelting of high-Cr V-Ti pellet burden with the increasing TiO2 contents.
It is well known that the gas permeability, one of the significant indexes, is greatly related to the differential pressure, and the higher the differential pressure, the worse the gas permeability. In practical production, good or bad gas permeability directly influences the BF product yield and the operation condition, and it is of great need to optimize the melting-dripping properties with certain TiO2 ranges. Table V shows the maximum differential pressure \( (\Delta P_{ \hbox{max} } ) \) and the corresponding temporary temperature \( (T_{{\Delta P_{\hbox{max} } }} ) \), dripping differential pressure \( (\Delta P_{\text{d}} ) \) , and permeability index (S) of different furnace burdens. S value is calculated by the equation \( S = \mathop \smallint \limits_{{T_{\text{s}} }}^{{T_{\text{d}} }} (\Delta P - \Delta P_{\text{s}} ){\text{d}}T \). On one hand, the maximum differential pressure increased to the maximum value of 19924 Pa when TiO2 in the high-Cr V-Ti pellets is 6.18 pct and then began to decrease. On the other hand, the permeability index increased with the increasing TiO2 contents as a whole. In comparison with the maximum differential pressure and the permeability index of ordinary V-Ti pellet burden with 9.02 mass pct TiO2 investigated in Yang et al.’s study,[27] both values of high-Cr V-Ti pellet burden with 9.22 pct TiO2 in this study are much higher. The worse reducibility in the case of higher TiO2-content enabled surface formation of wustite and metallic iron before the material was reduced to magnetite.[19] With the increasing wustite, more generated fayalite appeared, and this contributes to the disappearance of the microporosities and low porosity which can result in the burden shrinking velocity increasing and the diffusion difficulty of reducing gas and gas products increasing. Also, the formation of metallic iron can also cause the burden to shrink and prevent the gas diffusion. Thus, the permeability resistance was raised largely during the burden shrinking process. However, the wustite in the ilmenite is more difficult to reduce to metallic iron than absolute wustite. It is proposed that the influencing ratio of metallic iron increasing with the separation of wustite and TiO2 from the ferrum-titanium oxides.
Valuable Element Transformation
It is of great significance to investigate the migration behavior and distribution pattern of valuable elements including Fe, V, Cr, and Ti in the slag and melted iron, which can be a guideline to the production practice. By making XRF and ICP-AES analysis, the chemical composition of not-dripped products still in the bottom of crucible at the end of dripping stage is shown in Table VI and of dripped products away from the crucible is shown in Table VII. By comparison, it is found that the mass percent of Fe, V, Cr, and Ti in the dripped products is lower than that in the not-dripped products. Moreover, from the experimental investigations, most slag was splashed to the top layer in the crucible and the dripped products are most melted iron. It is necessary to analyze the distribution of different elements in the slag and melted iron to describe the transformation regulation of valuable elements, so it is studied by separating the slag and melted iron in the latter analysis.
It was observed that most not-dripped slag–iron was in the upper layer of the reduction matter in the crucible in which the slag accounts more than the melted iron. This slag-iron did not drip smoothly in the experiments due to the fact that foamed slag with the generation of TiC and TiN appeared in the melting-dripping process. In practical production, it is of vital importance that the appearance of TiC and TiN should be avoided as much as possible so that the productivity is guaranteed. To the not-dripped slag–iron for original pellets with 9.22 and 12.14 pct TiO2, the melted iron and slag were separated at 1623 K (1350 °C) for 60 minutes, and the slag is on the top of the melted iron. Table VIII presents the chemical composition of separated iron and slag. Through the comparison of Cr, V, and Ti between the dripped products and separated products of slag and iron for original pellets with 9.22 and 12.14 pct TiO2, it is found that V content changed from 0.011 and 0.016 pct in the separated melted iron to 0.030 and 0.048 pct in the dripped products which is mainly melted iron, respectively, and Cr content changed from 0.043 and 0.041 pct in the separated melted iron to 0.079 and 0.083 pct in the dripped products, respectively. However, Ti content changed from 0.134 and 0.295 pct in the separated melted iron to 0.078 and 0.103 pct in the dripped products, respectively. It is proposed that in the process of slag–iron’s dripping and separating, amounts of Cr and V moving to the melted iron are obviously more than those moving to the slag, while amount of Ti moving to slag is much greater than that moving to the melted iron, and it is further validated with the following microscopic examination. Besides, great quantities of TiC and TiN appeared, resulting in the difficulty of dripping in the experiments. It is consistent with the phenomenon that large amounts of foamed slag flowed to the top of not-dripped products while only small amounts of melted iron dripped through the crucible holes intermittently. Corresponding measures should be taken to reduce the high viscosity caused by TiC and TiN in the slag. Also, it is significant to make the valuable elements’ transformation and movement more clear to improve and perfect the utilization efficiency of vital V, Cr, and Ti, and in the following part, the element compositions and transformation patterns of these three elements during the formation and separation of slag and melted iron were further analyzed with the SEM–EDS analysis.
Microstructure
It is well known that the slag is sticky, and it is of great difficulty to separate the slag from melted iron with high contents of TiO2. Furthermore, the dripping condition became worse and worse with the increasing TiO2 contents, as mentioned above. From this investigation, the amount of dripping melted iron was less than that for ordinary iron ore pellets due to the fact that the melted iron with relatively more slag splashed to the top layer of the crucible. Also, a layer of products with multivariate colors including golden yellow, purplish red, and light blue, appeared in the surface of the crucible. It is proposed that the multivariate-color substance is Ti(C,N) generated through the reaction of slag and coke or slag and graphite crucible, which is in accordance with the previous study.[1] Moreover, it was observed in the not-dripped products presented in Figures 13 and 14. Figures 13(a) and (b) present the not-dripped product for high-Cr V-Ti pellets with 9.22 pct TiO2 at 200× and 800×, together with the EDS analysis of area A presented in Figure 13(c). Similarly, Figures 14(a) and (b) present the not-dripped product for high-Cr V-Ti pellets with 12.14 pct TiO2 at 200× and 1000×, together with the EDS analysis of area A presented in Figure 14(c). It can be seen obviously from the images that generated Ti(C,N) product, probably generating through the reaction of slag and coke, accumulated as especial regular rigid granules on the surface of coke (area B). In Figure 15, the XRD pattern of separated slag for high-Cr V-Ti pellets with 9.22 and 12.14 pct TiO2 is shown, and from the XRD analysis, it is found that generated Ti(C,N) increased with the increasing TiO2 contents. Also, it is consequent that Ti(C,N) generates from the reduction reaction of TiO2 with the existence of carbon at elevated temperatures based on thermodynamics analysis.[1,3] From the thermodynamics calculation, XRD analysis, presentative SEM images, and EDS analysis together with the element composition of Ti, C, and N, it is demonstrated that Ti(C,N) generates increasingly as more C and N combined with Ti were also detected with the increasing TiO2 contents. The C and N contents were 5.54 and 4.17 pct for not-dripped smelting products with 9.22 pct TiO2 in the original pellet samples, and 12.45 and 5.28 pct for those with 12.14 pct TiO2. The generated Ti(C,N) has great relations with the melting-dripping characteristics referred to in the above softening-melting-dripping behavior. Therefore, decreasing the generated amount of Ti(C,N) and finally decomposing these chemical compounds containing C and N, also appropriate for the smelting of high-Cr V-Ti magnetite in the blast furnace, are of vital significance.
Figure 16 presents the SEM image of dripped melted iron for high-Cr V-Ti pellets with 6.18, 9.22 and 12.14 pct TiO2 at 5000×, and Figure 17 presents the SEM image at 10000×. As shown in Figures 16 and 17, it can be seen clearly the size of melted iron decreased with the increasing TiO2 contents. This corresponds with the above experimental investigations of basic dripping characteristics of high-Cr V-Ti pellets that the dripping temperature increased with the increasing TiO2 contents as it is well known that the bigger the size, the less the dripping difficulty will be. Furthermore, on the basis of above investigations, the slag and melted iron gathered together more vigorously, and it was difficult for them to separate due to the formation of more Ti(C,N) with the increasing TiO2 contents, thus contributing to the melted iron being more disperse and the amount of separate melted iron without slag being less, which result in the increased dripping difficulty. From the macroscopic existed form and microscopic examination of melted iron, the decrease in the melted iron size with the increasing TiO2 contents is consistent with the dripping difficulty increasing. By analyzing the EDS analysis of areas A, B, and C shown in Figures 18(b) through (d) for the dripped melted iron of high-Cr V-Ti pellets with 12.14 pct TiO2 at 20000× shown in Figure 18(a), it is examined that the element distribution percent of Fe, V, Ti, and Cr for different areas of metallic iron are different. However, it can still be noticed that few amounts of Ti were found in the melted iron phase as most of the titanium was not reduced and remained in the slag, while Fe, V, and Cr were relatively easily reduced to hot metal. The atomic number of Cr and V is next to each other in periodic table of chemical elements, and the crystal systems of both are body-centered cubic. These factors indicate that Cr and V are more difficult to separate than Cr and Ti. The element transformation revealed the EDS analysis is correlated with the above analysis of valuable element transformation. Consequently, in the process of slag–iron’s dripping and separating, it can be further proposed that amounts of Cr and V moving to the melted iron are obviously more than those moving to the slag, while amount of Ti moving to slag is much greater than that moving to the melted iron. In addition, V, Ti, and Cr, especially V and Cr, are difficult to examine in certain areas due to low contents. Thus, the work of detecting the element distribution of melted iron is of great necessity. Figure 19 presents the element distribution of melted iron for high-Cr V-Ti pellets with 12.14 pct TiO2. Tiny amounts of Cr and V can be found as each total amount for Cr and V is limited, and only just small amounts of Ti can be found in some areas as most Ti is distributed in the slag. Furthermore, it is observed that Cr and V are more evenly dispersed than Ti in the melted iron, and this could probably be illustrated that Cr and V are inclined to stay in the melted iron, while Ti is to stay in the slag from another point of view.
Conclusions
The effect of TiO2 on the crushing strength and on the smelting mechanism of high-Cr V-Ti pellets was investigated in the present work. From the studies carried out, the following conclusions can be drawn:
-
1.
The crushing strength decreases with the increasing TiO2 contents at the range of 2.47 to 12.14 pct both before and after grinding the original ores of high-Cr V-Ti magnetite and titanium concentrate. It is beneficial to improve the crushing strength by grinding treatments in order to meet the BF production requirements. The crushing strength has great relations with the mineral phases and microstructures.
-
2.
With the increasing TiO2 contents at the range of 2.47 to 12.14 pct, the softening start temperature and softening temperature gradually increase, and the softening zone gradually narrows down; the melting start temperature and the dripping temperature increase, and the melting-dripping temperature zone also increases. The permeability index increased with the increasing TiO2 contents as a whole. The maximum differential pressure decreases after increasing to the maximum value of 19924 Pa when TiO2 in the high-Cr V-Ti pellets is 6.18 pct. It is still of great need to optimize the properties with certain TiO2 range.
-
3.
In the process of slag–iron’s dripping and separating, it is proposed that amounts of Cr and V moving to the melted iron are obviously more than those moving to the slag, while amount of Ti moving to slag is much greater than that moving to the melted iron by investigating the valuable elements’ transformation and movement.
-
4.
It is demonstrated that Ti(C,N) generates increasingly with the increasing TiO2 contents and accumulates as especial regular rigid granules on the surface of coke. The size of melted iron decreases with the increasing TiO2 contents, and this is in accordance with the present investigations that the dripping difficulty increases with the increasing TiO2 contents.
References
H.G. Du: Principle of smelting vanadium-titanium magnetite in the blast furnace, 1st ed., p. 1, Science Press, Beijing, China, 1996.
G.-J. Cheng, J.-X. Liu, Z.-G. Liu, M.-S. Chu, X.-X. Xue: Ironmaking and Steelmaking, 2015, vol. 42(1), pp. 17-26.
J.X. Liu, G.J. Cheng, Z.G. Liu, M.S. Chu, X.X. Xue: Steel Res. Int., 2015, vol. 86(7), pp. 808-816.
G.B. Qiu, L. Chen, J.Y. Zhu, X.W. Lv, C.G. Bai: ISIJ Int., 2015, vol. 55(7), pp. 1367-1376.
S.K. Gupta, V. Rajakumar, and P. Grieveson: Metall. Trans. B, 1989, vol 20B, pp. 735-745.
T. Hu, X.W. Lv, C.G. Bai, Z.G. Lun, and G.B. Qiu: Metall. Mater. Trans. B, 2013, vol 44B, pp. 252-260.
G.D. McAdam: Ironmaking and Steelmaking, 1974, vol 1(3), pp. 138-150.
J.B. Zhang, Q.S. Zhu, Z.H. Xie, L. Chao, and H.Z. Li: Metall. Mater. Trans. B. 2013, vol 44B, pp. 897-905.
K. Sun, R. Takahashi, J. Yagi: ISIJ Int., 1992, vol 32(4), pp. 496-504.
L. H. Zhou, and F. H. Zeng: Ironmaking and Steelmaking, 2011, vol 38(1), pp. 59-64.
R. Huang, X.W. Lv, C.G. Bai, K. Zhang, and G.B. Qiu: Steel Research Int., 2013, vol 84(9), pp. 892-899.
S.Z. El-Tawil, I.M. Morsi, A. Yehia, and A.A. Francis: Can. Metall. Quart., 1996, vol 35(1), pp. 31-37.
E. Park, and O. Ostrovski: ISIJ Int., 2004, vol 44(6), pp. 999-1005.
J. Dang, X.J. Hu, G.H. Zhang, X.M. Hou, X.B. Yang, and K.C. Chou: High Temperature Materials and Processes, 2013, vol 32(3), pp. 229-236.
K. Sun, T. Akiyama, R. Takahashi, and J. Yagi: ISIJ Int., 1995, vol 35(4), pp. 360-366.
J.-Y. Hwang: Characterization of Minerals, Metals, and Materials 2013, Minerals, Metals and Materials Society, Warrendale, 2013, pp. 363–69.
L.B. Xu: Master’s Thesis, Northeastern University, 2012.
H.Z. Ou: Master’s Thesis, Northeastern University, 2012.
T. Paananen, K. Kinnunen: Steel Research Int., 2009, vol 80(6), pp. 408-414.
M.S. Chu: Raw fuels and auxiliary materials in Ferrous Metallurgy, 1st ed., p. 158, Metallurgical Industry Press, Beijing, China, 2010.
Y.M. Chen, and R. Chen: Microstructure of sinter and pellet, 1st ed., p. 111, Central South University Press, Changsha, China, 2011.
D.Q. Zhu, D. Chen, J. Pan: J Central South Univ. (Sci. Technol.), 2011, vol 42(7), pp. 1825–32.
X.L. Chen, S. Liu, M. Gan, and X.H. Fan: Chin. J. Eng., in press.
S. Hayashi, and Y. Iguchi: Ironmaking and Steelmaking, 2005, vol 32(4), pp. 353-358.
H.T. Wang, and H.Y. Sohn: Ironmaking and Steelmaking, 2011, vol 38(6), pp. 447-452.
G.H. Li, Z.K. Tang, Y.B. Zhang, Z.X. Cui, and T. Jiang: Ironmaking and Steelmaking, 2010, vol 37(6), pp. 393-397.
G.Q. Yang, J.L. Zhang, J.G. Shao, Y.C. Wen, J.T. Rao, and W.G. Fu: Iron Steel Vanadium Titanium, 2012, vol 33(5), pp. 30-34.
M.H. KHEDR: ISIJ Int., 2000, vol 40(4), pp. 309-314.
W.Z. Luo, Y.W. Mao, L. H, and Y.K. Zhu: Iron and Steel, 1987, vol 22(1), pp. 1–4.
G.Y. Wen, Y.Z. Yan, S.J. Zhao, J.J. Huang, G.H. Jiang, and X.M. Yang: Iron and Steel, 1996, vol 31(2), pp. 6-11.
W.Z. Wang, and Y.X. Shi: Iron Steel Vanadium Titanium, 1989, vol 10(2), pp. 13-15.
N. Saito, N. Hori, K. Nakashima, and K. Mori: Metall. Trans. B, 2003, vol 34B, pp. 509-516.
G.Y. Wen, Y.Z. Yan, P.T. Zhou, Y.C. Zhou, D.H. Liao, and G. Wang: Iron Steel Vanadium Titanium, 1996, vol 17(3), pp. 24-29.
H.G. Du, and Z.P. Zhang: Iron Steel Vanadium Titanium, 1994, vol 15(4), pp. 1–3, 27.
P. Liu, and W.Z. Ding: Ferro-Alloys, 2004, vol 2, pp. 8-11.
Acknowledgments
The authors are especially thankful to the Major Program of National Natural Science Foundation of China (Grant No. 51090384), 863 Program (Grant No. 2012AA062302 and No. 2012AA062304) and Fundamental Research Funds for the Central Universities (Grant No. N110202001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 23, 2015.
Rights and permissions
About this article
Cite this article
Cheng, G., Xue, X., Jiang, T. et al. Effect of TiO2 on the Crushing Strength and Smelting Mechanism of High-Chromium Vanadium-Titanium Magnetite Pellets. Metall Mater Trans B 47, 1713–1726 (2016). https://doi.org/10.1007/s11663-016-0628-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0628-7