Abstract:
The present work calculated the heat capacity, enthalpy change, and slag temperature of CaO–MgO–Al2O3–SiO2 slags after adding Na2O or K2O, and investigated the influences of Na2O and K2O on the slag viscosity under given temperatures and heat quantities. It was found that the slag viscosity decreases with the addition of Na2O and tends to increase with K2O additions at the same temperature. The heat capacity of the slag increases, while the enthalpy change decreases obviously with the increasing addition of Na2O or K2O. Under the constant heat quantity, an increase in content of Na2O or K2O of the slag leads to an appreciable increase in slag temperature, whereas the viscosity decreases significantly. Besides, the Na2O or K2O additions also help to stabilize the slag fluidity and lower energy consumption of blast furnace.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viscosity is one of the most important physicochemical properties of blast furnace slags that determines the productivity and stability in the blast furnace operation.[1,2,3] As the main by-product of blast furnace ironmaking, the slag temperature has an important influence on mass transfer, heat transfer, and chemical reactions between the slag and hot metal. Moreover, when the slag is periodically discharged outside the furnace, the heat carried away by the slag is also highly correlated with the slag composition. In recent years, many blast furnaces have used low-grade ores due to the shortage of high-quality ore resources and increased economic and environmental pressures. This causes not only an increase in the slag volume but also of alkali oxides such as Na2O and K2O in typical blast furnace slags. For instance, owing to the use of the distinctive ores, along with special raw fuel conditions, the BF slags in some China’s steel enterprises contain considerable concentrations of Na2O and K2O, while the alumina content and MgO/Al2O3 ratio are about 9 pct and 0.9, respectively. Generally, such changes in slag composition will inevitably affect the slag viscosity, the operational stability, and energy utilization of blast furnace. Therefore, it is necessary to further investigate the influences of Na2O and K2O on the viscous behavior and the thermodynamic properties of the BF slags. Sukenaga et al.[4] investigated the effects of Na2O and K2O on the viscosity of CaO-SiO2-masspct20Al2O3 slag system by using solid-state Magic Angle Spinning-Nuclear Magnetic Resonance (MAS-NMR). The results showed that the slag viscosity decreased with Na2O additions while increased with the addition of K2O. The work done by Kim et al.[5] indicated that the viscosity of CaO-SiO2-masspct10MgO-masspct20Al2O3-Na2O slags decreased with the addition of Na2O. Meanwhile, Kim et al.[6] studied the CaO-SiO2-masspct10MgO-masspct20Al2O3-K2O slag system and observed an increase in the viscosity with K2O additions. Zhang et al.[7] also measured the viscosity of CaO-SiO2-Al2O3 melt and found that viscosity increased first and then decreased with increasing additive content of K2O.
However, to the knowledge of the authors, there are few detailed studies regarding the influences of Na2O and K2O on the viscosity and thermodynamic properties of blast furnace-type slags in particular under the condition of high MgO/Al2O3 ratio. Accordingly, insights into the viscosity of slag with relatively low alumina are meaningful to further optimize the blast furnace operation. As a matter of fact, the heat quantity of the slag is usually steady when the conditions of fuels and raw materials as well as blast furnace operations are stable over a given period. Furthermore, because the heat capacity of the slag system varies with the compositions of slag, the effects of Na2O and K2O on the viscosity may be different at this time. In this study, the viscosities of CaO-SiO2-8masspctMgO-9masspctAl2O3 were measured after adding Na2O or K2O under the assumption that the heat quantity of the slags was constant. The heat capacity, enthalpy change, and temperature of the slag system were also calculated based on the thermodynamic data of the slag components.
Experimental Procedures
Thermodynamic Calculations
The compositions of the slags used in the calculation and experiment are shown in Table I, and the total mass of each group of slags is 125 g. Theoretically, the heat capacity and enthalpy change of the slags can be estimated according to the formulas [1] through [12]. In view of the inevitable existences of crystal transformation heat, phase transformation heat, and chemical reaction heat during the melting process, the calculations were carried out using FactSage computer package[8] to improve the accuracy. The software package has been successfully used to predict thermodynamic data for metallurgical melts.[9,10,11,12]
In the calculation process, it was assumed that the initial conditions of each component of the slags were 298 K (25 °C) and 101.325 kPa. The heat capacity and enthalpy change of each group of slags were calculated at 1673 K, 1723 K, 1773 K, 1823 K, and 1873 K (1400 °C, 1450 °C, 1500 °C, 1550 °C, and 1600 °C). The first law of thermodynamics shows that under the conditions of constant pressure and only volume work, the enthalpy change of the slag at a certain temperature is equal to the heat absorbed during the heating process. Therefore, the average enthalpy changes of the slags with different contents of Na2O and K2O at 1823 K (1550 °C) were set as Q1 and Q2, respectively, and then were used as given heat inputs to calculate the corresponding equilibrium temperatures of the slags, wherein the equilibrium temperature refers to the temperature at which the slag attains thermal equilibrium under the given heat quantity. Finally, the heat inputs can be changed to 98 pct Q1, 96 pct Q1, 98 pct Q2, 96 pct Q2, and the equilibrium temperatures of the slags with different contents of Na2O and K2O were calculated again.
where \( C_{\text{pCaO}} \), \( C_{{{\text{pSiO}}_{ 2} }} \), \( C_{\text{pMgO}} \), \( C_{{{\text{pAl}}_{ 2} {\text{O}}_{ 3} }} \), \( C_{{{\text{pNa}}_{ 2} {\text{O}}}},\) and \( C_{{{\text{pK}}_{ 2} {\text{O}}}} \) are the specific heat capacities of CaO, SiO2, MgO, Al2O3, Na2O, and K2O, respectively,[13,14,15,16,17] J/g K; \( m_{i} \) represents the mass of each slag component, g; \( C_{pi} \) refers to the specific heat capacity of each slag component, J/g K; \( C_{\text{p}}^{\text{EM}} \) refers to the mixed excess heat capacity of multicomponent system at the target temperature, J/K; \( C_{\text{p}} \) denotes the heat capacity of the slag system at the target temperature, J/K; \( \Delta_{\text{tr}} H_{i} \), \( \Delta_{s}^{l} H_{i},\) and \( \Delta H_{i} \) refers to the crystal transition enthalpy, melting enthalpy, and enthalpy change of unit mass component \( i \) during the heating process, J/g; \( \Delta_{\text{mix}} H_{i} \) is the enthalpy change of mixing of unit mass component of multicomponent system at the target temperature, J/g; \( \Delta H_{T} \) is the enthalpy change of each group slag at target temperature, J; \( T_{\text{tr}} \) and \( T_{\text{M}} \) are the crystal transition temperature and melting temperature of slag component \( i \), respectively, K; \( T \) is the target temperature, K; \( i \) denotes slag components of CaO, SiO2, MgO, Al2O3, Na2O, and K2O, respectively.
Materials for Viscosity Measurement
Slags were prepared using reagent-grade chemicals of CaO, MgO, Al2O3, SiO2, Na2CO3, and K2CO3 powders. The reagents of CaO, MgO, Al2O3, and SiO2 powders were calcined at 1273 K (1000 °C) in a muffle furnace for 10 hours to decompose any impurities such as hydroxide and carbonates, while the Na2CO3 and K2CO3 were baked at 773 K (500 °C) for 10 hours before use. The slags were weighed 125g precisely according to the desired compositions shown in Table I and then mixed thoroughly with an agate mortar. The well-mixed samples were premelted in a Mo crucible for 2 hours at 1873 K (1600 °C) under 1 L/min high-purity Ar atmosphere to homogenize the mixtures. Subsequently, the slags were quenched and crushed for the following experiments. According to the studies of Kim[18] and Zhang et al.,[7] the vaporization of Na2O and K2O in aluminosilicate melts at high temperature can be negligible. As a result, it can be concluded that the contents of oxides, including Na2O and K2O, are almost unchanged before and after the measurements.
Apparatus and Procedure
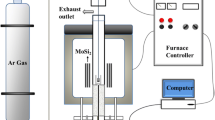
The viscosities of the slags at high temperature were measured by the typical rotating cylinder method using a rotating viscometer (RTW-10 Type), and schematic diagram is shown in Figure 1. The experimental apparatus consists of a heating system, a rotating system, and a measuring system. The electric resistance furnace was equipped with Six U-shaped MoSi2 heating elements for heating and melting and the hot zone was monitored by a Pt-6 pctRh/pt-30pctRh thermocouple. The thermocouple that was protected by an alumina tube aims to ensure the temperature deviation within ± 2 K using a PID controller. The viscosity measurements in this experiment were carried out with a Mo spindle, a Mo crucible coated with a graphite crucible, and a suspending corundum rod. Before starting each viscosity measurement, the viscometer was calibrated using castor oil with a known viscosity at room temperature.
Schematic diagram of experimental apparatus for viscosity measurements. Reprinted from Ref. [19], with permission
In the experiment, the Mo crucible and Mo spindle should be properly realigned along the axis of the viscometer. To prevent the oxidation of Mo crucible and Mo spindle, the high-purity Ar with a flow rate of 1.0 L/min was used as a protective gas throughout the entire experiment. Additionally, the Mo spindle should be carefully immersed into the slag melts until the tip was placed approximately 10 mm above the base of the crucible. All the viscosity measurements were performed during a 5 K/min cooling cycle under an Ar atmosphere with an equilibration time of 30 minutes at each target temperature. The duration was sufficient to homogenize the slag melt and attain thermal equilibrium. The three rotating speeds of 100, 150, and 200 rpm were taken for viscosity readings, and the equilibration time for each viscosity measurement was chosen as 3 minutes. Then the average viscosity value of different rotating speeds was adopted as the final viscosity. The viscosity deviation value by different rotating speeds was less than 2 pct, confirming that the slag melts was Newtonian and homogeneous.
Results and Discussion
Effect of Na2O on Viscosity and Thermodynamic Properties of the Slags
As shown in Figure 2, as the Na2O content increases, the viscosity of the slag decreases gradually, which is basically in accordance with the work done by others.[4,5,20] This is mainly because Na2O in the slag melts acts as a basic oxide and can be dissociated to produce Na+ and free oxygen ion (O2−), and the O2− ion depolymerizes the network structure of the slag to decrease the viscosity.[4] The results by Kim et al.[5] and Xu et al.[20] were also provided for comparison. It can be seen that, although the variation trend of viscosity is similar, the viscosity measured by Kim et al. is significantly higher than that in this experiment, which may be attributed to the high content of alumina in the slag. Because of the high similarity of slag composition, the results of Xu et al. are more close to the experimental values.
The study by Kim et al.[5] indicated that Na+ and O2− ions provided by Na2O preferred to modify [AlO4]5−-tetrahedrons to satisfy their ion compensation effect compared with [SiO4]4−-tetrahedrons. However, based on the findings of Xu et al.,[20] the silicate structures were depolymerized with the increase of Na2O content from 0 to 5 mass pct, and the decrease of average number of bridging oxygen of the slag was also confirmed. The main difference of the two studies may be related to the different Al2O3 contents, which probably determined the concentrations of Al3+ ion and [AlO4]5−-tetrahedral structure in the slags. Usually, the positive + 1 cations have ion compensation effect in the aluminosilicate melt,[21,22] and there is a strict order for which cations carry out the charge compensation of Al3+ ions.[23,24,25] Relative to Ca2+ and Mg2+ ions, Na+ ion also has the advantage of space configuration because one Ca2+ or Mg2+ ion has to compensate for two Al3+ ions.[26] Therefore, Na+ preferentially compensates with [AlO4]5−-tetrahedron to achieve charge balance. Furthermore, the addition of Na2O will stabilize the [AlO4]5−-tetrahedral structure due to the stronger chemical bonds, thereby increasing the degree of polymerization of the slag structure.[27] On the other hand, the Na+ ion provided by Na2O in the silicate melt has a smaller radius and is more likely to diffuse when the interaction force is neglected,[28] and it will help to reduce the viscosity. The molecular dynamics simulation results of aluminosilicate by Li et al.[29] show that the viscosity of slag tends to decrease with the addition of Na2O because of the increase of diffusion coefficient of Na+ ion. It can be found that the effect of Na2O on lowering viscosity is usually dominant. Hence, slag viscosity for current slag system decreases with increasing Na2O additions.
The effect of Na2O on the heat capacity and enthalpy change of the slag is provided in Figure 3. It can be observed that the heat capacity of the slag increases for the Na2O addition. Nevertheless, the heat capacity of the slag decreases with higher temperature when the Na2O content exceeds 1 mass pct. As an important thermo-physical property of the slag melt, the heat capacity of the slag is strongly dependent on the composition and temperature of the melt. As shown in Figure 3(b), the enthalpy change increases with an increase in slag temperature as expected. Besides, the enthalpy change of the slag at a certain temperature decreases with the increase of Na2O content. Obviously, the heat quantity taken away by slag discharge can be decreased with increasing the additive content of Na2O. In this sense, the addition of Na2O may help to reduce the fuel ratio and energy consumption of blast furnace especially as the slag temperature is relative stable.
In order to further explore the influences of Na2O on the properties of the slag under constant heat quantity, the slag temperature and viscosity as functions of Na2O content at different fixed heat quantities are demonstrated in Figure 4. For the convenience of discussion, the aforementioned equilibrium temperature of the slag is simply referred to as the slag temperature. It may be noted that under different fixed heat quantities, the slag temperature increases obviously with the Na2O additions, while the slag viscosity significantly decreases. It can be understood from the change in network structure of the slag. In addition to the effect of O2− ions provided by Na2O on the depolymerization of slag structure, the increasing temperature has a critical influence on the viscosity due to its strong thermal depolymerization by sufficient excess thermal energy.[30] Simultaneously, as the slag temperature increases, the structural units of the slag can gain more kinetic energy, so the motions speed up and move away from each other. As a consequence, more space is available for the structural units to move around easily and the restrictive forces reduce resulting in the decrease in viscosity.[31] Additionally, it can also be found that as the Na2O content increases, the viscosity fluctuation caused by the changes in heat quantities of the slag decreases. As we know, when operating conditions or raw materials and fuels change, it will inevitably cause heat fluctuations of the slag in actual BF production. From this point of view only, the fluidity and stability of the slag will be improved with the addition of Na2O.
Effect of K2O on Viscosity and Thermodynamic Properties of the Slags
The effect of K2O on the slag viscosity is presented in Figure 5 and compared with the results of Kim et al.[6] It can be found that the viscosity of the slag tends to increase with the addition of K2O, which is consistent with the results of previous studies.[4,6,7] According to the study by Kim et al.,[6] the O2− ion provided by K2O has two main roles, namely, destroying the silicate network structure or forming [AlO4]5−-tetrahedron with Al3+ ion. Similarly, the work done by Higo et al.[32] also reported that the fraction of NBO (non-bridging oxygen) increased monotonically with an increase in the K2O content, though slag viscosity increased. Based on the previous literature,[6,33,34] it can be speculated that some silicate structures change from a three-dimensional network to discrete anionic groups containing simple chains and/or rings because of the influence of basic oxides (such as CaO and MgO) in high-temperature aluminosilicate melt. Hence, the roles of K2O in the slag can be expressed by Eqs. [13] through [16] on the basis of previous researches.[35,36,37] Further, K+ ions are most preferentially compensated with [AlO4]5−-tetrahedrons to maintain electro-neutrality according to Zhang et al.[23] Therefore, K2O usually tends to promote the formation of [AlO4]5−-tetrahedron due to the strong compensation ability of K+ ion, which leads to an increase in slag viscosity.
In addition, it may be noted that the slag viscosity decreases with the initial increase of K2O content, which corresponds to the results of Kim and colleagues as shown in Figure 5.[6] Combined with the study by Higo et al.,[32] it may be deduced that as the K2O content increases to 1 mass pct, the increase in the average bond strength of the formation of aluminosilicate network due to the compensation effect of K+ was less than the decrease in the average bond strength owing to the formation of NBOs connected with Ca2+ and Mg2+ ions. As a result, the effect of increasing free oxygen ions (O2−) on the depolymerization of the network structure may be more prominent at this time. In contrast, when the K2O content exceeds 1 mass pct, the contribution of K2O additives in the formation of the [AlO4]5−-tetrahedron is dominant, and thus the overall average bond strength increase. However, the inference should also be approached with caution due to lack of solid evidence, and it will be essential to perform further investigations on the structural analysis of the slag.
Figure 6 shows the effect of K2O on the heat capacity and enthalpy change of the slag. One can see that the heat capacity of the slag generally increases with the increasing addition of K2O. Unlike Na2O, the higher slag temperature results in the greater heat capacity when the K2O additions are constant. But from the absolute value of heat capacity, K2O has much less influence on the heat capacity of the slag compared to Na2O. Furthermore, the enthalpy change of the slag, as shown in Figure 6(b), decreases almost linearly with increasing K2O content at a certain temperature. As a consequence, heat quantity supply needed to maintain the high slag temperature can be decreased with the increase of K2O additions. On the other hand, the heat quantity carried away by the slag discharge is also reduced. In this perspective, the increase of K2O content, as well as Na2O, may be beneficial to reduce the heat quantity consumption of the blast furnace in particular when the slag temperature needs to be kept stable.
When the slag heat quantities are constant, the effect of K2O on the slag temperature and viscosity is displayed in Figure 7. It can be seen that when the supply of heat quantities is fixed, the slag temperature increases linearly with the increase of K2O content, whereas the viscosity decreases appreciably. Compared to the effect of K2O additions on the increase of degree of slag polymerization, the increase of the slag temperature is more effective in improving the fluidity, thus accounting for the decrease in viscosity. Meanwhile, it can also be seen that the fluctuations of slag viscosity at different heat quantities tends to decrease as the K2O content increases. It appears that the K2O additions in the slag is favorable to weaken the viscosity change caused by the heat fluctuations, and thereby improves the stability of the slag. Regarding the mass transfer, heat transfer, and chemical reactions between the slag and hot metal, the corresponding kinetic and thermodynamic conditions will also be improved due to the decrease of viscosity and the striking increase in slag temperature, respectively. Thus, the increasing additive content of K2O or Na2O helps facilitate the mass transfer, heat transfer, and chemical reactions between the slag and hot metal.
Conclusions
In the present study, the heat capacity, enthalpy change, and slag temperature of the slag system were calculated. Meanwhile, the viscosities of CaO–SiO2–MgO–Al2O3 slags under constant temperatures or heat quantities were measured after adding Na2O or K2O. From the experimental and calculating results, it can be concluded that the slag viscosity decreases with the increase of Na2O content whereas tends to increase with K2O additions at the same temperature. However, further detailed investigations are required to verify the structural changes of the slag. With the increasing addition of Na2O or K2O, the heat capacity of the slag increases progressively, while the enthalpy change significantly decreases, thereby helping to reduce the heat taken away by the slag discharge. When the heat quantity of the slag keeps constant, the slag temperature increases remarkably with an increase in Na2O or K2O content, and the corresponding viscosity decreases evidently. The Na2O or K2O additions are not only conducive to stabilize the fluidity of the slag and reduce the energy consumption of blast furnace, but also promote the mass transfer, heat transfer, and chemical reactions between the slag and hot metal.
References
H. Kim, W.H. Kim, I. Sohn, and D.J. Min: Steel Res. Int, 2010, vol. 81, pp. 261–64.
I. Sohn and D.J. Min: Steel Res. Int., 2012, vol. 83, pp. 611–30.
H. Kim, H. Matsuura, F. Tsukihashi, W.L. Wang, D.J. Min, and I. Sohn: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 5–12.
S. Sukenaga, N. Saito, K. Kawakami, and K. Nakashima: ISIJ Int., 2006, vol. 46, pp. 352–58.
H. Kim, W.H. Kim, J.H. Park, and D.J. Min: Steel Res. Int, 2010, vol. 81, pp. 17–24.
W.H. Kim, I. Sohn, and D.J. Min: Steel Res. Int, 2010, vol. 81, pp. 735–41.
G.H. Zhang and K.C. Chou: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 841–48.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R.B. Mahfoud, J. Melancon, A.D. Pelton, and S. Petersen: CALPHAD, 2002, vol. 26, pp. 189–228.
M.Y. Kou, S.L. Wu, X.D. Ma, L.X. Wang, M. Chen, Q.W. Cai, and B.J. Zhao: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 1093–1102.
A. Kondratiev and E. Jak: Metall. Mater. Trans. B, 2005, vol. 36B, pp. 623–38.
M. Suzuki and E. Jak: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 1435–50.
M. Suzuki and E. Jak: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 1451–65.
Barin, O. Knacke, and O. Kubaschewski: Thermochemical Properties of Inorganic Substances, Springer-Verlag, Berlin, 1977.
I Barin: Thermochemical Data of Pure Substances, VCH, Weinheim, Germany, 1989.
R.C. Weast: Handbook of Chemistry and Physics, 55th ed., CRC Press, Cleveland, OH, 1974–1975, pp. D58–59.
JANAF Thermochemical Tables, 3rd ed., J. Phys. Chem. Ref. Data, 1985, vol. 14, suppl. 1.
G. Eriksson, A.D. Pelton: Metall. Mater. Trans. B, 1993, vol. 24B, pp. 795–805.
K.D. Kim: J. Am. Ceram. Soc., 1996, vol. 79, pp. 2422–28.
Z.Y. Chang, K.X. Jiao, J.L. Zhang, X.J. Ning, and Z.Q. Liu: ISIJ Int., 2018, vol. 58, pp. 2173–79.
R.Z. Xu, J.L. Zhang, W.X. Han, Z.Y. Chang, and K.X. Jiao: Ironmak Steelmak., 2018, in press.
B. Mysen: Contrib Mineral Petrol, 1997, vol. 127, 104–118.
Y. Sasaki and K. Ishii: Tetsu-to-Hagane´, 2002, vol. 88, pp. 419–29.
G.H. Zhang, K.C. Chou, and K. Mills: Metall. Mater. Trans. B, 2014, vol. 45B, pp. 698–706.
A. Navrotsky, G. Peraudeau, P. McMillan, and J.P. Coutures: Geochim. Cosmochim. Acta, 1982, vol. 46, pp. 2039–47.
F. Domine and B. Piriou: Am. Mineral., 1986, vol. 71, pp. 38–53.
G.H. Zhang, W.W. Zheng, and K.C. Chou: Metall. Mater. Trans. B, 2017, vol. 48B, pp.1134–38.
Y.L. Zhen, G.H. Zhang, X.L. Tang, and K.C. Chou: Metall. Mater. Trans. B, 2014, vol. 45B, pp.123–130.
G.H. Zhang and K.C. Chou: J. Min. Metall. B., 2012, vol. 48, pp. 1–10.
K.J. Li, R. Khanna, M. Bouhadja, J.L. Zhang, Z. J. Liu, B.X. Su, T.J. Yang, V. Sahajwalla, C.V. Singh, and M. Barati: Chem. Eng. J, 2017, vol. 313, pp. 1184–93.
J.B. Kim and I. Sohn: ISIJ Int., 2014, vol. 54, pp. 657–63.
D.R. Rohindra, R.A. Lata, and R.K. Coll: Eur. J. Phys., 2012, vol.33, pp. 1457–64.
T. Higo, S. Sukenaga, K. Kanehashi, H. Shibata, T. Osugi, N. Saito, and K. Nakashima: ISIJ Int., 2014, vol. 54, pp. 2039–44.
J.O.M. Bockris and D.C. Lowe: Proc. R. Soc. Lond., 226A, 1954, pp. 423–35.
L. Zhang and S. Jahanshahi: Metall. Mater. Trans. B, 1998, vol. 29B, pp.177–86.
N. Sano: Advanced Physical Chemistry for Process Metallurgy, Academic Press, New York, NY, 1997, pp. 45–48.
Y. Waseda and J.M. Toguri: The Structure and Properties of Oxide Melts, World Scientific, Singapore, 1998.
J.H. Park, D.J. Min, H.S. Song: ISIJ Int., 2002, vol. 42, pp.344–51.
Acknowledgments
The authors appreciate the financial support from the National Science Foundation for Young Scientists of China (51704019), and thank the anonymous reviewers for valuable comments helping us improve the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted December 13, 2018.
Rights and permissions
About this article
Cite this article
Chang, ZY., Jiao, KX., Ning, XJ. et al. Novel Approach to Studying Influences of Na2O and K2O Additions on Viscosity and Thermodynamic Properties of BF Slags. Metall Mater Trans B 50, 1399–1406 (2019). https://doi.org/10.1007/s11663-019-01565-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01565-5