Abstract
In order to improve the efficiency of slag and iron separation, a new idea of “the separation of slag (solid state) and iron (molten state) in rotary hearth furnace process at lower temperature” is put forward. In this paper, the forming process of iron nuggets has been investigated. Based on those results, the forming mechanisms and influencing factors of iron nugget at low temperature are discussed experimentally using an electric resistance furnace simulating a rotary hearth furnace process. Results show that the reduction of iron ore, carburization of reduced iron, and the composition and quantity of slag are very important for producing iron nuggets at lower temperature. Reduction reaction of carbon-containing pellets is mainly at 1273 K and 1473 K (1000 °C and 1200 °C). When the temperature is above 1473 K (1200 °C), the metallization rate of carbon-containing pellets exceeds 93 pct, and the reduction reaction is substantially complete. Direct carburization is the main method for carburization of reduced iron. This reaction occurs above 1273 K (1000 °C), with carburization degree increasing greatly at 1473 K and 1573 K (1200 °C and 1300 °C) after particular holding times. Besides, to achieve the “slag (solid state) and iron (molten state) separation,” the melting point of the slag phase should be increased. Slag (solid state) and iron (molten state) separation can be achieved below 1573 K (1300 °C), and when the holding time is 20 minutes, C/O is 0.7, basicity is less than 0.5 and a Na2CO3 level of 3 pct, the recovery rate of iron can reach 90 pct, with a proportion of iron nuggets more than 3.15 mm of nearly 90 pct. This study can provide theoretical and technical basis for iron nugget production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ironmaking process of direct reduction and smelting reduction using non-coking coal as a fuel and reductant is widespread around the world. A variety of methods are emerging.[1–3] Rotary hearth furnaces (RHF), direct reduction processes using carbon-containing pellet feeds, with its improved environmental footprint, extensive raw material and, flexible operating performance, are developing rapidly around the world.[4–6] In addition, the rotary hearth furnace iron nuggets process (ITmk3) is preferred by the domestic and international metallurgical workers due to the advantages of the final product.[7,8]
In the ITmk3 process, iron ore powder, pulverized coal, and binders are mixed in the required proportions and subsequently briquetted, and the briquettes are reduced at 1623 K and 1723 K (1350 °C and 1450 °C).[9–12] Under these conditions, both the iron and slag phases are liquid, and separation occurs with sufficient holding time at temperature. Finally, iron nuggets with a similar composition to pig iron, fewer impurities (such as S), and without gangue would be produced.[13–15] However, separation of metallic iron was conducted by melting both the metal and slag phases, and the high separation temperature is a disadvantage. In addition, the surface tension of melting slag is low, and it would cover the iron nuggets, hindering from coalescing and growing into much larger particles. Therefore, there are difficulties in the separation of small size iron nuggets and slag.[16–20] In recent years, in order to reduce the separation temperature, reducing carbon ratio to generate low melting point compound from the reaction of FeO and slag can achieve the separation of melting iron and slag, but people encountered the same difficulties in the separation of iron nuggets and slag.[21,22]
Separation of iron and slag is closely related to iron phase structures (reduction level, degree of carburization) and slag phase structure. In theory, whether the slag is in a solid state or molten state, the separation of iron and slag can be realized when the metallic iron is in a molten state. When the temperature is above 1424 K (1151 °C), metallic iron can transform from solid state to molten state by carburization. As a consequence, a new idea of “separation of slag (solid state) and iron (molten state) at low temperature during the formation process of iron nuggets” is put forward. Under the guidance of this new approach, the iron nugget formation process is discussed, based on which the mechanism and influencing factors of iron nuggets forming in RHF at a relatively low temperature are investigated, providing the theoretical and technical basis for this new method of producing iron nuggets in an RHF.
Materials and Methods
Materials
Main materials included in this study are iron ore and graphite. The main chemical composition of the materials is shown in Tables I and II. The iron ore is hematite from Australia with high iron grade and high loss-on-ignition (LOI). The SiO2 and Al2O3 contents are also relatively high. The fixed carbon content of the graphite is 99.50 pct and the ash component is below 0.05 pct. The CaO and Na2CO3 used in the experiments were of reagent grade.
Methods
The experimental process is shown in Figure 1. The experiments were conducted by a high-temperature electric resistance furnace which is shown in Figure 2. The iron ore and graphite were first ground separately to less than 200 mesh and then put into an electric drying oven, where they were dried for 12 hours at 378 K (105 °C) to remove the water contained in the materials. After drying, the iron ore and graphite were mixed with water and binder in the required proportions (C/O) and subsequently extruded into a cylindrical sample with a diameter of 60 mm and a height of 15 mm. After that, the extruded samples were placed in the resistance furnace and heated to the designated temperature in a pure nitrogen atmosphere. The samples were heated to the designated temperature in 60 minutes, and held at that temperature for the required time. Then the samples were cooled to room temperature in the furnace.
After cooling, the percentage of iron nugget at different sizes was determined by magnetic separation and screening. Then the samples were divided into two portions. One portion was crushed and milled to below 200 mesh for chemical analyses, and the other portion of reduced samples was used for microstructure analysis. The chemical composition analyses were conducted by the China University of Geosciences (Beijing) analysis laboratory, and the microstructure of the samples was observed with a scanning electron microscope and X-ray diffraction.
In this study, the C/O, metallization rate, and iron recovery rate were defined as follows:
where M c refers to the carbon level in the carbon-containing pellets, and M o refers to the oxygen level in iron oxides.
where η 1 refers to the metallization rate (pct), M·Fe refers to the metallic iron content in reduced samples (pct), and T·Fe refers to the total iron content in reduced samples (pct),respectively.
where η 2 refers to the iron recovery rate (pct), M 1 refers to the mass of iron nugget samples, M 2 refers to the mass of the raw materials, T·Fe n refers to the total iron content of iron nugget samples (pct), and T·Fe s refers to the total iron content of the raw materials (pct), respectively.
Results and Discussion
Discussion of the Iron Nuggets Forming Process
The formation process of iron nuggets and the morphology of carbon-containing pellets at different temperatures are shown in Figures 3 and 4.
The morphology of carbon-containing pellets at different temperatures and holding time. The upper row: from left to right, the temperatures are 1373 K, 1473 K, 1573 K, and 1623 K (1100 °C, 1200 °C, 1300 °C, and 1350 °C). The lower row: from left to right, the temperatures are 1573 K (1300 °C) holding time 5, 20, and 60 min
As shown in Figures 3 and 4, in the interior of carbon-containing pellets, carbon granules and iron oxide particles are evenly distributed, and are in close contact with each other. Direct reduction, namely solid-solid contact reaction between carbon particles and iron oxide particles, occurs at the beginning.[8,9] Since the CO produced through the direct reduction reaction can react with iron oxide, the indirect reduction reaction takes place, which is accompanied by the carbon solution loss reaction.[14,15]
When the temperature is above 1273 K (1000 °C), the reaction rate is higher and iron oxides become fully reduced. At this time, small metallic iron particles begin to appear inside the pellets. Less reduction occurring at these higher temperatures means less CO2 is produced along with the amount of endothermic reaction.[11] So the heating rate of the pellets is accelerated, the metal phase begins to soften, and diffusion and the carburization rate are accelerated.[23] When the temperature is about 1473 K and 1573 K (1200 °C and 1300 °C), the carburization rate of reduced iron increases greatly, the melting point of reduced iron decreases, and iron coexists as solid and liquid.[24] Some network pores appear inside of the pellets due to the consumption of carbon, which will improve the condensation of iron phase and form some larger iron particles; finally, high quality of iron nuggets is formed.
Analysis of Iron Nuggets Forming Mechanism
There are three stages from carbon-containing pellets to iron nuggets—reduction, carburization, and separation of slag and iron. Therefore, the formation mechanism of iron nuggets should be discussed from these three parts.
Reduction process
The reduction of iron oxide to metallic iron is a step-by-step process, including direct reduction and indirect reduction. In theory, metallic iron is generated at 993 K (720 °C); however, due to limitations in dynamic conditions, the reaction cannot be completed.[15] To clarify the reduction situation at different temperatures, the reduction rate and metallization rate of roasted pellets were measured, with the results as shown in Table III. When the temperature is below 973 K (700 °C), the iron oxide reduction rate is under 1.05 pct. When temperature rises to 1173 K (900 °C), the reduction rate is only 30 pct. It appears that the reaction of iron oxide below 1273 K (1000 °C) is mainly “Fe2O3 → Fe3O4 → FeO”, and the reaction “FeO → Fe” occurs primarily above 1273 K (1000 °C).[20] On increasing temperature from 1273 K to 1373 K (1000 °C to 1100 °C), the reaction rate is relatively high, the metallization rate has increased from 28 to 87 pct, but the reduction becomes slower when the temperature is above 1373 K (1100 °C). When the temperature reaches 1473 K (1200 °C) and above, metallization rate of sample is over 93 pct and the reduction reaction is almost completed.
From the result of reduction rate, metallization rate, XRD, and SEM (Figures 5 and 6), large amount of iron oxides exists at 1273 K (1000 °C), and there is a little metal iron in form of bars. With the temperature increasing, reduction constantly increases and iron oxide phases almost disappear while the metallic iron phase increases. The pores gradually become small, and reduction nearly ceases. At the same time, iron nuggets do not form because the formation of iron nuggets requires the following steps: carburization, iron melting, and separation of iron and slag.
In a word, reduction of iron oxides almost does not occur below 973 K (700 °C). The process “Fe2O3 → Fe3O4 → FeO” occurs under 1273 K (1000 °C), the process “FeO → Fe” mainly happens at 1273 K and 1373 K (1000 °C and 1100 °C), and the whole process is finished above 1473 K (1200 °C) while the metallization rate is above 93 pct. That is to say, the reduction rate of the pellets is slow at lower temperatures, whereas it is relatively fast at higher temperatures. Therefore, in actual production, in order to improve the reaction rate of the pellets, the furnace temperature should be raised above 1273 K (1000 °C) rapidly.
Carburizing process
Carburizing process is the key step of the formation of iron nuggets. With the progress of carburizing reaction, the melting point of metallic iron is reduced, which will speed up diffusion and aggregation, so it is a prerequisite for the formation of large and uniform iron nuggets. Carburizing process of reduced iron is mainly through the following reactions: gasification carburizing, direct carburizing, and slag carburizing as shown in Figure 7.
There are three different sources of carbon in metallic iron due to the mentioned three carburizing reactions:[23,25,26]
-
(1)
Surface contamination: At 673 K and 873 K (400 °C and 600 °C), the carbon formed from CO pyrolysis has a good absorbability on the surface, the reaction equation is as follows:
$$ 2{\text{CO}} = {\text{CO}}_{ 2} + {\text{C,}} $$(4)$$ 3{\text{Fe}} + {\text{C}} = {\text{Fe}}_{ 3} {\text{C}} . $$(5) -
(2)
Dissolution carburizing: At 1000 K and 1426 K(727 °C and 1153 °C), carbon dissolves into DRI through direct carburizing Eq. [6] and gasification carburizing Eq. [7]
$$ {\text{C}}_{{\left( {\text{s}} \right)}} = \left[ {\text{C}} \right], $$(6)$$ 2{\text{CO}} = {\text{CO}}_{ 2} + \left[ {\text{C}} \right]. $$(7) -
(3)
Cementation: Cementite produced from the reaction between DRI particles and carbon.
$$ 3{\text{Fe}}_{\alpha } + {\text{C}} = {\text{Fe}}_{ 3} {\text{C,}} $$(8)$$ 3{\text{Fe}}_{\gamma } + {\text{C}} = {\text{Fe}}_{ 3} {\text{C,}} $$(9)$$ 3{\text{Fe}} + 2{\text{CO}} = {\text{Fe}}_{ 3} {\text{C}} + {\text{CO}}_{ 2} . $$(10)
Carburization of the reduced iron in iron nuggets process mainly depends on dissolution carburizing and cementation.[27] When the reduced iron exists in the form of ferrite, the starting temperature of the carburizing reaction Eq. [8] is 1035 K (762 °C); in this case, dissolved carbon is very small, up to a maximum of 0.002 pct. As the temperature rises, ferrite converts to austenite and the carbon dissolving capacity is greatly improved. So most of the carburization reactions in the iron nuggets process occur between 1273 K and 1573 K (1000 °C and 1300 °C) through direct carburization.[27] In order to further verify the carburizing process, carburization of the pellets under different temperatures was measured in this study; test results are shown in Figure 8 and Table IV.
As shown in phase diagram,[5] the carburization rate of reduced iron is only 0.03 pct at 1273 K (1000 °C). As temperature increases, carburization rate increases rapidly. When the temperature is 1473 K (1200 °C), carburization rate is 1.09 pct; and when the temperature reaches 1573 K (1300 °C), the carburization rate has reached 2.32 pct. In addition, constant holding time will promote the carburizing reaction. When the temperature is 1473 K (1200 °C) and holding time is 20 minutes, the carburization rate reaches 2.46 pct. While the carburization rate reaches 3.92 pct at 1573 K (1300 °C) with holding time 20 minutes, the reduced iron is in a liquid state.
Meanwhile, SEM analysis of samples roasted at different temperatures is shown in Figure 9. The carburizing of reduced iron is investigated through Figures 6 and 9. At 1273 K (1000 °C), the carburizing reaction happens, but it is limited because of the lower temperatures and lower amount of reduced iron. As the temperature increases, iron oxides are gradually reduced and metallic iron content increases, which increase the contact area between metallic iron and carbon.[28] When the temperature rises to 1573 K (1300 °C), the carburizing reaction is enhanced, the iron phase melts together, and the pores in pellets become smaller.[29]
In short, the carburizing of carbon-containing pellet mainly depends on direct carburizing. Under the experimental conditions, the carburizing reaction mainly occurs above 1273 K (1000 °C), while the carburizing reaction will be enhanced at 1473 K and 1573 K (1200 °C and 1300 °C) and a certain holding time. Therefore, in order to improve the carburization rates of the carbon-containing pellets, a certain holding time is necessary when the furnace temperature is above 1473 K (1200 °C).
Separation of iron and slag
The separation of iron and slag is the key to formation of iron nuggets. Under the circumstance of fast reduction at low temperatures, adjusting the structure of slag, increasing the melting point of slag, and controlling the amount of slag will be helpful to the separation of slag (solid state) and iron (molten state).
The growth process of reduced iron is shown in Figure 10, as temperature increases and metallic iron grains integrate into iron particles. Larger iron particles will improve the degree of dissociation between iron and slag, which leads to an increase of interfacial tension of iron and slag and finally achieves the separation of iron and slag.[30]
Under higher temperature and longer holding time, the growth rate of metallic iron is higher and easily forms big particles, which makes the separation of iron and slag easier. However, the temperature cannot be too high—too high temperature makes the iron and slag in the pellets all liquid, so the separation of iron and slag will be limited by slag coating on iron (surface tensions of slag is 0.2 to 0.4 N/m, and surface tension of iron is 1.6 to 1.8 N/m).[31] Therefore, to achieve the separation of slag (solid state) and iron (molten state) effective, improving the slag melting point above the roasting temperature by adjusting the slag components is important and the quantity of slag should be as little as possible. In addition, in order to avoid the formation of low melting point compounds containing FeO, the reduction of carbon-containing pellets must be completed at relatively low temperatures, so the reduction is also the basis of separation.
The results of separation of iron and slag at 1573 K (1300 °C) under different holding time are shown in Table V.
Under the condition of 1573 K (1300 °C) and holding time 5 minutes, iron nuggets will be generated, but recovery rate of iron is relatively low, and iron nuggets particle size is relatively small. As holding time increases, recovery rate of iron increases, as does the iron nuggets particle size. According to the experimental results, the suitable holding time is 20 minutes; under this condition, the recovery rate of iron is about 90 pct and the proportion of iron nuggets more than 3.15 mm is about 75 pct.
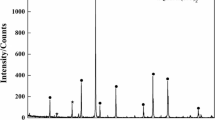
The XRD results of slag after the separation of iron and slag are shown in Figure 11.
As shown in Figure 11, except for the large amount of carbon and small amount of Fe, the contents of SiO2 and Al2O3 are relatively high, and there is a certain amount of Al2O3·SiO2. Considering the slag system diagram of CaO-SiO2-Al2O3, under the roasting temperature, slag phase is in solid state, which contributes to the separation of slag (solid) and iron (molten). In actual production, if the melting point of slag of carbon-containing pellets is low, adding some CaO will increase the melting point of slag.[32]
In a word, the separation of slag (solid state) and iron (molten state) can be achieved at 1573 K (1300 °C). But the reduction of carbon-containing pellets completed at low temperatures, carburizing with a certain holding time, little quantity of slag, and higher melting point of slag are necessary. In actual production, the appropriate holding time is 20 minutes, the iron yield is about 90 pct, and the proportion of iron nuggets more than 3.15 mm is about 75 pct.
Influencing Factors of Iron Nuggets Forming
Temperature and holding time are important influencing factors for iron nuggets formation, but besides temperature and holding time, these are many factors (such as C/O, roasting atmosphere, basicity, etc.) that also effect iron nuggets formation. In this study, the influencing factors of iron nuggets formation are discussed.
Effect of carbon ratio
The experimental results at different carbon contents are shown in Table VI. The experimental temperature is 1573 K (1300 °C) and the holding time is 60 minutes.
Iron recovery rate and the iron nuggets proportion of large particle size increase at first and then decrease with rising in carbon content. When the C/O is 0.5, the separation of slag and iron cannot be achieved; when the C/O is 0.7, iron recovery rate is 90.29 pct and the proportion of iron nuggets more than 3.15 mm is 81.21 pct; if the C/O increases to 1.2, iron recovery rate is 54.91 pct and iron nuggets more than 3.15 mm do not appear. As the carbon content increases, the Boudouard reaction proceeds at a higher rate to provide the reductant CO. It increases the ratio of CO/CO2 and accelerates the reduction process. But when the carbon content is excessive, there is a surplus of carbon after consumption as reducing agent, which inhibited coalescence of iron phase and reduced the size distribution of the iron nuggets. In short, to achieve a better separation of slag and iron, C/O about 0.7 is more appropriate.
Effect of roasting atmosphere
The experiment at different roasting atmospheres (in a pure nitrogen atmosphere and air atmosphere) is investigated when the C/O is 0.7, and the holding time is 60 minutes. The experimental results at different roasting atmosphere are shown in Table VII.
It can be seen from the results, in a pure nitrogen atmosphere, iron recovery rate is 90.29 pct, and the proportion of iron nuggets more than 3.15 mm is 81.21 pct. But in the air atmosphere, the pellet is still a clump and it is difficult to form iron nugget at 1573 K (1300 °C). When the temperature is 1673 K (1400 °C) in the air atmosphere, iron nugget can be formed. The iron recovery rate is 85.51 pct, and the proportion of iron nuggets larger than 3.15 mm is more than 87 pct. This is because in the conditions of air atmosphere, the reduced metal at the surface of pellets is easily oxidized, and an oxide layer can be formed, which is not conducive to reduction, carburization, and slag and iron separation of carbon composite pellets, so it is necessary to increase temperature to achieve separation of slag and iron. In summary, if the conditions permit, the protective atmosphere is more appropriate.
Effect of basicity
Different basicities (natural basicity, 0.2, 0.5, 0.8, 1.0, 1.5, and 1.8) were investigated for a temperature of 1573 K (1300 °C), C/O of 0.7, and the holding time of 20 minutes. The experimental results at different basicities are shown in Table VIII. In this paper, the natural basicity is the ratio of CaO content and SiO2 content, and the natural basicity is 0.017.
Not only the amount of iron reduces gradually with increasing basicity, but also the particle size of iron nuggets is getting smaller and smaller. When basicity is less than 0.5, the iron recovery rate is higher, and the size composition of iron nuggets is good relatively; but when the basicity exceeds 0.5, the iron recovery rate is small, and the iron nuggets proportion of large particle size is fewer. When basicity is natural basicity, the iron recovery rate is about 90 pct, and the proportion of iron nuggets more than 3.15 mm is 73.11 pct; when basicity is 0.5, the iron recovery rate is 88.40 pct and the proportion of iron nuggets more than 3.15 mm decreases to 53.25 pct. However, when basicity is 1.8, the iron recovery rate only is 36.95 pct, and the proportion of iron nuggets more than 3.15 mm only is 13.41 pct. In the case that no low-melting compound is produced in the slag phase which is thus not melted, the distance between reducing agent and iron ore fines increases with basicity. Since a large distance is less conducive to the reduction, carburizing, gathering and growth up of iron, there is little iron nuggets generated and the particle size is small. In short, under the experimental raw material conditions, basicity less than 0.5 is more suitable.
Effect of NaCO3 addition
Different NaCO3 additions (0, 1.5, 2.5, 3.0, 4.0, 5.0, 6.0, and 7.0 pct) were investigated for a temperature of 1573 K (1300 °C), C/O of 0.7, and the holding time of 20 minutes. The experimental results at different NaCO3 additions are shown in Table IX. In this paper, the NaCO3 addition is the percentage of the total feed mix.
As shown in Table IX, increasing the ratio of Na2CO3 from 0 to 3 pct causes an increment in the iron recovery rate and a significant increase in iron nuggets proportion of large particle size. The iron recovery rate increases about 1 pct, and the proportion of iron nuggets more than 3.15 mm increases from 73.11 to 89.64 pct. When the Na2CO3 addition is 5 pct, the iron recovery rate decreases to 89.92 pct, but the proportion of iron nuggets more than 3.15 mm increases to 91.36 pct. With a further increment in Na2CO3, the iron recovery rate and iron nuggets proportion of large particle size tend to decrease. This is a result of Na2O formation through dissociation of Na2CO3. Sodium oxide can participate in a reaction with SiO2 and fayalite (2FeO·SiO2). So the formation of fayalite can be suppressed and the FeO in the fayalite can be replaced by Na2CO3.[33] This increases the reduction activity of FeO and reinforces the reduction of iron oxide. However, excess Na2CO3 does not help the reduction of iron oxide because Na2O reacts with slag phase to form low melting point compounds.[34] The abundant liquid phase will hinder the internal diffusion of reducing gas. It can therefore be concluded that an appropriate level of Na2CO3 can reinforce the reduction of iron ore and improve the separation efficiency of slag and iron. The recommended Na2CO3 dosage is a mass ratio of 3 pct.
Comparison with Other Iron Nugget Production Methods
The conditions of typical iron nugget production method,[35–37] the method suggested in this paper, and other low-temperature method[21,22] are shown in Table X.
The typical iron nugget production method and other low-temperature method were conducted by melting both the iron and slag phases, while the slag is still in solid state in the method put forward in this paper. The conditions achieved in this study for iron nugget production are identified as a reduction temperature of below 1573 K (1300 °C), a holding time of 20 minutes, a C/O mass ratio of 0.7, a basicity of less than 0.5, and a NaCO3 addition of 3 pct. However, for the typical method, the reduction temperatures are 1623 K and 1723 K (1350 °C and 1450 °C), the holding time is 15 to 30 minutes, the C/O is 0.8 to 1.2, and the basicity is about 1.0. So compared with the typical method, the method put forward in this paper has some advantages in reduction temperature, C/O, and basicity. Besides, for the other low-temperature method, the reduction temperatures are 1573 K and 1593 K (1300 °C and 1320 °C), the holding time is 40 to 60 minutes, and the C/O is 0.55 to 0.65. The method put forward in this paper is also advantageous in reduction temperature and holding time.
The product quality of these different iron nugget production methods is shown in Table XI. The method put forward in this paper was conducted by reduction temperature of 1573 K (1300 °C), holding time of 20 minutes, C/O mass ratio of 0.7, natural basicity, and NaCO3 addition of 3 pct. The results of the typical method were the experimental results simulated in the laboratory at the reduction temperature of 1673 K (1400 °C), the holding time of 20 minutes, the C/O of 1.0, and the basicity of 1.0. And the results of other low-temperature method were the result in the References 21 and 22.
As shown in Table XI, the method put forward in this paper can produce high quality product. Compared with the other low-temperature method, the method put forward in this paper possesses higher TFe contents and iron recovery rate. Besides the iron recovery rate, the proportion of iron nuggets of size higher than 3.15 mm and higher than 1 mm of the method in this paper is also higher than that of the typical methods.
In short, compared with other methods, the method put forward in this paper has some advantages both in conditions and product quality.
Conclusions
This study presents a new idea of “the separation of slag (solid state) and iron (molten state) in rotary hearth furnace process at lower temperature” to improve separation efficiency of slag and iron during the formation of iron nuggets. Through the simulation of RHF, the formation process of iron nuggets is discussed, based on which the mechanism and influence factors of iron nuggets formation in RHF at low temperature are investigated. Conclusions are summarized as follows:
-
1.
The process of carbon-containing pellets transforming into iron nuggets is mainly through reduction, carburization, and separation of slag and iron. The reduction is the foundation; carburizing and separation of iron and slag are also critical steps. When the carbon-containing pellets have been fully reduced, the carburizing process will be improved to a large extent, and the melting temperature of iron reduced. On the other hand, through full reduction, the content of FeO in slag is small, and the melting point of slag is relatively high, resulting in the separation of iron (molten state) and slag (solid state).
-
2.
Little reduction of iron oxides occurs under 973 K (700 °C). The reactions Fe2O3 → Fe3O4 → FeO occur under 1273 K (1000 °C), and the process FeO → Fe is mainly occured at 1273 K and 1373 K (1000 °C and 1100 °C). When the temperature is above 1473 K (1200 °C), the metallization rate of carbon-containing pellet is over 93 pct and the reduction reaction is almost completed. However, the formation of iron nuggets also needs the process of carburizing, iron melting, and separation of iron and slag.
-
3.
In the formation process of iron nuggets, direct carburizing is the main method of carburizing, and the carburizing reaction mainly occurs above 1273 K (1000 °C), while the carburizing reaction will be enhanced at 1473 K and 15783 K (1200 °C and 1300 °C) and a certain holding time. Under the condition of 1573 K (1300 °C) and holding time 20 minutes, the carburization rate can reach 3.92 pct, above the melting point of the metallic iron-carbon mixture.
-
4.
In order to achieve the separation of slag (solid state) and iron (molten state), the reduction of carbon-containing pellets completed at low temperatures, carburizing with a certain holding time, little quantity of slag, and higher melting point of slag are necessary.
-
5.
The recommended conditions for iron nugget process are identified as a reduction temperature of 1573 K (1300 °C), a holding time of 20 minutes, a C/O mass ratio of 0.7, a basicity of less than 0.5, and a Na2CO3 mass ratio of 3 pct. Under this condition, the iron recovery rate is over 90 pct and the proportion of iron nuggets more than 3.15 mm is about 90 pct.
References
R. Seffen: Revue de Metallurgie, 2009, vol. 5, p. 171.
W.K. Lu, X. Jiang, and J.L. Yang: The 5th International Congress on the Science and Technology of Ironmaking, 2009.
H. Mitsutaka, K. Isao, and K. Shoichi: United States Patent, US6592647, 2003.
K. Shoichi, T. Yasuhiro, and T. Koji: United States Patent, US6592649, 2003.
K. Ishizaki, K. Nagata, T. Hayashi. ISIJ Int., 2006, vol. 46, p. 1403.
Z. Qi, T. Murakami and E. Kasai: ISIJ Int., 2012, vol. 52, p. 1778.
S. Sun and W.K. Lu: ISIJ Int., 1999, vol. 39, p. 123.
S. Kang and W.K. Lu: Metall. Mater. Trans. B, 2009, vol. 40, p. 91.
Y. Iguchi and S. Yokomoto: ISIJ Int., 2004, vol. 44, p. 2008.
J. Yang, T. Mori and M. Kuwabara: ISIJ Int., 2007, vol. 47, p. 1394.
K. Nagata and R. Kojima: ISIJ Int., 2001, vol. 41, p. 1316.
Y. Iguchi and S. Endoi: ISIJ Int., 2004, vol. 44, p. 1999.
[13] I. Sohn and R.J. Fruehan: Metallurgical & Materials Trans. B, 2005, vol. 36, p. 605.
S. Halder and R.J. Fruehan: Metallurgical & Materials Trans. B, 2008, vol. 39, p. 784.
S. Halder and R.J. Fruehan: Metallurgical & Materials Trans. B, 2008, vol. 39, p. 796.
H. L. Han, D. P. Duan, X. Wang and S. M. Chen: Metallurgical & Materials Trans. B, 2014, vol. 45, p. 1634.
Y. G. Ding, J. S. Wang, S. Ma, G. Wang and Q. G. Xue: Iron and Steel, 2011, vol. 46, p. 16.
Y.H. Guo, J.J. Gao, H.J. Xu, K. Zhao and X.F. Shi: Journal of Iron and Steel Research, International, 2013, vol. 20, p. 24.
Y.G. Ding, J.S. Wang, G. Wang, S. Ma and Q.G. Xue: Journal of Iron and Steel Research, International, 2012, vol.19, p. 9.
Z.L. Xue, D. Yang, L.G. Zhou and Y.Y. Li: Journal of Wuhan University of Science and Technology, 2009, vol. 32, p. 1.
B.X.Zhu, G.Wei and X.Jiang: Journal of Northeastern University, 2012, vol. 33, p. 247.
B.X.Zhu, G.Wei and X.Jiang: Journal of Northeastern University, 2012, vol. 33, p. 537.
Y. Iguchi and S. Endo: ISIJ Int. 2004, vol. 44, p. 1991.
K. Ohno, T. Miki, Y. Sasaki and M Hino: ISIJ Int., 2008, vol. 48, p. 1368.
K. Ohno, T. Miki and M. Hino: ISIJ Int., 2004, vol. 44, p. 2033.
R. Khanna, F. McCarthy, H. Sun, V. Sahajwalla and N. Simento: Metall. Mater. Trans. B, 2005, vol. 36, p. 719.
H. Kim, D. Min and S. Jung: ISIJ International, 2013, vol. 53, p. 199.
H. Ono, K. Tanizawa and T. Usui: ISIJ International, 2011, vol. 51, p. 1274.
F. McCarthy, V. Sahajwalla, J. Hart and N. S. Chaudhury: Metall. Mater. Trans. B, 2003, vol. 34, p. 573.
K. Ohno, A. Babich, J. Mitsue, T. Maeda, D. Senk, H. W. Gudenau and M. Shimizu: ISIJ Int., 2012, vol. 52, p. 1482.
S.T. Cham, R. Khanna, V. Sahajwalla, R. Sakurovs and D. French: ISIJ Int., 2009, vol. 49, p. 1860.
H. Kim, J. Kim and Y. Sasaki: ISIJ Int., 2010, vol. 50, p. 1099.
H. M. Long, J. X. Li and P. Wang: Ironmaking and Steelmaking, 2012, vol. 39, p. 585.
W. Yu, T. C. Sun, J. Kou, Y. X. Wei, C. Y. Xu and Z. Z. Liu: ISIJ Int., 2013, vol. 53, p. 427.
T. Murakami, T. Nishimura and N. Tsuda: ISIJ Int., 2013, vol. 53, p. 1763.
B.Burak and S. M. Nezihi: Miner. Process. Extr. Metall. Rev., 2013, vol. 34, p. 195.
B. Anameric and S. K. Kawatra: Miner. Metall. Process., 2006, vol. 23, p. 52.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 5, 2014.
Rights and permissions
About this article
Cite this article
Han, H., Duan, D., Chen, S. et al. Mechanism and Influencing Factors of Iron Nuggets Forming in Rotary Hearth Furnace Process at Lower Temperature. Metall Mater Trans B 46, 2208–2217 (2015). https://doi.org/10.1007/s11663-015-0420-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0420-0