Abstract
Cyclic voltammetry, chronopotentiometry, and square wave voltammetry were used to investigate electrochemical behavior of Ti(II) ion in a purified CaCl2 melt at a temperature of 1173 K (900 °C). The result indicated that the cathodic reduction of Ti(II) ion in the melt was a one-step quasi-reversible process controlled by the diffusion. The diffusion coefficient was determined in a CaCl2-TiCl(0.5 mol/dm3) at 1173 K (900 °C). The work also demonstrated the feasibility of producing metallic titanium in the as-prepared CaCl2-TiCl2 melts through galvanostatic electrolysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanium is ranked as the ninth most abundant element and fourth among common metals in the earth’s crust. Primary titanium metal, sponge titanium, is produced through a process invented by Dr. Kroll in the 1940s.[1] The current industrial practice includes multiple steps. The procedure is complex and is a batch process, factors that drive up the production cost substantially. Much research has been focused on developing a technique to produce low-cost and high-purity titanium.[2–8] Electrolysis from TiO2[9–13] and related methods[14–17] have been investigated extensively. In the oxide electrolysis process, TiO2 serves as the cathode and is reduced in a CaCl2 electrolyte. Unfortunately, very long electrolyzing times are required for significant conversion, and current efficiencies are low. Furthermore, production is still restricted to a batch process. Japanese research groups[14–17] proposed a similar method involving a combination of electrolysis and calciothermic reduction. Calcium, electrodeposited on the cathode, is used to reduce the TiO2. However, problems remain associated with the high oxygen content of titania and with difficulties in the extraction of the product titanium from its mixture with TiO2, CaO, Ca, and solvent CaCl2. Zhu etc. proposed USTB process.[18] High-purity titanium was prepared from titanium dioxide through a combination of low-temperature carbothermic reduction and electrolysis. The problem for the process is unstable dissolution of anode. Therefore, we reported a new process in which a dual electrolytic cell had been used to prepare titanium from ores or titanium oxides.[19] The flowchart for the process is shown in Figure 1.
The reaction of the first step can be expressed as follows:
The cathode reaction:
The anode reaction:
The reaction between the chlorine gas and the mixture of TiO2 and carbon powder at the bottom of the electrolytic cell is as follows:
TiCl4 is reduced in situ by the cathodic dissolved calcium:
The results show that ΔG0 of the Reaction [4] and [5] are −712.8 and −419. J/mol at 1123 K (850 °C), respectively. Therefore, the Reaction [4] can be taken place first than Reaction [5]. However, the formed metallic Ti by Reaction [4] can react with TiCl4 according to the Reaction [6].
By summing Reaction [4] and Reaction [6], the Reaction [7] is expressed as follows:
According to Reaction [7], the chloride melts of CaCl2-TiCl x are obtained, and it is used into an electrolyte in the second step. When the average titanium oxidation state in the prepared CaCl2-TiCl x melt is near to 2, the reaction of the second step can be expressed as follows:
The cathode reaction:
The anode reaction:
Based on Reaction [8], metallic titanium is obtained, and the anodic gas can be recycled into the first step.
The main challenge with titanium production from molten salts is the multiple valence states of titanium. All those different oxidation states, and the possible cycling between them, can ruin the current efficiency at the cathode. The kinetics for electrodeposition and electrocrystallization of titanium were studied in alkali chloride melts such as KCl-LiCl, KCl-NaCl, KCl-LiCl-NaCl, KCl-MgCl2-NaCl, and CaCl2-NaCl etc.[20–23] A.M. Martinez et al.[23] demonstrated that the electrochemical systems of Ti(III)/Ti(II) and Ti(II)/Ti in the equimolar mixture CaCl2 + NaCl at 823 K (550 °C) were quasi-reversible, and the Ti(III)/Ti(II) exchange was closer to the reversibility than the titanium electrodeposition process. In comparison of Ti(III) ion, it is easier to produce metal with high quality from Ti(II) ion in a one step in molten salts. This work demonstrates the feasibility of producing metallic titanium in CaCl2-TiCl2 melts. Importantly, the electrochemical behavior of Ti(II) ion in such melts at 1173 K (900 °C) has also been investigated using a series of electrochemical techniques such as cyclic voltammetry, chronopotentiometry, and square wave voltammetry.
Experimental
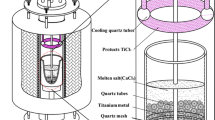
Preparation of CaCl2-TiClx
The calcium chloride with an excess amount of sponge titanium was dried at 623 K (350 °C) in a vacuum for 24 hour. The mixture was then heated up to melt at 1123 K (850 °C) under a high-purity argon (Ar) atmosphere (99.999 pct.). A high-purity hydrogen chloride gas (HCl, 99.999 pct.) was bubbled into the melt for 1 hour to remove oxygen.[23] And then, the high-purity Ar gas was charged into the melt to remove the remanent of HCl and moisture. Subsequently, the titanium tetrachloride vapor was flowed into the purified melt and reacted with sponge titanium to form the soluble TiCl2 under a slight negative pressure. Following reactions can occur after introducing titanium tetrachloride into the CaCl2 molten salt.
Therefore, titanium dichloride and titanium trichloride can exist together in CaCl2-TiCl x melt. The concentration ratio between the subchlorides of titanium is mainly concerned on the equilibrium of disproportionation Reaction [12].[24–27]
The concentrations of Ti2+, Ti3+, and total titanium dissolved in CaCl2-TiCl x melt were determined by H2 volumetric analysis, titration.[28] The total concentration of titanium ions was controlled through changing the amount of titanium tetrachloride. Finally, the prepared salts was cooled to room temperature and transferred into a glove box for keeping, where the content of water and oxygen was maintained under 0.2 ppm.
Electrochemical Test
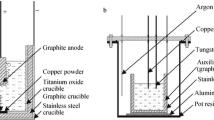
Cyclic voltammetry, square wave voltammetry, chronopotentiometry, and galvanostatic electrolysis were performed using a Princeton Potentiostat/Galvanostat Model 263A controlled with the powersuite software package. The working electrodes are glass carbon (d = 3 mm) rod sealed by a quartz tube in an alumina crucible. In order to maintain the area of working electrode and decrease the error margin of electrochemical experiments, the working electrodes were cleaned by applying an anodic polarization between each measurement.
Spectral pure graphite rod (d = 6 mm) was served as counter electrode. The reference electrode was a titanium rod (d = 3 mm). All electrochemical tests were performed in dry argon atmosphere.
Results and Discussion
The Chemical Analysis for Titanium Ions in CaCl2-TiClx Melt
The average titanium oxidation state determined by the chemical analysis of titanium ions in CaCl2-TiCl x sample is given in Table I.
The corresponding result indicates that the average titanium oxidation state of the prepared CaCl2-TiCl x melt is close to 2. The equilibrium of the disproportionation reaction between the subchlorides of titanium and the metallic titanium is shifted to form the titanium dichloride in the presence of excess amount of metallic titanium in CaCl2-TiCl x melt. The composition of subchloride of titanium in the prepared CaCl2-TiCl x is in agreement with the results of previous works.[26–28]
Cyclic Voltammetry
The cyclic voltammogram obtained on the glass carbon electrode in CaCl2-TiCl2 (0.5 mol/dm3) at 1173 K (900 °C) is shown in Figures 2(a) and (b). The scan rate was 50 mv/s. Figure 2(a) exhibits the one pair of peaks (A’ and A) in the observed scan rate range; the reduction current peak A can observe clearly, which is attributed to the reduction deposit of titanium. During positive scanning, the oxidation current peak A’ exhibits, which is corresponding to the dissolution of the deposited titanium. With extending the potential positively, one small pair of the redox current peaks are also observed (shown in Figure 2(b)). The current peaks B’ and B are likely to be caused by the oxidation of Ti2+ to Ti3+ and the corresponding reduction of Ti3+.
The cyclic voltammograms under the various scan rates are shown in Figure 3. It exhibits that the peak current density is increasing with the increase of the scan rate in the range of 25 to 150 mV/s. The peak separation on the voltammogram in Figure 3 is around 300 mV, which is larger than value for the reversible process ([E pc − E p/2] = 56.5/2). The cathodic peak potential, E pc, shifts negatively, when the scan rate increases. All of these results confirm that the reduction process of Ti2+ is close to quasi-reversible.
The relationship between the peak current density and the square root of the scan rate is shown in Figure 4. The curve has a good linear relationship. The linear relationship observed in Figure 4 demonstrates Eq. [14] for the reduction of titanium in CaCl2-TiCl2 melt, and proves that the electrochemical deposition is controlled by the diffusion of Ti2+. The Eq. [14] exhibits the relationship between the peak current, the diffusion coefficient, and the scan rate.[29]
where \( i_{\text{p}} \) is the peak current, A/cm2; F the Faraday constant, C; R the ideal gas constant, J/mol K; T temperature, K; n the electron exchange number; A electrode area, cm2; D o diffusion coefficient, cm2/s; \( C_{\text{o}}^{*} \) Ti2+ ion concentration, mol/cm3; \( v \) scan rate, V/s.
By using Eq. [14] and the straight line slope in Figure 4, the diffusion coefficient of Ti2+ at 1173 K (900 °C) in CaCl2-TiCl2 melt has been calculated as 1.14 × 10−5cm2/s (±10 pct).
Chronopotentiometry
The chronopotentiograms of the cathodic behavior of Ti2+ in CaCl2-TiCl2 melt under various current densities are shown in Figure 5. Figure 5 exhibits that the reduction potential range of Ti2+ is mainly same as that observed on the cyclic voltammogram; the transition time is decreasing with the increase of the current density.
The Eq. [15] demonstrates the relationship between the transition time and the current density.[29]
where \( i \) is the current density, mA/cm2; \( \tau \) the transition time, s; \( C_{\text{o}}^{*} \) the concentration of Ti2+ ion, mol/cm3; n the electron charged number; A electrode area, cm2; D o diffusion coefficient, cm2/s.
The transition time was determined by measuring the duration of the first part of the chronopotentiogram, applying methodology indicated in Reference 30.
From the Eq. [15], \( i\tau^{\frac{1}{2}} \) is constant, when the concentration of Ti2+ is constant. Figure 6 exhibits the relation of \( i\tau^{\frac{1}{2}} \) and current density; the data plotted agree well with the theories in Reference 31. By using the average value \( i\tau^{\frac{1}{2}} \), the diffusion coefficient was calculated using the Eq. [15] as 1.05 × 10−5cm2/s (±10 pct), which is in agreement with the results from the cyclic voltammetry.
Square Wave Voltammetry
In order to further confirm the above results, the cathodic behavior of Ti2+ in CaCl2-TiCl2 was also studied by the square wave voltammetry. The results observed by the square wave voltammetry under the various frequencies in CaCl2-TiCl2 (0.5 mol/dm3) melt at 1173 K (900 °C) are shown in Figure 7(a). The scan frequency varied from 5 to 25 Hz. Figure 7(a) shows that the square wave voltammograms exhibit the peak current density in the same potential range as that in the cyclic voltammograms, and then, the peak current density is increasing with the increase of the scan frequency; however, the each peak potential is observed in the same position. In square wave voltammetry, scanning potential of working electrode proceeds by staircase with superimposition, on each step of the staircase, of two potential pulses, forward and backward, with the same intensity, plotting the difference currents observed on each step between the successive pulses of the potential associates to each electrochemical reaction peak with Gaussian shape.[32] The result observed by the mathematical analysis of square wave voltammograms is shown in Figure 7(b).
Square wave voltammogram on a glass carbon electrode (S = 0.35 cm2) in CaCl2-TiCl2 (C Ti(II) = 0.5 mol/dm3) at 1173 K (900 °C) and 5 to 25 Hz. (a) experiment results, (b) square wave voltammogram after Gauss fitting, (c) experimental data and the width of the half peak of Gauss fitting data at 25 Hz
In case of reversible electrode reaction, the relationship between the peak current and scanning frequency is the same as the Eq. [16] in square wave voltammetry.[32–34]
where \( I{\text{p}} \) is the peak current, A; n the electron charged number; F the Faraday constant, C; A the electrode area, cm2; \( C_{0} \) the concentration of Ti2+ion, mol/cm3; D the diffusion coefficient, cm2/s; \( f \) the scanning frequency, Hz; R the ideal gas constant, J/mol·K; T temperature, K; \( E_{\text{SW}} \) square wave amplitude, V.
By using the Eq. [16], the diffusion coefficient of Ti2+ in CaCl2-TiCl2 (0.5 mol/dm3) melt at 1173 K (900 °C) was calculated as 1.14 × 10−5cm2/s (±10 pct).
Table II presents the diffusion coefficients of Ti2+ obtained in different melts.[20–22]
Using the half peak (W 1/2) of Gaussian wave, the number of exchanged electrons can be calculated.[32–34] The relationship between the half peak of Gaussian wave and the number of exchanged electrons is the same as the Eq. [17].
The relationship between the square wave voltammogram and the half peak of the Gaussian wave is shown in Figure 7(c).
By using Eq. [17], the average number of electrons transferred of the cathode reduction reaction in the range of 5 to 25 Hz was calculated as n = 1.98. The number of electrons transferred is close to 2. This exhibits that the cathodic reaction of Ti2+ ion in CaCl2-TiCl2 melt precedes a one-step electrochemical reaction:
Electrochemical Deposition
All the electrochemical tests proved that Ti2+ in the CaCl2-TiCl2 can be successfully reduced to titanium. The reduction of Ti2+ proceeds in one step exchanging two electrons. In order to obtain the titanium deposit on the cathode, the titanium deposit was carried out using the CaCl2-TiCl2 melt at 1173 K (900 °C). In the set-up, two titanium plates (15 mm × 50 mm × 5 mm) were used as anodes, and a stainless plate (15 mm × 50 mm × 1 mm) was used as cathode. After electrolysis, the cathode was coated with a mixture of titanium deposit and salt. The cathode was washed in an ultrasonic tank containing the dilute hydrochloric acid (1 mol/dm3) for dissolving salt, and then was washed by water and filtrated, and was dried. Table III indicates the changes of current efficiency with varying current density. As can be seen in Table III, the current efficiency is increasing when the current density is in the range of 0.1 to 0.5 A/cm2 while decreasing in the range of 0.5 to 0.9 A/cm2 and a peak value of current efficiency appears at a current density of 0.5 A/cm2.
Figure 8 gives the titanium deposited under the current density of 0.5 A/cm2 in CaCl2-TiCl2 (5.0 wt pct) at 1173 K (900 °C). It can be seen from Figure 8(a) that the deposited has dendritic structures, and it was also proved by the SEM micro-morphology showing in Figure 8(b). The grain sizes of dendritic crystals are 500 μm.
Figure 9 shows XRD pattern of cathode deposit. The XRD pattern confirms that the obtained cathodic deposit is metallic titanium. The compositions of the obtained sample were determined by ICP-AES and Chemiluminescent Nitrogen/Oxygen Analyzer showing in Table IV. The experimental results show that the impurity elements content can meet the standard of high-purity titanium powder (4N5 grade).
Conclusions
Titanium dichloride was prepared by using a titanium sponge to reduce titanium tetrachloride in CaCl2 melt. When the titanium tetrachloride is reduced by the excess amount of titanium sponge in CaCl2 melt, and the average titanium oxidation state in CaCl2-TiClx melt is close to 2. The results detected by different electrochemical techniques such as cyclic voltammetry, chronopotentiommetry, and square wave voltammetry show that the cathodic reduction of Ti2+ ion is a one-step quasi-reversible process controlled by the diffusion. The diffusion coefficient of the Ti2+ ion is determined at 1173 K (900 °C). Furthermore, the work demonstrates the feasibility of producing metallic titanium in the as-prepared CaCl2-TiCl2 melts through galvanostatic electrolysis.
References
W.J. Kroll: J. Electrochem. Soc., 1940, vol. 78, pp. 35-47.
F.R. Clayton, G. Mamantov, and D.L. Manning: J. Electrochem. Soc., 1973, vol. 120, pp. 1193-99.
D. Wei, M. Okido, and T. Oki: J. Appl. Electrochem., 1994, vol. 24, pp. 923-29.
T. Oishi, H. Kawamura, and Y. Ito: J. Appl. Electrochem., 2002, vol. 32, 819-24.
J.H. von Barner, P. Noyé, A. Barhoun, and F. Lantelme: J. Electrochem. Soc., 2005, vol. 149, pp. C20-26.
F. Lantelme and A. Salmi: J. Electrochem. Soc., 1995, vol. 142, pp. 3451-56.
D.R. Sadoway: JOM, 1995, vol. 43, pp.15-19.
N.A. Fried, K.G. Rhoads, and D.R. Sadoway: Electrochim. Acta, 2001, vol. 46, pp. 3351-58.
G.Z. Chen, D.J. Fray, and T.W. Farthing: Nature, 2000, vol. 407, pp. 361-64.
D.J. Fray: JOM, 2001, vol. 53, pp. 27-31.
S.Q. Jiao and D.J. Fray: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 74-79.
G.Z. Chen and D.J. Fray: J. Electrochem. Soc., 2002, vol. 149, pp. E455-67.
S.L. Wang and Y.J. Li: J. Electroanal. Chem., 2004, vol. 571, pp. 37-42.
T.H. Okabe, M. Nakamura, T. Oishi, and K. Ono: Metall. Trans. B, 1993, vol. 24B, pp. 449-55.
K. Ono and R.O. Suzuki: JOM, 2002, vol. 54, pp. 59-61.
T.H. Okabe, T. Oishi, and K. Ono: Metall. Trans. B, 1992, vol. 23, pp. 583-90.
R.O. Suzuki: J. Phys. Chem. Solids, 2005, vol. 66, pp. 461-65.
S.Q. Jiao and H.M. Zhu: J. Mater. Res., 2006, vol. 21, pp. 2172-75.
S.Q. Jiao, Y.J. Hu, Y. Liu and H.D. Jiao: CN103290433A, 2013.
G.M. Haarberg, W. Rolland and J. Thonstad: J. Appl. Electrochem., 1993, vol. 23, pp. 217-24.
K. Matsmoto, H. Numata, S. Haruyama and I. Ohno: Mater. Trans., 1999, vol. 40, pp. 1429-35.
D.M. Ferry, G. S. Picard and B. L. Trémillon: J. Electrochem. Soc., 1988, vol. 135, pp. 1443-47.
A.M. Martinez, Y. Castrillejo, E. Barrado, G.M. Haarberg and G. Picard: J. Electroanal. Chem., 1998, vol. 449, pp. 67-80.
F. Lantelme, K. Kuroda and A. Barhoun: Electrochim. Acta, 1998, vol. 44, pp. 421-31.
W.C. Kreye and H.H. Kellogg: J. Electrochem. Soc., 1957, vol. 104, pp. 504-08.
C.H. Baumgartner: Anal. Chem., 1992, vol. 64, pp. 2001-02.
O. Takeda and T. Okabe: J. Alloy. Compd., 2008, vol. 457, pp. 376-83.
Q.Y. Wang, J.X. Song, G.J. Hu, X.B. Zhu, J.G. Hou, S.Q. Jiao and H.M. Zhu; Metall. Mater. Trans. B, 2013, vol. 44B, pp. 906-13.
A.J. Bard and L.R. Faulkner: Electrochemical Methods: Fundamentals and Applications, John Wiley and Sons press, New York, 2000, pp. 231-310.
R.W. Laity and D.E. Mclntyre: J. Am. Chem. Soc., 1965, vol. 87, pp. 3806-12.
R.K. Jain, H.C. Gaur and B.J. Welch: J. Electroanal. Chem., 1977, vol. 79, pp. 211-36.
J.J. O’Dea, J. Osteryoung and R. Osteryoung: Anal. Chem., 1981, vol. 53, pp. 695-701.
L. Ramaley, S. Matthew and J.R. Krause: Anal. Chem., 1969, vol. 41, pp. 1362-65.
S. Matthew, J.R. Krause and L. Ramaley: Anal. Chem., 1969, vol. 41, pp. 1365-69.
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (No.51322402), the Program for New Century Excellent Talents in University, Ministry of Education of China (NCET-2011-0577), the National High Technology Research and Development Program of China (863 Program, No. 2012AA062302), and the Fundamental Research Funds for the Central Universities (FRF-TP-12-002B) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Min Ho Kang and Jianxun Song had equal contribution to this work.
Manuscript submitted June 3, 2014.
Rights and permissions
About this article
Cite this article
Kang, M.H., Song, J., Zhu, H. et al. Electrochemical Behavior of Titanium(II) Ion in a Purified Calcium Chloride Melt. Metall Mater Trans B 46, 162–168 (2015). https://doi.org/10.1007/s11663-014-0191-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0191-z