Abstract
The cathodic behavior of Ti(III) ions in a NaCl-2CsCl melt was investigated by cyclic voltammetry, chronopotentiometry, and square wave voltammetry with a tungsten electrode being the working electrode at different temperatures. The results show that the cathodic behavior of Ti(III) ion consists of two irreversible steps: Ti3+ + e = Ti2+ and Ti2+ + 2e = Ti. The diffusion coefficient for the Ti(III) ion in the NaCl-2CsCl eutectic is 1.26 × 10−5 cm2 s−1 at 873 K (600 °C), increases to be 5.57 × 10−5 cm2 s−1 at 948K (675°C), and further rises to 10.8 × 10−5 cm2 s−1 at 1023 (750 °C). Moreover, galvanostatic electrolysis performed on a titanium electrode further presents the feasibility of electrodepositing metallic titanium in the molten NaCl-2CsCl-TiCl3 system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanium and its alloys have attracted great interests for their desirable properties such as high strength, low density, excellent flexibility, strong spring-back characteristics, corrosion resistance, and biocompatibility. However, its use has been restricted because of its high processing cost of the traditional Kroll process. To extract the titanium and its alloys in a simpler and lower cost way, attractions have been drawn on molten salt electrolysis process, such as FFC-Cambridge process,[1] OS process,[2,3] molten oxide electrolysis (MOE),[4] and the electrolysis of titanium oxycarbides (the USTB process[5–7]). The electrochemistry of titanium subchlorides in molten salts and the deposition of metallic titanium from titanium chlorides have been the subject for many investigators.[8–12] The electrowinning of titanium metal has been realized by dissolving titanium chlorides (TiCl4, TiCl3) into chlorides, fluorides, and mixtures of chlorides and fluorides. In all-fluoride melts, especially the LiF-NaF-KF eutectic melt, a two-step reduction of Ti(IV) to titanium metal, Ti(IV) → Ti(III) → Ti, is known to proceed, which means the presence of fluorides leads to the stabilization of Ti(III), and the deposition of titanium may occur directly from a trivalent species.[13] Many investigations of the electrochemistry have also been involved in pure chloride melts. For example, in molten LiCl-KCl or NaCl-KCl, the reduction of Ti(IV) follows the steps of Ti(IV) → Ti(III) → Ti(II) → Ti,[14–16] which are more complicated. Despite the extensive investigations, as well as the advantages in economics and product quality, the electrolysis of titanium from chloride melts has not yet been commercialized as the different valencies of titanium in pure chloride electrolyte may undergo reoxidation and disproportionation reactions, which results in a very low current density. Therefore, a better understanding of the equilibria between the different oxidation states of titanium in the chloride molten salts is of great significance.
The previous work has found that Ti(III) is stable in molten CsCl at 973 K (700 °C) but the metal deposition takes place via Ti(III) → Ti(II) → Ti.[17] However, when the temperature is lowered to 673 K (400 °C), the Ti(III) is reduced to titanium directly. Although the preliminary work investigated the effects of temperature for the stability of trivalent titanium ions and indicated that in alkali chloride baths the stability of valence states Ti(IV) and Ti(III) of titanium increases with the radius of the cations in the melts,[18,19] the kinetic parameters for the charge transfer steps and the mechanism of electrodeposition of titanium in the NaCl-2CsCl eutectic have not been reported. The purpose of the present work is to investigate the mechanism and the kinetics of electrodeposition of titanium from a NaCl-2CsCl melt and to disclose the nature of the kinetics as a function of electrolyte temperature.
Experimental Section
The preparation of Titanium Trichloride
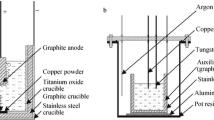
All chemical reagents used were analytical grade. A schematic illustration of the representative experimental apparatus for the preparation of TiCl3 is shown in Figure 1. TiCl3 was obtained by the reaction of TiCl4 (TiCl4, Sinopharm Chemical Reagent Co., Ltd., analytical grade ≥99.0 pct) with titanium metal in the CaCl2 (CaCl2, Sinopharm Chemical Reagent Co., Ltd. analytical grade ≥99.99 pct, 200 g) melt. CaCl2 was dried at 573 K (300 °C) in a vacuum status for 24 hours. Subsequently, the salts were heated up to melt at 1173 K (900 °C) in a high-purity argon atmosphere (Ar, Sinopharm Chemical Reagent Co., Ltd., 99.999 pct), and the flowrate is 30 mL min−1. Additionally, high-purity hydrogen chloride gas (HCl, Sinopharm Chemical Reagent Co., Ltd. analytical grade ≥99.999 pct) was bubbled into the salt to remove the O2−. Titanium sponge (Sinopharm Chemical Reagent Co., Ltd., 99.9 pct, average particle diameter (d 50): 10 mm, 6.3 g) was placed into the premelted salt. According to Eq. [1], a stoichiometric mass of liquid TiCl4 (Sinopharm Chemical Reagent Co., Ltd., analytical grade ≥99.0 pct, 26 g) was fed into this reaction container by dropping it from the top of the chamber through a quartz feed tube. It was expected that the TiCl4 supplied in the form of a gas bubble at elevated temperatures reacted with the titanium sponge in the molten CaCl2. TiCl3 was synthesized by the reaction of TiCl2 and TiCl4 (gas). The TiCl2 was firstly generated by the reaction between titanium metal and TiCl4 (gas). The product of pure TiCl3 was condensed at the lower temperature part of cooling quartz tubes. After the experiment, the quartz tube was removed from the molten salt, the product was gradually cooled in the furnace in an argon (Ar) atmosphere with the flowrate of 30 mL min−1, and the product was collected within 30 seconds.[20]
The Cell, the Electrode, and the Tests
NaCl (Sinopharm Chemical Reagent Co., Ltd., analytical grade ≥99.8 pct) and CsCl (Sinopharm Chemical Reagent Co., Ltd., analytical grade ≥99.99 pct) were mixed at a molar ratio of 1:2 in an argon filled glove box and were then transferred into an alumina crucible. The electrolyte was melted in the alumina crucible after being placed in a furnace (SKL10, Yun Jie electric furnace factory, Baotou, China) controlled by a CHIND DI3000 programmable device. It should be noted that the experiments were performed in an inert argon (Ar, Sinopharm Chemical Reagent Co., Ltd., 99.999 pct) atmosphere previously dehydrated by heating in a vacuum. All measurements were carried out under the atmosphere of dried argon (Ar, Sinopharm Chemical Reagent Co., Ltd., 99.999 pct), and the temperature was fixed at 873 K (600 °C).
Some of electroanalytical techniques were used for test. All the electrochemical tests are carried out in a three electrode configuration with a tungsten wire with an apparent area of 0.03295 cm2 being the working electrode, a spectral pure graphite rod with a diameter of 6 mm being counter electrode, and an Ag/AgCl electrode being the reference electrode, respectively. The Ag/AgCl electrode was prepared by putting a silver wire with a diameter of 0.5 mm in a mullite tube contained silver chloride solution (4 wt pct) in the NaCl-2CsCl molten mixture. A schematic illustration of electrochemical test is shown in Figure 2. The potential of the reference electrode was calibrated relative to chlorine gas emission by using galvanic square wave technology at 873 K (600 °C). The results are shown in Figure 3. Figure 3(a) showed the potential variation of Ag/AgCl relative to chlorine gas emission with time and Figure 3(b) showed the enlarged red dotted region of Figure 3(a). \( E_{{{\text{Cl}}_{ 2} /{\text{Cl}}^{ - } }} \) values were 1.156V (vs AgCl/Ag). All of the following potentials are calibrated to standard chlorine electrode potential.
(a) The potential variation of Ag/AgCl relative to chlorine gas emission with time. (b) The enlarged red dotted region of Figure 3(a)
Production of Titanium Deposit
The deposition of titanium was carried out in the NaCl-CsCl melt containing TiCl3 to assess the feasibility of titanium electrodeposition in an electrolyte of NaCl-2CsCl-TiCl3 (2.0 wt pct). The temperature was 873 K (600 °C), the cathodic current density is 0.3 A cm−2, and time duration of electrolysis is 10 hours. A titanium plates (20 mm × 40 mm) were used as the anodes, and a stainless plate (10 mm × 10 mm) was used as a cathode. The cathode is much smaller than the anode to obtain a uniform electrical field. The electrochemical tests were performed using a Electrochemical workstation (Model263A, Princeton institute, America) controlled with the powersuite software package. The obtained products on the cathode were characterized by scanning electron microscope (JSM-6701F, JEOL, Japan) and X-ray diffraction (D/max-RB, Rigaku, Japan).
Results and Discussion
Cyclic Voltammogram
The cyclic voltammogram performed in a blank NaCl-2CsCl melt with a tungsten wire being the working electrode is presented by the dotted line in Figure 4. The potential was scanned from the −0.5 V (vs Cl2/Cl−) to the more negative direction, reversed at −3.0 V (vs Cl2/Cl−), and returned to the −0.5 V (vs Cl2/Cl−) after the positive direction scanning. It can be seen that a gradual increase of reduction current appeared from about −2.7 V (vs Cl2/Cl−), indicating the generation of liquid alkaline metal. Apparently, there are no significant reactions associated with the redox of electrolyte components as well as the W electrode material in the range from −0.5 V (vs Cl2/Cl−) to −2.7 V (vs Cl2/Cl−), and credible test results can be achieved within limits.
To further investigate the electrochemical behavior of Ti(III) ion in the NaCl-2CsCl melt, cyclic voltammetry is performed on a W working electrode, and the corresponding result is shown by the solid line in Figure 4. It exhibits two pairs of peaks (R1, O1; R2, O2), and the reduction current peak R1 at −1.55 V (vs Cl2/Cl−) is not observed obviously, which is corresponding to the reduction of Ti(III) to Ti(II), and the other peak R2 at −1.81 V (vs Cl2/Cl−) can be attributed to the deposition of titanium from Ti(II). During positive scanning, the oxidation current peaks O2 and O1 are observed, which are corresponded to the dissolution of the deposited titanium and the oxidation of Ti(II) to Ti(III).
Moreover, cyclic voltammograms of NaCl-2CsCl-TiCl3 system are recorded at various scan rates, which are shown in Figure 5. There is a slight peak shift toward the negative direction for the cathodic reaction and toward the positive direction for the anodic reaction with an increase of scan rate, revealing a quasi-reversible or irreversible process is involved. In addition, the value of |E pa − E pc| is always larger than that calculated from 2.3RT/nF, demonstrating that this electrochemical process is irreversible.[21] Actually, the kinetics of the electrochemical process largely depends on the time span involved in the scanning, so an irreversible shape is generally more easily observed at a short time span.[22]
Chronopotentiometry
In order to further confirm the above results, chronopotentiometry has been applied to investigate the cathodic process of Ti(III) ion in the NaCl-2CsCl-TiCl3 melt. A transient curve shown in Figure 6 shows the existence of two potential Platforms in the potentials of −1.55 V and −1.8 V (vs Cl2/Cl−). They are corresponded to the consecutive reactions Ti(III) → Ti(II) → Ti.
Square Wave Voltammogram
A typical square wave voltammogram is applied to determine the transfer electron number and calculated the diffusion coefficient. In Figure 7, two peaks can be clearly observed during potential negative sweep. By measuring the width at mid height of each peak, it is possible, using Eq. [2], to determine the electron number that must have been exchanged (n):[23,24]
where R is the ideal gas constant, J mol−1 K−1; T is the absolute temperature, K; F is the Faraday constant, C mol−1; and 3.52 is dimensionless.
The calculated result proposes that (R1) correspond to a soluble–soluble system Ti(III)/Ti(II), while (R2) are associated with the formation of metallic Ti. It can thus be concluded that the electro-reduction of Ti(III) ions in a NaCl-2CsCl molten salt proceeds via the following two-step process:
In order to study the effect of temperature on the behavior of Ti(III), square wave voltammograms tested at different temperatures are given in Figure 8, which indicates that the peak current density increased with the increasing temperature. There is a slight peak shift toward the positive direction for a cathodic reaction.
The specific value of the diffusion coefficient was determined by changing the frequency signal of the square wave voltammograms. The results show that the square wave voltammograms exhibit the peak current density of R1 in the same potential range as that in the cyclic voltammogram, as shown in Figure 9(a). Moreover, the peak current density is increasing regarding to the increase of the scan frequency. The height of the peak and the square root of frequency indicate the relationship as Eq. [5]:[25,26]
where i p is the peak current, A; n the electron charged number; F the Faraday constant, C; A the electrode area, cm2; C0 the concentration of Ti3+ ion, mol cm−3; D the diffusion coefficient, cm2 s−1; f the scanning frequency, Hz; R the ideal gas constant, J mol−1 K−1; T temperature, K; and ∆E is the square wave amplitude, V.
The maximum current of the peak (R1) vs the square root of the frequency of the square wave signal presents a linear relationship. The straight line obtained is shown in Figure 9(b) which confirms that Eq. [3] can be applied in the frequency range studied. Furthermore, the diffusion coefficient of Ti(III) ions in NaCl-2CsCl at 873 K (600 °C) can be calculated as 1.26 × 10−5 cm2 s−1. Meanwhile, the diffusion coefficient of Ti(III) ions in NaCl-2CsCl at 948 K and 1023 K (675 °C and 750 °C) was determined to be 5.57 × 10−5 and 10.8 × 10−5 cm2 s−1, by using the above-mentioned method, respectively (Table I).
Electrolysis
A galvanostatic electrolysis was performed with using a cathodic current density is 0.3 A cm−2 in the an electrolyte of NaCl-2CsCl-TiCl3 (2.0 wt pct). After electrolysis, the cathodic product was washed in an ultrasonic tank containing the dilute hydrochloric acid (1M) for dissolving salt and then was washed with water, filtrated, and dried. The photograph with the titanium deposited is shown in Figure 10(a). SEM images of electrolytic Ti are shown in Figure 10(b) which demonstrates a relatively large grain obtained. The XRD pattern confirmed that the Ti metal is successfully deposited which can be seen in Figure 11(a). The potential–time curve is shown in Figure 11(b), the decreasing potential during the electrolysis is due to the increases of surface area of the cathode surface during the generation and growth of the new grain, and the surface of anode is much larger than that of cathode.
Conclusion
The electrochemical behavior of Ti(III) ion in a NaCl-2CsCl melt was investigated on a tungsten electrode. The results demonstrated that the reduction of Ti(III) was a two-step irreversible process which is controlled by the diffusion. The diffusion coefficient (D) for the reduction process of Ti(III) ion in such melt at different temperatures was different. With the temperature increasing, the diffusion coefficient was enhanced: the diffusion coefficient of Ti(III) ions in NaCl-2CsCl at 873 K (600 °C) was calculated to be 1.26 × 10−5 cm2 s−1 and the diffusion coefficients of Ti(III) ions in NaCl-2CsCl at 948 K and 1023 K (675 °C and 750 °C) were calculated to be 5.57 × 10−5 and 10.8 × 10−5 cm2 s−1, respectively. Furthermore, the galvanostatic electrolysis demonstrated the feasibility of depositing titanium in the NaCl-2CsCl-TiCl3 melt.
References
G.Z. Chen, D.J. Fray and T.W. Farthing: Nature, 2000, vol. 407, pp. 361-364.
Ono K, Suzuki RO (2002) JOM, vol. 54, pp. 59-61.
R.O Suzuki, K. Ono and K. Teranuma: Metall and Materi Trans B, 2003, vol. 34, pp. 287-295.
D.R. Sadoway: JOM, 1991, vol. 43, pp. 15–19.
S.Q. Jiao and H.M. Zhu: J. Mater. Res., 2006, vol. 21, pp. 2172-2175.
S.Q. Jiao, X.H. Ning, K. Huang and H.M. Zhu: Pure Appl. Chem, 2010, vol. 82, pp. 1691-1699.
S.Q. Jiao and H.M. Zhu: J.Alloy Compd., 2007, vol. 438, pp. 243-246.
S. Senderoff and G.W. Mellors: Science, 1966, vol. 153, pp. 1475-81.
E. Chassaing, F. Basile and G. Lorthioir: J. Appl. Electrochem., 1981, vol. 11, pp. 193-196.
O.Q. Leone, H. Knudsen and D. Couch: J. Metalls, 1967, vol. 19, pp. 18-23.
Martínez AM, Castrillejo Y, Barrado E, Haarberg GM, Picard G (1998) J Electroanal Chem 449:67-80.
S.N. Flengas and T.R. Ingraham: Can. J. Chem., 1960, vol. 38, pp. 813-817.
H. Wendt, K. Reuhl and V. Schwarz: J. Appl. Electrochem., 1992, vol. 22, pp. 161-165.
B.N. Popov, M.C. Kimble, R.E. White and H. Wendt: J. Appl. Electrochem., 1991, vol. 21, pp. 351-357.
G.M. Haarberg, W. Rolland, Å. Sterten and J. Thonstad: J. Appl. Electrochem., 1993, vol. 23, pp. 217-224.
F. Lantelme and A. Salmi: J. Electrochem. Soc., 1995, vol. 142, pp. 3451-3456.
F. Lantelme, K. Kuroda and A. Barhoun: Electrochim. Acta, 1998, vol. 44, pp. 421-431.
B.F. Markov and B.P. Podafa: Uzb. Khim. Zh., 1970, vol. 36, pp. 881-&.
E. Chassaing, F. Basile and G. Lorthioir: Journal of the Less Common Metals, 1979, vol. 68, pp. 153-158.
J.X Song, Q.Y. Wang, M.H. Kang and S.Q. Jiao: Int. J. Electrochem. Sci, 2015, vol. 10, pp. 919-930.
A.J. Bard and L.R. Faulkner: 2001.
R.S. Nicholson: Anal. Chem., 1965, vol. 37, pp. 1351-1355.
G.C. Barker: Anal. Chem. Acta, 1958, vol. 18, pp. 118-131.
L. Ramaley and M.S. Krause: Anal. Chem., 1969, vol. 41, pp. 1362-1365.
J.J. O’Dea, J. Osteryoung and R.A. Osteryoung: Anal. Chem., 1981, vol. 53, pp. 695-701.
J.H. Christie, J.A. Turner and R.A. Osteryoung: Anal. Chem., 1977, vol. 49, pp. 1899-1903.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (No. 51322402), the National High Technology Research and Development Program of China (863 Program, No. 2012AA062302).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted January 30, 2015.
Rights and permissions
About this article
Cite this article
Song, Y., Jiao, S., Hu, L. et al. The Cathodic Behavior of Ti(III) Ion in a NaCl-2CsCl Melt. Metall Mater Trans B 47, 804–810 (2016). https://doi.org/10.1007/s11663-015-0521-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0521-9