Abstract
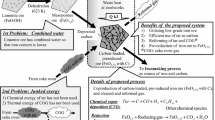
A new ironmaking concept is being proposed that involves the combination of a rotary hearth furnace (RHF) with an iron-bath smelter. The RHF makes use of iron-oxide-carbon composite pellets as the charge material and the final product is direct-reduced iron (DRI) in the solid or molten state. This part of the research includes the development of a reactor that simulated the heat transfer in an RHF. The external heat-transport and high heating rates were simulated by means of infrared (IR) emitting lamps. The reaction rates were measured by analyzing the off-gas and computing both the amount of CO and CO2 generated and the degree of reduction. The reduction times were found to be comparable to the residence times observed in industrial RHFs. Both artificial ferric oxide (PAH) and naturally occurring hematite and taconite ores were used as the sources of iron oxide. Coal char and devolatilized wood charcoal were the reductants. Wood charcoal appeared to be a faster reductant than coal char. However, in the PAH-containing pellets, the reverse was found to be true because of heat-transfer limitations. For the same type of reductant, hematite-containing pellets were observed to reduce faster than taconite-containing pellets because of the development of internal porosity due to cracking and fissure formation during the Fe2O3-to-Fe3O4 transition. This is, however, absent during the reduction of taconite, which is primarily Fe3O4. The PAH-wood-charcoal pellets were found to undergo a significant amount of swelling at low-temperature conditions, which impeded the external heat transport to the lower layers. If the average degree of reduction targeted in an RHF is reduced from 95 to approximately 70 pct by coupling the RHF with a bath smelter, the productivity of the RHF can be enhanced 1.5 to 2 times. The use of a two- or three-layer bed was found to be superior to that of a single layer, for higher productivities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rotary hearth furnace (RHF) is a donut-shaped refractory-lined reactor that makes use of waste oxides, particulate fines, and millscale generated in the steel mills and that produces direct-reduced iron (DRI), by mixing the oxides with a carbonaceous reductant such as coal into a composite. The composite material is usually charged into the RHF (hearth) in the form of pellets[1,2] or as a powder mixture.[3] The temperature and atmosphere inside the furnace are controlled by means of burners positioned along the walls and, sometimes, along the roof of the furnace. The energy for reduction is principally due to the thermal radiation from the furnace walls and roof and from the burner flames. It is known that the heat-transport phenomenon limits the productivity of the RHF. Most of the RHFs typically use a single layer or, at most, two layers of composite pellets on the hearth. This is because when a multilayer bed is used, the amount of energy reaching the lower layers of the multilayer bed is substantially reduced.
A new ironmaking concept is being investigated that involves the combination of an RHF with an iron-bath smelter.[4] The idea is to prereduce the iron oxide to approximately 70 pct metallization in the RHF and carry out the remaining smelting of the DRI obtained from the RHF and gangue removal in the smelter. By doing so, the productivity of the RHF can be increased due to the lower target degree of metallization, and the energy requirements of the smelter will be substantially reduced due to the prereduced feed. Because of the lower target degree of metallization, there exists the possibility that the number of layers of the pellet bed may be increased. Therefore, it is possible to overcome the individual limitations of both of these reactors and enhance the productivity of the overall process.
Previously,[5] the reaction-rate constants for the chemical reactions, namely, the carbon gasification by CO2 and the wüstite reduction by CO were determined. Two different carbonaceous reductants, coal char and devolatilized wood charcoal, and two kinds of ferric oxide, an artificial highly porous hematite and naturally occurring hematite ore tailings, were the subjects of the investigation for the rate constants. It was found that the rate constants for the carbon oxidation for wood charcoal were almost an order of magnitude higher than coal char because of the highly porous morphology of wood charcoal; the rate constants for the wüstite reduction, on the other hand, were found to be higher for the porous artificial hematite (PAH) in comparison to the naturally occurring hematite ore. Moreover, both the carbon oxidation and the wüstite reduction were found to control the chemical kinetics of the overall reduction at the temperatures of interest.
In this article, the primary subject is the reduction of spherical iron-oxide-carbon composite pellets under heat-transfer conditions similar to those in an RHF. The reduction in composite pellets is fairly complex and is influenced by several factors. These include the chemical kinetics of the heterogeneous surface-chemical reactions and the external and internal heat-transport steps to and within the composites.[6–8] The reduction kinetics of both a single-layer and a multilayer bed was measured.
Fortini and Fruehan[6] investigated the reduction of composite pellets under temperature conditions corresponding to the operation of the RHF. In their experiments, Fortini and Fruehan relied on the mass-loss measurements of a single composite pellet made using a thermal gravimetric analyzer. The experimental data were interpreted using a mathematical model developed for the reduction of composite pellets. The present work on the reduction of a multilayer pellet bed in an RHF simulator is an extension of the work of Fortini and Fruehan.[4] The RHF simulator represents the reduction conditions around the composite pellets similar to those that exist in an RHF. The reduction rate was measured using off-gas analysis, which is a significant improvement over the mass-loss technique.
Sohn[9] developed an RHF simulator to study the role of volatiles in coal in the reduction of iron-oxide-coal composite pellets. High-intensity infrared (IR) emitting lamps were employed to simulate the radiation heat transport to the pellet bed. Similar equipment was used in the present work, to simulate the external heat transport to the pellets.
Experimental
RHF Simulator
The RHF simulator is designed to perform the reduction of iron-oxide-carbon composite pellets by simulating heat-transfer conditions similar to those that exist in an actual RHF. Figure 1 shows a schematic of the simulator; as is evident from the figure, it is comprised of two primary components: a heat source and a reaction chamber.
The reaction vessel is comprised of two concentric tubes: an internal copper tube 165 mm in external diameter and 12.7 mm in thickness made out of electronic-grade copper and an outer low-carbon steel tube 241 mm in diameter and 6.35 mm in thickness. Both of the tubes were welded and soldered on top of a low-carbon steel base plate. The annular region between the two tubes was bridged by a low-carbon steel annulus, which was welded to both of these tubes. This annular region between the tubes acted as a cold-water jacket around the central copper reaction chamber through which cold water was constantly circulated to keep the temperature within reasonable limits. The copper tube had a carbon steel lid that could be screwed on top of it. Radiation heating from an IR-emitting lamp positioned on top of the pellet bed was used to heat up the bed to temperatures ranging between 1423 and 1573 K. There was a circular opening (∼102 mm in diameter) at the center of the lid, through which the radiation would enter the reaction vessel. To make the system airtight, a polished quartz disk (115 mm in diameter × 1.6 mm in thickness) was held against the circular hole by means of a stainless steel O-ring. This O-ring was screwed to the lid and held the quartz disk firmly in place. A high-temperature ceramic-fiber gasket was also present between the quartz disk and the O-ring. This provided additional sealing and avoided cracking of the quartz disk, in case of the overtightening of the O-ring screws.
Argon gas of ultrahigh purity (UHP) (grade 5.0) was passed through the reaction chamber using mullite injector tubes at a known flow rate of 0.006 m3/h (STP). The argon acted as both a carrier and a tracer gas. Inside the reaction vessel, there was a circular steel disk that rested on four height-regulating screws inside the central copper tube. This disk acted as both a radiation shield and the supporting base of the reaction crucible. This disk was covered with high-temperature ceramic-fiber paper and an alumina brick, to avoid oxidation.
Individually weighed iron-oxide-carbon composite pellets were arranged in the form of layers in a refractory-brick crucible and reduced in the RHF simulator. R-type thermocouples were used to measure the temperature of the different layers and the crucible bottom. The readings were monitored using a data acquisition system and were logged into a computer. However, it must be noted that the thermocouples were not embedded inside the pellets. They were placed at locations such that the distance between their tips and the pellet surface was approximately 1 to 2 mm before the start of the experiment. During the reduction, the composite pellets were observed to shrink in all directions. Thus, the pellet-to-pellet distance of a particular layer increased, with the whole bed migrating downward in the vertical direction. However, the temperature readings were good enough to provide a reasonable estimate of the temperature of the pellets belonging to a certain layer.
The source of the radiation heat was an IR-heating assembly (model 5090-06-01) manufactured by Research, Inc. (Eden Prairie, MN). This assembly involved two heater modules, each holding four tungsten-halide vapor lamps arranged side by side in parallel, such that a uniform coherent beam of IR radiation was incident over the pellet bed. Each of the lamps was rated at 144 V, with a rated power output of 1200 W. Every lamp had an inner tungsten-filament wire surrounded by a halogen vapor in a quartz envelope. As the lamp operates, tungsten vaporizes slowly from the filament and reacts with the halogen to form a tungsten halide. When the halide comes in contact with the filament, it undergoes decomposition because of the heat and forms tungsten again, which redeposits on the filament. This cyclic process is repeated again during the operation. Each heating panel was comprised of an aluminum reflector (water cooled), which directed the IR energy emitted by the lamps toward the pellet bed below.
Under normal operating conditions, the distance between the lamps and the top layer of the pellet bed was approximately 6 to 7 mm. The radiation entered the chamber through the quartz window on the lid of the vessel, between the lamps and the pellet bed. Invariably, the temperature of the top layer of the bed reached approximately 1523 K in approximately 6 to 7 minutes. This time usually varied depending on the condition of the lamps and the transparency of the quartz window. New lamps could help attain this temperature in approximately 4 to 5 minutes, while older ones could take as much as 10 minutes for the temperature to reach this threshold. Usually, the quartz window recrystallized after several experiments, which presented an impediment to the amount of incoming radiation. Efforts were therefore made to change the quartz window after every experiment, to avoid significant differences in the heating rates from one experiment to the other.
The power to the lamps was controlled by a silicon-controlled rectifier power controller (model 1029C-240V-120A-RO-5V-IPOT) manufactured by Control Concepts, Inc. (Chanhassen, MN). The voltage output of the controller to the lamps was controlled by an external feedback signal (0 to 5 V) from a National Instruments (Austin, TX) data acquisition (DAQ-NI6024E) card, using a built-in proportional integral derivative algorithm. The feedback signal was controlled by the temperature of the top-layer thermocouple. The lamps operated in a pulsating mode when the set-point temperature was attained, to maintain a more or less uniform top-layer temperature. The resulting temperature of the top layer of the bed pulsated within ±20 K of the set-point temperature, with the average being within ±5 K of the set-point value.
Off-Gas Measurements
Off-gas measurements enabled us to determine the amounts of CO and CO2 formed during the reaction and compute the degree of reduction as a function of time. This was made possible because of the fixed known flow rate of the carrier gas (UHP argon at 0.006 m3/h (STP)). This mode of measurement was a significant improvement over the mass-loss monitoring technique for studying the kinetics of the overall process. An Ametek (Pittsburgh, PA) (model LCM 100) residual gas analyzer was used for this purpose.
During the reduction, the release of extremely fine carbon and ore particles from the pellets into the off-gas stream exhibited a tendency toward entrainment; this resulted in a problem when analyzing the gas using the mass spectrometer, due to the blockage of the spectrometer’s capillary. In order to avoid this, a medium-porosity filter paper (FisherbrandFootnote 1 P5 type) was stationed in the off-gas conduit at the mouth of the capillary. The pressure buildup ahead of the filter paper that resulted from the impediment in the gas flow was relieved by directing the gas away, along a bypass line. It was found that directing the gas in this manner does not alter the off-gas composition measurement significantly, except for a slight delay of a few minutes (∼3 minutes) in the measurement. More details about the experiments can be found elsewhere.[10]
Composite Pellet-Making Procedure
The number of moles of carbon required to reduce 1 mole of Fe2O3 is between 1.5 and 3.0, depending on the relative amounts of CO and CO2 formed. Usually, industrial composite pellets are richer in the carbon needed for complete reduction and as an additional carbon source. Thus, to ensure there is enough carbon, the pellets containing Fe2O3 were composed of Fe2O3 and carbon such that Fe2O3:C = 1:3 on a molar basis. Pellets containing taconite concentrate (TAC) as the iron oxide were also looked into in this study. Taconite is primarily magnetite (Fe3O4). Following a similar argument, the taconite-containing pellets corresponded to a composition such that Fe3O4:C = 1:4 (molar basis).
The naturally occurring hematite ore tailings (HEM) contained 87.55 mass pct Fe2O3, 0.054 mass pct carbon, 0.01 mass pct S, and 61.24 mass pct total Fe. The PAH was essentially pure Fe2O3 with a negligible amount of impurities. The taconite used to make the pellets contained 4.43 mass pct SiO2, 0.02 mass pct Al2O3, 0.02 mass pct P2O5, 0.01 mass pct K2O, 0.01 mass pct sulfur, and 68.6 mass pct total Fe. Bentonite clay (73 mass pct SiO2, 14 mass pct Al2O3, and 2 to 3 mass pct Fe2O3) (Acros Organics, Morris Plains, NJ) was used as a binder for the taconite and hematite ore-containing pellets. Due to the fact that the fine particles of the ores were clogging the capillary of the mass spectrometer, the amount of binder addition was approximately 10 mass pct, which is somewhat higher than the conventional 1 to 2 mass pct for commercial pellets. It should be noted that no binder addition was made to the PAH-containing pellets because of their superior agglomeration properties.
Two reductants were investigated in this work: coal char and devolatilized wood charcoal. The coal (low-volatile bituminous) that was used to make the coal char contained 5.1 mass pct ash, 19 mass pct volatile matter, and 75.8 mass pct fixed carbon on a dry basis. The coal was supplied by Consol Energy (Canonsburg, PA). It is important to note that the same coal was used for making both the composite pellets and the powder mixtures that were used, in turn, to determine the reaction-rate constants.[5] The eucalyptus wood charcoal (Vallourec & Mannesmann Tubes, Belo Horizonte, MG, Brazil) contained 71.49 mass pct fixed carbon, 24.67 mass pct volatiles, 0.85 mass pct ash, and 2.99 mass pct moisture. After mixing, the constituents were emptied into a quartz bowl and mixed with the appropriate amounts of distilled water. The mixture was then kneaded into a dough from which pellets of similar sizes were hand rolled and baked in an oven at 473 K for approximately 5 days. Most of the pellets measured 15 to 17 mm in diameter.
Overall Experimental Setup for Reduction Experiments
Individually weighed pellets were placed in the crucible inside the reaction chamber of the RHF simulator. The chamber was properly sealed, using a polished fused-quartz disk and alumina cement at the top, and purged with UHP argon gas for approximately 15 to 20 minutes before turning on the IR lamps. The off-gas was continuously analyzed with the mass spectrometer, until the profiles of all the constituents in the off-gas stream became uniform before the start of every experiment. In the different experiments, the layers under consideration were occupied by the self-reducing pellets, while the other layers were occupied by inert dense alumina balls (CoorsTek brand, 15 mm in diameter, Golden, CO), that were similar in size to the composite pellets. The carrier UHP argon flow rate through the reaction chamber was maintained at 0.006 m3/h (STP).
Once the reference background gas composition was obtained, the lamps were turned on. A set point of 1523 K was fixed for the top-layer temperature, for all the reduction experiments. Both the temperatures of the different layers and the base of the crucible were monitored; the off-gas was continuously analyzed. The heating rates for the different experiments were observed to vary within the limits of experimental error. The lamps were turned off when the reduction was almost complete, as indicated by the off-gas composition measurements (when the partial pressure of Ar exceeds that of CO). The pellets were cooled to room temperature under the argon flow, with the mass spectrometer analyzing the off-gas continuously. The reduced pellet bed was taken out and weighed again, to obtain the total mass loss. The mass loss was also calculated from the mass-spectrometer data, for comparison with the actual experimental value. Both these mass losses agreed to within 1 to 5 pct of each other. The differences arise either because of the loss of fine particulate matter from the pellets into the gas stream or due to the reoxidation of the DRI caused by small air leakages after the lamps are turned off.
The off-gas composition measurements were used to compute the amounts of CO and CO2 and the degree of reduction. Since reduction experiments were carried out using single-, double-, and triple-layer arrangements, it was possible to estimate the reduction rates of each individual layer.
Interrupted-Reduction Experiments
In these experiments, a single composite pellet of PAH-coal-char was placed on the top layer of the pellet bed in the RHF simulator, with six other pellets of the same kind surrounding it on the same layer. This was done in order to simulate ambient conditions similar to those in the actual reduction. The middle and lower layers were occupied by inert alumina balls, similar to the top-layer-reduction experimental simulations. The pellets were heated for predetermined intervals of time following which the lamps were turned off. Heating rates similar to the ones used during the actual reduction experiments were also used for these tests. It was ensured that the reduction did not go to completion. The pellets were then cooled under a rapid flow of argon for approximately 45 minutes, to minimize surface reoxidation. After partial reduction, the pellets were taken out after cooling and mounted in an epoxy-based resin. The pellets were then cut along their diameter, to reveal the partially reduced cross section.
Results and discussion
Measurement of Reduction Rates
Figures 2 through 4 represent the various significant experimental data for one experimental scenario using PAH-coal-char composite pellets. Nineteen pellets were placed on the top layer, while the middle and bottom layers were occupied by 12 and 20 pellets, respectively. The lamps were turned on for approximately 2000 seconds. The reduced pellets were seen to undergo significant amounts of sintering; as a result, they were welded to one another, making it very difficult to weigh them individually after the reduction. So, the weight of the entire bed was measured after reduction. The total measured mass loss (ΔW) of all the three layers taken together after reduction was close to 79.65 grams; the mass loss obtained from the mass-spectrometer data was approximately 77.0 grams. Figure 2 depicts the off-gas composition.
Soon after the lamps are turned on, the CO2 begins to build up abruptly, once the temperature for the initiation of the fixed carbon reduction is attained. This is expected, because the reduction of hematite to metallic iron is known to occur in stages.[11] In the first stage, hematite reduces to magnetite, during which the primary gaseous species evolved is more or less completely CO2, which is consistent with equilibrium conditions for the Fe2O3-to-Fe3O4 transformation stage. The initial observation, therefore, supports this argument. The CO results in the subsequent stages of reduction, i.e., when magnetite reduces to wüstite, followed by the reduction of wüstite to iron. This is characterized by an increasing trend of CO during the later stages of the reduction. It can be seen that the CO partial pressure remains higher than the CO2 during these stages, because CO is the primary gaseous reaction product at the reaction temperature. The partial-pressure profiles of CO and CO2 show a change in slope at two different points (indicated by “a” and “b” along the respective profiles), which can be attributed to the onset of the reduction of the middle layer and lower layer, respectively. The middle-layer reduction probably begins at approximately t = 500 s, while the bottom layer starts to reduce at around t = 900 s. This is because of the unidirectional mode of heat transport, from the top to the bottom of the bed. The temperature of the middle layer at approximately t = 500 s and of the bottom layer at approximately t = 900 s is approximately 1273 K (Figure 4). Therefore, both these observations support this argument. An almost complete reduction of the three layers was complete in ∼30 to 35 minutes. This is evident from Figure 3, which plots the degree of reduction as a function of the reduction time. The percentage degree of reduction was computed according to Eq. [1], given here as
It may be noticed that the final degree of reduction is slightly in excess of 100 pct, based on the amounts of CO and CO2 generated. This may be possible for two reasons. First, there is always some moisture present in the reaction chamber, due to the water vapor present in the carrier gas or the release of some chemisorbed moisture from the pellets during reduction. This moisture can react with the carbon in the pellets and form CO + H2. Second, the leakage of air into the reaction chamber, which can react with the carbon in the pellets to form CO and CO2, could be responsible.
In another experiment, the simulation of a single-layer reduction using PAH-coal-char-composite pellets took approximately 15 to 20 minutes to go to completion, which is reasonably consistent with the residence time of the composite pellets in an industrial RHF.[12,13] In the single-layer-reduction simulation, the top layer of the bed was occupied by the composite pellets, while the middle and bottom layers were substituted with inert alumina balls, as described earlier.
Figure 5 shows the mass-loss curves for a three-layer bed of PAH-wood-charcoal pellets. The amount of CO generation in proportion to CO2 is much higher during the reduction of wood-charcoal-containing pellets, relative to the case in which coal char was used as the reductant. This is because of the higher rate of the carbon gasification reaction by CO2 (higher intrinsic reactivity) for wood charcoal, as reported elsewhere.[5]
Figure 6 shows the mass-loss curves for three layers of taconite-coal-char composite pellets. It may be noticed that the relative proportion of CO to CO2 generated in this case is higher than the scenario (Figure 3) in which three layers of PAH (Fe2O3)-coal-char pellets were reduced. This is because the reduction of taconite does not involve the reduction of the hematite-to-magnetite stage, unlike the reduction of Fe2O3. It is this particular transformation that generates a substantial amount of CO2.
Some experiments were performed in which PAH-wood-charcoal composite pellets were used to assess the reproducibility of the experimental data. Two single-layer tests, each with 19 pellets, were replicated under similar experimental conditions. The comparison of the degree of reduction as a function of time for these two experiments is depicted in Figure 7.
Figure 7 indicates that the two degree-of-reduction curves are somewhat displaced from one another. However, the reduction rates for the two trials are fairly close to one another; the reduction begins earlier in test 1 than in test 2, however, because there exists a horizontal displacement in the curves. The different onsets of the reduction in the two cases are due to the difficulty in controlling and replicating the exact experimental conditions during the experiments. Operating characteristics such as the heating rate of the pellet bed are subject to vary because of the degradation of the lamps from one experiment to another. However, the parameter of interest is the reaction rate (the slope of the curve), which appears reasonably consistent for both. Corrective measures adopted in the experiments using TAC-coal-char, TAC-wood-charcoal, HEM-coal-char, and HEM-wood-charcoal pellets involved changing the lamps more frequently and using a new quartz window for every experiment. In these experiments, the experimental error due to the mass spectrometer and the flow controller for the carrier UHP argon was computed to be less than 1 pct for the degree of reduction.
An important point to consider is the fact that, during these experiments involving composite pellets, the reduction is controlled by both the chemical-kinetic steps and the heat-transport mechanisms. Because it is difficult to decouple both of these aspects to study the reduction of the composite pellets, an effort was made to study the chemical kinetics of the carbon-oxidation reaction by CO2 and the wüstite reduction by CO in composite mixtures in another work.[5] Knowledge of the chemical-kinetic steps would be useful in obtaining a deeper appreciation of the role of the heat-transport mechanisms in the reduction of composite pellets.
Comparison of Reaction Kinetics of Various Composite Pellets
Understanding the kinetics of reaction of the different kinds of composite pellets is important in determining the combinations of raw materials most likely to reduce faster in the RHF. In working this out, the degree of reduction is plotted against time for all the pellet types experimented with. The following important considerations need to be taken into account while making a comparison of this sort.
-
(a)
In an actual industrial RHF, the composite pellets are charged on top of a heated-up hearth, whereas in the RHF simulator, the bottom of the crucible is at room temperature before starting an experiment. Therefore, the reaction times, as obtained experimentally, are a higher-end estimate because of the low-temperature environment around the bottom-layer pellets, which is unlike what happens in the RHF.
-
(b)
There is a time delay between the gas sampling time and the moment at which the mass spectrometer actually analyses the sample because of the additional resistance to the gas flow across the inline filter. It was estimated that this lag time could be as much as 3 to 5 minutes, depending on the gas flow rate. So, the experimentally obtained reaction times must be offset with this time delay, to come up with a better estimate of the actual reaction time under the current experimental environment.
-
(c)
In some cases, the external heat-transfer conditions vary from one experiment to the other. These contribute to a subtle difference of 5 to 8 pct on the degree-of-reduction scale between identical experiments, even though the reaction rates did not change much.
-
(d)
There is a considerable amount of dust and soot generated in the RHF because of the fine particles in the pellets and the volatile evolution from coal during the preheating stage. Due to the presence of this particulate matter above the bed, the external heat transport to the bed may not be substantial from the roof or the walls of the furnace. The radiation from the furnace gas, which occupies the volume above the bed, becomes more dominant. The current experimental schemes discount these factors because of the use of devolatilized reductants and the small size of the bed.
From the experimental observations, it is imperative that the overall reduction rate is dependent on the interplay of the following factors:
-
(a)
chemical kinetics of the carbon-oxidation reaction by CO2;
-
(b)
chemical kinetics of the wüstite-reduction reaction by CO;
-
(c)
internal heat transfer within the pellets, which has a significant dependence on the thermal conductivity of the composite pellets; and
-
(d)
external heat transfer to the pellets, a condition that is influenced by the changes in the size of the pellets during the course of reduction and becomes important for a multilayered-bed arrangement.
Reaction kinetics for top-layer reduction
In Figure 8, the degree of reduction curves for the different kinds of composite pellets are shown as a function of time corresponding to the top-layer-reduction configuration. For the top-layer-reduction scheme, the initial reduction rate for the different combinations was observed to follow the following sequence: PAH-coal-char > PAH-wood-charcoal ∼ HEM-wood-charcoal > TAC-wood-charcoal ∼ HEM-coal-char > TAC-coal-char.
These observations indicate that PAH-coal-char is faster than the PAH-wood-charcoal, despite the higher reactivity of wood charcoal as compared to coal char. This is primarily because of the relatively slow heat transfer (lower thermal conductivity) in PAH-wood-charcoal in comparison to PAH-coal-char, owing to the high internal porosity of wood charcoal and the denser morphology of coal char. The PAH-wood-charcoal appears to be initially faster than the HEM-wood-charcoal. However, after an approximately 33 to 35 pct reduction, the rates for both become comparable. This is because of the high porosity of the PAH, which allows it to reduce faster in the initial stages. However, as time passes, the PAH-wood-charcoal pellets undergo “swelling,” which decreases the internal heat transport within the pellets, causing the rate to become equal to that of HEM-wood-charcoal. In general, the reduction of the PAH is faster than that of the taconite and hematite ores because of the higher rate constants for the wüstite reduction at higher temperatures, as has been reported elsewhere.[5] This is true as long as the resistance due to the external and internal heat-transport mechanisms is lower than that for the chemical-kinetic steps. These temperatures are usually attained quite rapidly because of the high heating rate of the lamps. The higher rate constants are due to the widespread internal porosity in the PAH. The ores, on the other hand, are relatively much denser. The phenomenon of the swelling of PAH-wood-charcoal pellets can also be another reason for the slower reduction of the PAH-wood-charcoal pellets, as compared to the PAH-coal-char pellets. The volume changes of composite pellets will be addressed in detail in another article.[14]
When comparing the kinetics of TAC-wood-charcoal, TAC-coal-char, HEM-coal-char, and HEM-wood-charcoal, it was noticed that the reaction kinetics of the HEM-wood-charcoal is slightly faster than that of the HEM-coal-char and the reaction kinetics of the TAC-wood-charcoal is higher than that of the TAC-coal-char. This behavior in the ore-containing pellets with respect to wood-charcoal and coal-char seems to be different from that in the PAH-containing pellets. This could be because the denser nature of the ore particles enhances the internal heat transfer within the pellets and makes its relative influence on the overall reaction rate lower in comparison to the chemical kinetics of the carbon-oxidation reaction by CO2. Therefore, under those circumstances, wood-charcoal-containing pellets reduce faster than coal-char-containing pellets, as the carbon-oxidation reaction by CO2 has a larger influence on the overall rate.
The HEM-wood-charcoal reduces faster than the TAC-wood-charcoal, and HEM-coal-char is faster than TAC-coal-char, which is consistent with the fact that taconite (Fe3O4) is more difficult to reduce than hematite (Fe2O3). This happens because of the Fe2O3-to-Fe3O4 transition during the reduction of hematite, which causes crack formation and porosity development.[11] This indicates that the internal heat transfer in these pellets is faster relative to the kinetics of the wüstite-reduction reaction because of the denser structure of the ore particles.
The TAC-wood-charcoal and HEM-coal-char reveal similar reduction rates for the greater duration of the reduction. It has already been stated that taconite (Fe3O4) is more difficult to reduce than hematite (Fe2O3). However, wood charcoal is more reactive than coal char. Therefore, it is probably the interplay between the kinetics of the carbon-oxidation and wüstite-reduction reactions that is responsible for governing the reduction rates in these two composites.
Thus, for the top-layer reduction, the primary rate-limiting mechanisms seem to be the chemical kinetics of the carbon-oxidation and wüstite-reduction reactions coupled with the internal heat-transport mechanisms. The role of the external heat transfer is important during the initial stage of the heating up of the pellets. During the intermediate and later stages, however, the external heat transport may not be that significant in demarcating the differences in the rate, as the top-layer pellets receive most of the radiation from the lamps uniformly. However, the pellets do undergo shrinkage during the reduction. This can have a significant influence over the internal heat transport, as the radius of the pellet decreases. Since different kinds of pellets undergo various degrees of shrinkage, the internal heat transfer is affected differently for each kind.
Reaction kinetics for top-middle-layer reduction
The reduction of a bed with two layers of pellets highlights the external heat-transfer mechanisms to the pellets. The external heat transport attains prominence when it comes to the onset of the reduction of the middle layer. This is because the volume changes of the top-layer pellets have a direct impact on the rate of the temperature rise of the middle layer. Figure 9 depicts the comparison of the reaction kinetics of the different composite pellets, for this reduction scheme. In this scenario, there is a surprising difference in the reduction rates, when they are compared with the top-layer-reduction configuration.
The reduction rate for this arrangement follows the following sequence: PAH-coal-char > HEM-wood-charcoal > TAC-wood-charcoal ∼ PAH-wood-charcoal ∼ HEM-coal-char > TAC-coal-char. The HEM-wood-charcoal appears to reduce faster than the PAH-wood-charcoal, which is in sharp contrast to what was observed during the single-layer reduction. This is contrary to the fact that the reduction kinetics of hematite ore is slower than for PAH, since PAH has a higher surface area than the ore because of its highly porous nature. This strange observation can be explained by the differences in the heat-transfer conditions. In order to look into the external heat transfer to the middle-layer pellets, the temperature profiles of the middle layer during the reduction of the PAH-wood-charcoal and HEM-wood-charcoal pellets were compared; Figure 10 shows the relative comparison of the two.
In Figure 10, there is a sharp inflection point in the middle of the profile, for the case of the PAH-wood-charcoal. The reason for this behavior was found to be the abnormal swelling of the top layer in the early stages of the experiment, followed by its shrinkage towards the later stages. The swelling observation was confirmed during the visual examination of the bed after reduction, for a three-layer arrangement of PAH-wood-charcoal, which will be discussed later. This swelling behavior of the top-layer pellets in the PAH-wood-charcoal curtails the external heat transfer to the middle-layer pellets, thus causing a lower reduction rate. Once the shrinkage of the top-layer pellets begins after the initial swelling phase, the temperature of the middle layer begins to rise, as can be seen in the figure. This kind of phenomenon is, however, absent or rather insignificant during the reduction of HEM-wood-charcoal pellets. Therefore, in that case, the temperature of the middle layer goes up steadily and the bed reduces faster than in the PAH-wood-charcoal scenario. Because of the significant amounts of shrinkage that the pellets undergo, the external heat transport to the middle layer is enhanced for all the pellet types, except for the PAH-wood-charcoal, as discussed earlier. The other kinds of pellets, in the context of the reaction rates, follow a trend similar to that in the top-layer reduction. Thus, the trends exhibited by the other types of pellets relative to one another can be explained on grounds similar to those of the top-layer configuration.
A picture of a reduced three-layer bed of PAH-wood-charcoal is shown in Figure 11. The bottom layer is clearly seen to undergo substantial swelling (shown by the dotted circle), while the top and middle layers undergo shrinkage after swelling. The bottom layer does not see temperatures high enough, which is why it does not shrink. The other combinations of oxide and carbon examined did not show any swelling effects similar to those shown by the PAH-wood-charcoal. It is exactly this behavior of PAH-wood-charcoal pellets that is responsible for the limitations in external heat transport to the middle and lower layers that stunt the reduction rate. This abnormal swelling of the PAH-wood-charcoal pellets was found to occur due to the formation of iron whiskers.[10]
Rate-Controlling Mechanisms
A schematic of the primary rate-controlling steps for each layer of a multilayer bed, as a function of process time, is represented in Figure 12. The rate-controlling mechanisms for each arrangement have been classified into three time regimes: initial, intermediate, and final. The different rate-limiting steps have been arranged in order of importance; it should be noted, however, that the relative importance of these steps is a function of the nature of the raw materials in the pellets, as well.
The representation in Figure 12 indicates the importance of the chemical-kinetic steps and the external and internal heat-transport steps during the initial stages, for all three pellet layers. It is to be noted that the chemical kinetics is more important during the initial stage of reduction of a single layer of pellets, particularly in the ore-containing pellets. Internal heat-transfer mechanisms may be more important than chemical kinetics in the initial stages of the reduction of a single layer of PAH-containing pellets. During the intermediate stage of the reduction of a single layer, the external heat transport becomes quite fast, as the reduction temperatures are attained rapidly, due to the high heating rate. In the final stages, due to the shrinkage of the pellets and the formation of metallic iron, the internal heat transport is greatly enhanced. Thus, the chemical kinetics is expected to control the rate at those stages.
For the reduction of the middle layer, the external heat transport is expected to be the most dominant during the early and intermediate stages, because it takes some time for the top layer to start reducing and shrink. In the case of PAH-wood-charcoal, the top layer swells first, before shrinking. Thus, the external heat-transport processes are likely to be the most important rate-limiting step under these conditions, as long as the top layer has not shrunk appreciably. Once the top layer has reduced and shrunk to a considerable extent, the external heat transport to the middle layer improves; thus, the chemical kinetics and internal heat transfer become more important rate-controlling steps during the intermediate stage. In the later stages, the chemical kinetics controls the rate, similar to what occurs in the single-layer reduction. A similar explanation can be furnished for the reduction of the bottom layer.
Impact of Degree of Reduction on Productivity
An important observation is the time that it takes to reach an approximately 95 pct reduction and an approximately 70 pct reduction of a multilayer bed. It was observed that, for all the bed arrangements, it takes approximately 1.5 to 2 times more time to reach a 90 to 95 pct reduction than it takes to attain a 70 pct reduction. This is quite evident from the two columns for t 70 pct and t 90 pct in Table I. Thus, if the target degree of the reduction in the RHF is decreased from 95 to 70 pct by virtue of having a bath smelter for processing the DRI, the productivity of the RHF can be nearly doubled. The choice of the optimum target degree of reduction in the RHF also depends on the processing times and economics of the smelter operations.
Optimal Pellet Composition and Bed Arrangement
Assuming a final reduction degree of 70 pct, Table I tabulates the productivity-per-unit time for the different composite pellets for the different bed arrangements. It is further assumed that the output (tonnage) varies linearly with the number of layers of the bed. The output-per-unit time is computed by dividing the number of layers by the time taken to reach 70 pct reduction for the designated number of layers. The normalized output for each case refers to the output normalized by the lowest output for a certain combination and layer configuration at a certain degree of reduction. The same has been done assuming a final reduction degree of 90 pct. Since the output-per-unit time to attain either a 70 or a 90 pct reduction is the least for a single layer of taconite-coal-char pellets, the same has been used to compute the normalized outputs shown in the last two columns of the table.
Table I suggests that using a two- or three-layer bed is more advantageous than using a single-layer bed, for the conditions in which the target degree of reduction is either 70 or 90 pct. This indicates that, even with a three-layer bed, 90 pct reduction can still be achieved without significantly compromising the reduction time. This is possibly because of the enhancement of the external heat transport to the lower layers of the bed, as a result of pellet shrinkage. Had the shrinkage of the pellets been “insignificant,” it would have taken much longer for two- and three-layer beds to reduce completely than it took under the present conditions. In that scenario, it would be expected that the output-per-unit time for a two-layer or three-layer bed would be lower than for a single layer under those conditions. Thus, pellet shrinkage is immensely important. Of all the composite pellets, the PAH-coal-char reduced the fastest; PAH, however, is not used in the industry. The TAC-wood-charcoal, HEM-wood-charcoal, and HEM-coal-char perform reasonably well, compared to the others. It may also be seen that the two-layer reduction is more beneficial than the three-layer bed, for HEM-wood-charcoal. Therefore, there is an optimum number of layers that comes into the picture because of the external heat-transfer limitations. This optimum number will depend on both the pellet composition and the thermophysical properties of the pellets.
Reduction Front during the Reduction
Figure 13 shows the diametrical cross section of a partially reduced pellet of PAH-coal-char reduced for 6.5 minutes obtained after an interrupted experiment in the RHF simulator. The figure is indicative of the fact that the reduction front moves from the top half of the pellet toward the bottom half. This is expected, due to the unidirectional mode of the radiation heat transfer from the lamps to the pellets. The top half of the pellet shows a shiny metallic iron layer, while the bottom of the pellet shows a dark region that is likely to be a mixture of hematite and magnetite. There is a middle band (gray), which is probably comprised of wüstite and magnetite mixed together. It may also be seen that prominent cracks are formed only in the upper side of the pellets, which is further indication that the reduction begins from the top portion of the pellet and proceeds downward. As a result, it may be inferred that the temperature distribution around the pellets is not spherically symmetric. The top hemisphere is exposed to the high-temperature environment earlier than the bottom hemisphere. Thus, the reduction initiates in the top half and gradually proceeds downward, as the thermal energy gets conveyed across the pellet.
Conclusions
A unique experimental apparatus was fabricated to simulate the external heat-transfer phenomenon inside an RHF on a laboratory scale. The apparatus employed IR heating to simulate the high heating rates achieved in the RHF by the radiation from the furnace refractory and burner flame. Off-gas analysis was used to compute the reaction rates and the degree of reduction as a function of time. The reduction times obtained during the experiments were found to be in reasonable accord with the residence times for RHFs operated under similar temperature environments. The following conclusions could be drawn from this part of the work.
-
1.
The reduction of Fe2O3 to metallic iron proceeds along a stepwise sequence according to which Fe2O3 is reduced to Fe3O4 in the first stage, followed by the subsequent reduction of Fe3O4 to FeO and of FeO to metallic iron. This was verified from the evolution of the off-gas composition during the reduction of the composite pellets.
-
2.
During the reduction of composite pellets, the overall reaction kinetics is controlled jointly by four rate-controlling mechanisms:
-
a.
the kinetics of the carbon oxidation by CO2;
-
b.
the chemical kinetics of the wüstite-reduction reaction by CO;
-
c.
the external heat transport to the pellets; and
-
d.
the internal heat transfer within the pellets.
-
a.
-
3.
The reduction of wood-charcoal-containing pellets yields much more CO in the off-gas than the in coal-char-containing pellets because of the higher rate of carbon oxidation for wood charcoal than for coal char.
-
4.
During the top-layer reduction of composite pellets, the external heat transport is usually much faster than the chemical-kinetic mechanisms. Under such circumstances, the kinetics of the carbon oxidation and wüstite reduction, along with internal heat transport, is likely to be the major rate-limiting step.
-
5.
The PAH-coal-char pellets were found to reduce faster than the PAH-wood-charcoal pellets, despite the higher reactivity of wood charcoal. This is primarily because of internal heat transfer limitations owing to initial swelling of PAH-wood-charcoal pellets and the lower thermal conductivity of wood charcoal compared to coal char, due to the highly porous structure.
-
6.
In ore-containing pellets, wood charcoal seems to be a faster reductant than coal char. The rate is primarily controlled by the kinetics of the carbon-oxidation reaction.
-
7.
Under conditions in which the external and internal heat-transport steps are comparatively faster, the taconite-containing pellets were found to reduce more slowly than hematite-containing pellets, for a particular reductant. This is consistent with the higher reducibility of hematite in comparison to magnetite because of the development of high internal porosity (caused by cracks and fissure formation) during the transformation of hematite to magnetite.
-
8.
For the two-layer pellet arrangement, PAH-wood-charcoal was found to reduce somewhat slower than HEM-wood-charcoal, contrary to what was observed during the reduction of the single-layer arrangement. The cause of this is that PAH-wood-charcoal pellets undergo significant amounts of swelling before shrinking. It is the swelling of the top-layer pellets in the case of PAH-wood-charcoal that does not allow the temperature of the second layer to rise quickly enough, resulting in a slower reduction rate for the overall bed.
-
9.
As the number of layers of the bed increases, the reduction becomes more limited by the external heat transport to the lower layers of the bed. This is likely to happen during the initial and intermediate stages of the reduction, when the top and middle layers have not shrunk appreciably. Toward the end of the reaction, the role of the external heat transport declines, because the bed becomes quite accessible to the radiation by then.
-
10.
All the composite pellets were found to shrink during reduction, which is critical in determining the external heat-transport conditions.
-
11.
The reduction in the composite pellets proceeds from the top of the pellets and moves toward the bottom because of the unidirectional heat transport in the RHF simulator.
-
12.
The reduction experiments indicated that it takes approximately 1.5 to 2 times more reduction time to attain an average reduction degree of 90 to 95 pct than it takes to reach a reduction degree of 70 pct. Therefore, if the RHF is coupled with a bath smelter, according to the proposed process, the productivity of the RHF can be almost doubled if the target degree of reduction in the RHF is reduced to 70 pct with the remaining reduction accomplished in the smelter.
-
13.
A two- or three-layer bed was undoubtedly found to be more advantageous, as far as productivity is concerned, in comparison to a single layer. This is due to the effect of the shrinkage of the pellets, which tends to enhance the external heat transport to the lower layers of the bed.
Notes
Fisherbrand is a trademark of Fisher Scientific, Pittsburgh, PA.
References
K.N. Pargeter, R.H. Hanewald, D.E. Dombrowski: Conserv. Recycl., 1985, vol. 8, pp. 363–75
R. Degel, O. Metelmann, and H. Lehmkuhler: Electric Furnace Conf. Proc., Iron & Steel Society, Warrendale, PA, 2000, pp. 519–33
Y. Sawa, T. Yamamoto, K. Takeda, H. Itaya: ISIJ Int., 2001, vol. 41, pp. S17–S21
O.M. Fortini: Doctoral Thesis, Carnegie Mellon University, Pittsburgh, PA, 2004
S. Halder and R.J. Fruehan: Metall. Mater. Trans. B, 2008. doi:10.1007/s11663-008-9200-4
O.M. Fortini, R.J. Fruehan: Metall. Mater. Trans. B, 2005, vol. 36B, pp. 709–17
S. Sun, W.-K. Lu: ISIJ Int., 1993, vol. 33 (10), pp. 1062–69
S. Sun, W.-K. Lu: ISIJ Int., 1999, vol. 39 (2), pp. 130–38
I. Sohn: Doctoral Thesis, Carnegie Mellon University, Pittsburgh, PA, 2005
S. Halder: Doctoral Thesis, Carnegie Mellon University, Pittsburgh, PA, 2007
J.O. Edström: J. Iron Steel Inst., 1953, vol. 175, pp. 289–304
R.L. Schultz, P. Fontana, and H. Lehmkuhler: Int. Conf. on New Developments in Metallurgical Process Technology, 1999, pp. 50–57
H. Ichikawa and H. Morishige: SEAISI Q., 2002, pp. 68–75
S. Halder and R.J. Fruehan: Metall. Mater. Trans. B, 2008. doi:10.1007/s11663-008-9201-3
Acknowledgments
The authors thank the member companies of the Center for Iron & Steel Making Research for the financing of this research. The authors express special thanks to the United States Steel Corporation (Pittsburgh, PA) and Companhia Vale do Rio Doce (Novo Lima, MG, Brazil) for the iron ores, to Consol Energy, Inc. for the coal, and to Vallourec & Mannesmann Tubes for the wood charcoal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted April 28, 2008.
Rights and permissions
About this article
Cite this article
Halder, S., Fruehan, R. Reduction of Iron-Oxide-Carbon Composites: Part II. Rates of Reduction of Composite Pellets in a Rotary Hearth Furnace Simulator. Metall Mater Trans B 39, 796–808 (2008). https://doi.org/10.1007/s11663-008-9203-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-008-9203-1