Abstract
A thermodynamic and kinetics investigation on the oxidation of MoS2 in molybdenite concentrate to MoO2 by water vapor was carried out as part of new process development. The kinetics of the reaction were determined by measuring the weight change of a sample with time in water vapor at temperatures between 700 °C and 1000 °C. The reaction rate followed the shrinking-unreacted-core model under chemical reaction control, which showed activation energy of 102 kJ/mol. In addition, the behavior of rhenium and selenium in molybdenum concentrate during the process was investigated. While most rhenium remained with the molybdenum dioxide during the water vapor oxidation, almost all selenium was volatilized in agreement with thermodynamic analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molybdenite (MoS2) is the major mineral for molybdenum. In the conventional pyrometallurgical process, oxidation roasting is applied to produce MoO3, one of the commercial products containing Mo. However, there are several serious problems in processing molybdenite by this method: Valuable elements in the concentrate such as rhenium and selenium cannot be recovered easily to high degrees and air pollution may occur due to the emission of SO2 gas.[1,2] The rhenium-bearing off-gas is scrubbed and the scrubbing solution is subjected to ion exchange to recover part of the rhenium contained in the concentrate. However, this process is expensive and has poor recovery efficiency.
Sohn[3–6] and Hakobyan[7] investigated a water-vapor oxidation process as an alternative to the conventional roasting process. This new process offers the possibility of lower emission of sulfur containing pollutants by recovering the sulfur in MoS2 in an elemental form. It also makes it easier to extract valuable minor elements from the ore.
The major objective of this research was to study the feasibility of this new process and to determine the kinetics of oxidation of MoS2 by water vapor.
Experimental
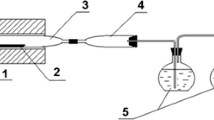
The experimental setup used in this study is shown in Figure 1. It consisted of a water-vapor feeding system with an evaporator, a movable vertical tube furnace with vertical trail, a reactor, and an off-gas system with a NaOH-solution scrubber. The reactor had a double wall: the outer wall was a stainless steel tube and the inner wall was a mullite tube with 45-mm inner diameter, which prevented hydrogen generation from the reaction between water vapor and stainless steel at high temperatures.
The sample used in this study was a molybdenite concentrate containing 80 pct MoS2 screened to – 60 mesh size. Figure 2 shows the size distribution of the sample measured using a Beckman Coulter laser diffraction analyzer model LS230 (Beckman Coulter, Inc., Fullerton, CA). This figure also shows the size distribution of the oxidation products to be discussed subsequently. The chemical composition of the concentrate is summarized in Table I. The sample was homogenized and kept in a desiccator after being dried to remove moisture and a small amount of oil used for flotation.
A layer of sample powder with a thickness of 1 mm was placed in an alumina tray and put into the vertical reactor. The reactor, purged with Ar gas, was positioned in the furnace by pulling up the furnace after the temperature of the furnace reached the desired temperature. Then, the Ar purging was stopped after the temperature of the sample was brought to the desired value, and liquid water was injected into the reactor for a predetermined length of time. All the water is evaporated in the top part of the reactor to 86.7 kPa pressure, the atmospheric pressure at Salt Lake City. The weight change during the reaction was measured from the weights of the solid sample before and after the reaction. Chemical analysis and microstructure observation were performed using X-ray diffraction (XRD), inductively coupled plasma (ICP), and scanning electron microscopy (SEM).
Results and Discussion
Thermodynamics
The equilibrium compositions in the MoS2-H2O (g) system at various temperatures were calculated using HSC Chemistry software developed by Outokumpu Research Oy, which is based on the principle of Gibbs free energy minimization. Figure 3 shows the equilibrium amounts of the major species present when 1 mol of MoS2 is reacted with 100 mol of H2O (g). It is seen that the main solid product at high temperatures is MoO2 rather than MoO3, which is the stable phase in a conventional roasting process using oxygen gas, and that the gaseous product is a mixture of H2 (g), H2S (g), and SO2 (g). Thus, the reaction between MoS2 and H2O can be represented by
the ratios among the gaseous products determined by thermodynamics at equilibrium and also by kinetics in general. According to this analysis, there is a possibility that elemental sulfur can be recovered from the off-gas by the Claus process and that hydrogen can be recovered as a byproduct. A detailed analysis including the amounts of gaseous products was beyond the scope of this work.
To predict the behavior of Re and Se in the MoS2 concentrate, the equilibrium compositions of Re and Se compounds in 1 mol MoS2-100 mol H2O (g)-0.001 mol ReS2-0.001 mol Se were calculated, as shown in Figure 4. While the stable Re phases are elemental Re at high temperatures and ReS2 at low temperatures, those of Se are gaseous phases such as SeS (g), SeH (g), and Se2 (g). Thus, it is expected that Re will remain in solid products after the water vapor oxidation and that Se should be collected from the off-gas system. (The result in Figure 4 shows just the lower part of the overall equilibrium calculation. Because of the low concentrations of the species shown in this part in the presence of the dominant species consisting of Mo, H, S, and O, the amounts shown here contain some round-off errors exemplified by the absence of any rhenium between 800 °C and 855 °C.)
Kinetics of Oxidation of Molybdenite by Water Vapor
Kinetic experiments were conducted by measuring the weight change of a sample with time in an environment containing 100 pct water vapor. The weight change during the reaction was measured from the weights of the solid sample before and after each run. Continuous weight measurement was difficult in this work for various reasons including the problem of vaporizing water without generating a pressure pulse. Pulsed water vapor flow is no problem in a batch run made in the absence of mass-transfer effects. It is important in determining the reaction rate of individual concentrate particles to eliminate the effect of external mass transfer between the bulk gas and particle surface as well as the effect of interparticle diffusion. Thus, the experiments were performed to determine the minimum water feeding rate above which a further increase does not affect the reaction rate, thus indicating the elimination of external mass-transfer effect. The interparticle diffusional effect was eliminated by using a shallow layer of particles, as mentioned previously. Figure 5 shows the results for five different water feeding rates at 1000 °C using approximately 300-mg samples. As the water feeding rate increased, the conversion rate increased due to the mass-transfer effects. However, the rate for 4 mL/min was essentially the same as that for 6 mL/min, which indicated that above 4 mL/min of water feeding rate, the mass transfer is sufficiently fast and thus does not affect the reaction rate. Based on this result, the feeding rate of 4 mL/min was applied to other experiments. The product of these experiments was MoO2, as shown in Figure 6, which is consistent with the thermodynamic analysis. The size distribution of the MoO2 particles thus produced is shown in Figure 2.
The effect of reaction temperature on the overall reaction rate was investigated with the water feeding rate of 4 mL/min and at six different temperatures. The weights of the samples were all approximately 300 mg except for the cases of 120-min reaction time in which approximately 800-mg samples were used. It was determined that the sample amount (even a 2-g sample) did not affect the conversion vs time relationship in the range tested. This condition must be met when the objective is to determine the kinetics unaffected by interstitial diffusion and external mass transfer. The results are shown in Figure 7 for the temperature range 700 °C to 1000 °C. As expected, the conversion rate increased with temperature. These results were applied to a shrinking-unreacted-core rate model.[8] This rate expression relates the conversion to the reaction time by
where k app is the apparent rate constant, t is the reaction time, X is the conversion, and F p is the shape factor whose value is 1, 2, and 3, respectively, for flat plates, long cylinders, and spheres (or equidimensional in all directions). To determine the shape factor, the morphologies of MoS2 and MoO2 were investigated by the use of SEM, as shown in Figure 8, which indicated that the shape was almost equidimensional in all directions. Thus, the value of 3 was chosen as the shape factor for these samples. The conversion data in Figure 7 were plotted according to Eq. [2] in Figure 9, which shows a straight line at each temperature. (The three highest conversion values were calculated to be slightly greater than 100 pct, because of small uncertainties in experimental measurements. Such points cannot be displayed in Figure 8 due to the nature of the vertical scale.) This rate equation was further verified after trying a number of different rate models such as the shrinking-core model with different shape factors and the nucleation-and-growth model. The values of k app were obtained from the slopes of the straight lines in Figure 9 and were subjected to an Arrhenius plot yielding an activation energy value of 102.4 kJ/mol, as shown in Figure 10.
Conversion-time curves prepared from Fig. 7 according to Eq. [1]: • 700 °C, ■ 800 °C, ▴ 850 °C, ○ 900 °C, □ 950 °C, and △ 1000 °C
Behavior of Rhenium and Selenium
To determine the behavior of rhenium and selenium, their concentrations relative to Mo in the MoS2 concentrate and MoO2 samples produced from it were measured by ICP. The MoO2 samples were prepared under the condition of 950 °C reaction temperature, 3.5 hours of reaction time, and 4 mL/min of water feeding rate to obtain essentially 100 pct conversion. While most of the rhenium remained in the MoO2 sample after the water vapor oxidation, almost all of the selenium disappeared from the sample, as shown in Table II. These experimental results agreed well with the thermodynamic analysis, that is, Re remained in the MoO2 product as a solid compound and Se was evaporated as gaseous compounds from the solid sample. Thus, Re can be extracted from this solid product without a serious loss during the oxidation process and Se can be extracted from the off-gas scrubber.
Treatment of the Sulfur-Containing Off-Gas
The equilibrium off-gas from the oxidation of MoS2 with water vapor contains H2S and SO2, as can be seen in Figure 3. When the gas is separated from the solid and cooled, the ratio of H2S and SO2 contents becomes higher, which was confirmed by thermodynamic calculations.[9] These gases can then be converted to elemental sulfur by the following Claus reaction:
A more detailed description of this topic in conjunction with the development of a process based on the treatment of molybdenite concentrates is the topic of another article.[9]
Conclusions
The product of water-vapor oxidation of molybdenum sulfide at high temperature was MoO2, which is consistent with the thermodynamic analyses. Kinetic analyses showed that the shrinking-unreacted-core model under chemical reaction control was applicable to the reaction of molybdenum sulfide with water vapor at high temperatures. This reaction had an activation energy of 102.4 kJ/mol. Rhenium in the molybdenum sulfide concentrate remained in the solid product, and selenium was evaporated as gaseous selenium compounds.
References
C.K. Gupta: Extractive Metallurgy of Molybdenum, CRC Press, Inc., Boca Raton, FL, 1992, pp. 67-72
D. Kim: Ph.D. Thesis, University of Utah, Salt Lake City, UT, 1980
H.Y. Sohn: U.S. Patent No. 4,376,647, Mar. 15, 1983
H.Y. Sohn D. Kim: J. Met., 1984, vol. 36 (1), pp. 67–73.
H.Y. Sohn D. Kim: Metall. Trans. B, 1987, vol. 18B, pp. 451–57.
H.Y. Sohn D. Kim: Metall. Trans. B, 1988, vol. 19B, pp. 973–75
K. Hakobyan and A. Hakobyan: Eurasia Patent No. 002417, Aug. 12, 1998
J. Szekely J.W. Evans H.Y. Sohn: Gas-Solid Reactions, Academic Press, Inc., New York, NY, 1976, pp. 73–89
K.Y. Hakobyan, H.Y. Sohn, A.K. Hakobyan, V.A. Bryukvin, V.G. Leontiev, and O.I. Tsibin: Trans. Inst. Mining Metall., Sec. C, in press
Acknowledgment
This work was supported by the United States Civilian Research and Development Foundation under Project No. AE2-2526-KA-03.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted April 19, 2006.
Rights and permissions
About this article
Cite this article
Blanco, E., Sohn, H.Y., Han, G. et al. The Kinetics of Oxidation of Molybdenite Concentrate by Water Vapor. Metall Mater Trans B 38, 689–693 (2007). https://doi.org/10.1007/s11663-006-9001-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-006-9001-6