Abstract
Two conventionally solidified Al-0.2Ti alloys (with 0.18 and 0.22 at. pct Ti) exhibit no hardening after aging up to 3200 hours at 375 °C or 425 °C. This is due to the absence of Al3Ti precipitation, as confirmed by electron microscopy and electrical conductivity measurements. By contrast, an Al-0.2Zr alloy (with 0.19 at. pct Zr) displays strong age hardening at both temperatures due to precipitation of Al3Zr (L12) within Zr-enriched dendritic regions. This discrepancy between the two alloys is explained within the context of the equilibrium phase diagrams: (1) the disparity in solid and liquid solubilities of Ti in α-Al is much greater than that of Zr in α-Al; and (2) the relatively small liquid solubility of Ti in α-Al limits the amount of solute retained in solid solution during solidification, while the comparatively high solid solubility reduces the supersaturation effecting precipitation during post-solidification aging. The lattice parameter mismatch of Al3Ti (L12) with α-Al is also larger than that of Al3Zr (L12), further hindering nucleation of Al3Ti. Classical nucleation theory indicates that the minimum solute supersaturation required to overcome the elastic strain energy of Al3Ti nuclei cannot be obtained during conventional solidification of Al-Ti alloys (unlike for Al-Zr alloys), thus explaining the absence of Al3Ti precipitation and the presence of Al3Zr precipitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Group 4 transition metals (Ti, Zr, or Hf) constitute a group of alloying additions to Al that show particular promise for developing creep-resistant, thermally stable Al-based alloys.[1] In each of these systems, an ordered Al3M (M = Ti, Zr, or Hf) trialuminide may be precipitated from a supersaturated solid solution during post-solidification aging. While the equilibrium structure of these Al3M trialuminides is tetragonal (D022 or D023), decomposition of supersaturated Al-M solid solutions occurs initially by the formation of nanometer-scale metastable cubic Al3M (L12) precipitates exhibiting small lattice parameter mismatches with α-Al, which transform to their respective equilibrium tetragonal structures after prolonged aging (approximately 100 to 1000 hours) at elevated temperatures (>450 °C). Moreover, these transition elements are anomalously slow diffusers in α-Al, with very limited equilibrium solid solubilities, enabling precipitated Al3M to be resistant to Ostwald ripening in accordance with volume diffusion-controlled coarsening models.[1]
For high-temperature applications, Ti seems especially promising as an alloying addition among the Group 4 elements because it has the smallest atomic weight and the smallest diffusivity in α-Al.[1] Indeed, Al alloys reinforced by Al3Ti dispersions have garnered considerable interest as potential lightweight, high-temperature structural materials, and the thermal stability and strength at high temperatures of these alloys is well documented.[2–18] These studies, however, investigated alloys prepared by rapid solidification processing (RSP)[2–8] or mechanical alloying (MA)[2,9–18] techniques, whose nonequilibrium processing routes circumvent the difficulties encountered during conventional solidification of Al-Ti alloys.
The problems with casting these alloys arise from the fact that Al3M (M = Ti, Zr, or Hf) exhibits a peritectic phase equilibrium with the terminal α-Al solid solution. The first solid to form under equilibrium conditions is the properitectic, or primary, Al3M ordered phase (for alloys in the peritectic composition range, i.e., those enriched beyond the minimum liquid solubility of solute). These solute-rich primary phases readily nucleate and grow into coarse (approximately 100 μm) precipitates during conventional casting, leaving the remaining melt, and ultimately the solidified α-Al solid solution, substantially depleted in solute. This limits the potential for subsequent precipitation strengthening because the supersaturation—and therefore the chemical driving force for solid-state nucleation, as well as the equilibrium volume fraction of the dispersed phase, if formed—is significantly reduced.
Under certain conditions, primary Al3M may solidify from the melt with the metastable cubic L12 structure.[19] These metastable particles are isostructural to, and exhibit a small lattice parameter mismatch with, the α-Al solid solution, and therefore act as efficient heterogeneous nucleants during solidification of α-Al. The resulting grain refinement in cast alloys is frequently exploited industrially by Ti additions to Al[19–22] and has also been observed in Al-Zr[19,23,24] and Al-Hf[25–27] alloys when primary precipitation of Al3M (L12) occurs. The refined grain size, however, is undesirable for applications where creep resistance is required.
This study examines the feasibility of developing conventionally cast and aged, precipitation-strengthened, dilute Al-based alloys alloyed with the Group 4 elements Ti and Zr, exhibiting a coarse grain size suitable for creep-resistant applications. Most studies related to conventionally solidified Al-Ti alloys have focused on the thermal stability of the relatively coarse primary Al3Ti phase formed during solidification.[28–32] As noted in References 33 and 34, the scientific literature pertaining to decomposition or age hardening of Al-Ti solid solutions produced by conventional ingot metallurgy processing is rather limited. The primary objective, therefore, is to report and discuss the precipitation behavior in dilute Al-Ti alloys, with the data for Al-Zr provided primarily as a comparison. In a subsequent article,[35] we report in detail on the precipitation behavior and stability of Al3Zr in conventionally solidified Al-Zr alloys.
2 Experimental procedures
2.1 Alloy Compositions and Preparation
Two Al-Ti and one Al-Zr alloys were cast, with both solutes nominally alloyed at the 0.2 pct level (all compositions are given in at. pct); the exact alloy compositions are Al-0.18Ti, Al-0.22Ti, and Al-0.19Zr, with an estimated error of ±0.01 at. pct, as indicated in Table I. Small (approximately 7 g) buttons of these alloys were prepared by arc melting in a gettered purified argon atmosphere using a nonconsumable tungsten electrode and a water-cooled copper cathode as the crucible. The charges were melted a minimum of 10 times and inverted between melts to ensure alloy homogeneity. The moderately enhanced cooling rate associated with the chilled crucible was estimated to be of the order of 10–100 °C s−1. For dilute Al-Ti and Al-Zr alloys, as well as other peritectic systems, to a specific cooling rate corresponds a critical solute concentration below which primary precipitation of Al3M (M = Ti or Zr) does not occur.[36–38] The compositions in Table I were expected, based on prior solidification studies (Figure 8, discussed subsequently), to be near the threshold for avoiding primary precipitation of Al3M for the moderate cooling rates used here.
Both Al-Ti alloys were prepared by melting 99.95 at. pct Al (Atlantic Equipment Engineers, Bergenfield, NJ, and containing 257 at. ppm Fe and 260 at. ppm Si as impurities) with a monolithic Al3Ti master alloy. A primary advantage of this approach is that the melting point of Al3Ti (1350 °C) is lower than that of pure Ti (1688 °C). Additionally, this ordered structure is a line compound whose composition is entirely homogeneous. This is not true of commercial master alloys (containing typically 5 to 10 wt pct Ti) that exhibit coarse (approximately 100 μm) primary Al3Ti precipitates. For the dilute alloys in this investigation and the small mass of the button ingots, such inhomogeneous master alloys introduce unacceptable uncertainties in chemical composition. The monolithic Al3Ti master alloy was prepared by arc melting the requisite amounts of pure constituents (99.95 pct Al, Atlantic Equipment Engineers; 99.99+ pct Ti, Alfa Aesar, Ward Hill, MA) corresponding to the stoichiometry of the Al3Ti phase; chemical homogeneity and structural uniformity of the button ingot was verified using X-ray diffraction.
The Al-0.19Zr alloy was prepared from a dilute master alloy containing 1.9 wt pct Zr, which was dilution-cast from a commercial 10 wt pct Zr master alloy (KB Alloys, Reading, PA). The chemical compositions of both Al-Ti alloys were verified independently by Bodycote Materials Testing (Skokie, IL) and ATI Wah Chang (Albany, OR), while the composition of Al-0.19Zr was checked by ATI Wah Chang only.
2.2 Aging Treatments and Analytical Techniques
Prior to post-solidification aging, sections of each alloy were examined by optical microscopy and scanning electron microscopy (SEM) to verify the as-solidified microstructure (porosity and grain size) and to check for the presence of properitectic, or primary, Al3M precipitates. The SEM micrographs of the as-solidified specimens were obtained with an Hitachi S-3500 instrument (Hitachi High Technologies America, Inc., Pleasanton, CA) operated at an accelerating voltage of 20 kV, using a secondary electron detector. The as-cast alloys were isothermally aged at 375 °C or 425 °C, within the range of temperatures reported to exhibit a strong age hardening response for Al-Ti alloys produced by RSP[4,6,39,40] or MA[10] and for Al-Zr alloys produced by RSP[34,41–44] or chill casting.[45,46] Precipitation of Al3Ti or Al3Zr during aging was monitored by a number of techniques, described subsequently.
First, Vickers microhardness measurements were performed at room temperature on metallographically polished sections using a load of 200 g and a dwell time of 5 seconds. Second, precipitation of Al3Ti or Al3Zr was assessed directly by transmission electron microscopy (TEM). The TEM foils were prepared by mechanical grinding sections of aged specimens to a thickness of approximately 100 μm. Discs of 3-mm diameter were punched from these sections and thinned to perforation by twin-jet electropolishing at 20 Vdc (Struers TenuPol-5Footnote 1) using a 10 vol pct solution of perchloric acid in methanol at −40 °C. Conventional TEM was performed on a Hitachi H-8100 operating at 200 kV or a PHILIPSFootnote 2 CM 30 operating at 300 kV, using a double-tilt stage. Electropolished TEM foils were also examined using a LEOFootnote 3 1525 high-resolution field-emission gun scanning electron microscope (SEM) operated at 3 kV with a short working distance (3 mm) using a secondary electron in-lens detector. As discussed subsequently, the alloys are highly segregated on the micrometer scale, which is not easily discerned in TEM. The advantage of SEM as a complement to TEM is the much larger field-of-view from the larger range in magnification, which is compounded by the fact that there is no limitation that the foil be electron transparent. Furthermore, the resolution of the LEO 1525 is capable of resolving nanometer-scale Al3Ti or Al3Zr precipitates.
Finally, for the Al-Ti alloys, which did not exhibit detectable age hardening or evidence for precipitation of Al3Ti by electron microscopy (SEM and TEM), electrical conductivity measurements were also performed to monitor the decomposition of the supersaturated solid solutions during extended aging at 425 °C. These measurements were performed using a SIGMATEST 2.069 (Foerster Instruments, Pittsburgh, PA) eddy current apparatus at room temperature. Five measurements were recorded, each corresponding to a different frequency (60, 120, 240, 480, and 960 kHz), on each specimen. For consistency, a single specimen of each alloy (aged for various times) was used for conductivity measurements.
3 Experimental results
3.1 Optical Microscopy
Figure 1 shows the as-cast macrostructure of the alloys studied. The solidification macrostructure is typical of cast alloys, with coarse columnar grains, originating at the bottom surface of the ingot (which was in contact with the chilled copper crucible of the arc melter), growing upward toward a zone of equiaxed grains at the center of the ingot. The relative sizes of the columnar and equiaxed zones is strongly dependent on the solute content of the alloy and, more precisely, the extent of properitectic Al3M (M = Ti or Zr) precipitation because these primary phases are potent grain refiners, as discussed previously.
Alloy Al-0.18Ti is the most dilute of those studied (Table I), and the extent of the columnar zone, which comprises nearly half of the ingot cross section in this alloy, is also greatest. Within the equiaxed zone, the grains are relatively coarse, with diameters ranging from 0.5 to 1.0 mm. The effect of a small increase in Ti concentration in these conventionally solidified alloys is demonstrated for Al-0.22Ti, which exhibits a dramatic refinement in grain size with a fine columnar zone that adjoins an even finer equiaxed region of small (50 to 100 μm) grains in the upper half of the ingot. Moderate grain refinement is also exhibited for Al-0.19Zr, which is intermediate between that of alloy Al-0.18Ti and alloy Al-0.22Ti. The columnar zone of Al-0.19Zr extends for approximately one-third of the ingot cross section, with the bulk of the ingot consisting of equiaxed grains ranging in size from 0.15 to 0.30 mm in diameter.
The pronounced grain refinement observed in Figure 1 for Al-0.22Ti and Al-0.19Zr is due to copious precipitation of properitectic Al3Ti or Al3Zr precipitates, which were observed in metallographically polished as-solidified specimens by SEM (Figure 2). The primary properitectic precipitates are 10 to 20 μm in diameter and exhibit a petal-like morphology. This morphology is characteristic of the metastable L12 properitectic Al3M phase,[19] whose cubic structure is commensurate with fcc α-Al and acts as an effective heterogeneous nucleant of α-Al during solidification, resulting in the grain refinement observed in Figure 1. The refined grain structure is also apparent in Figure 2, as grain boundaries are apparent in both SEM micrographs. The grain boundaries are decorated with Fe-rich precipitates, confirmed by energy-dispersive X-ray spectroscopy in the SEM, and are thought to be a eutectic constituent formed at the end of solidification, as expected because the impurity Fe concentration is close to the maximum solubility of Fe in α-Al.[47]
SEM secondary electron micrographs of as-solidified (a) Al-0.22Ti and (b) Al-0.19Zr. Both alloys exhibit petal-like primary Al3Ti or Al3Zr precipitates, responsible for the grain refinement observed in Fig. 1. Grain boundaries are visible in both micrographs, and Zr-enriched dendritic cells are apparent in (b)
The presence of the primary precipitates in Figure 2 indicates also that (for the conventional casting conditions used in this study) exceeding approximately 0.2 at. pct solute (Ti or Zr) results in primary precipitation of Al3M and hence does not increase the amount of solute retained in solid solution. In addition to the primary precipitates described previously, Figure 2(b) reveals the Zr-enriched dendrites in as-solidified Al-0.19Zr, which appear lighter due to Z-contrast from backscattered electrons. A similar contrast is not as apparent in Al-0.22Ti (Figure 2(a)) because the difference in atomic number between Al (13) and Ti (22) is less than that of Zr (40). After aging, only the solute-enriched dendritic cells contain precipitates, as described subsequently.
3.2 Age Hardening
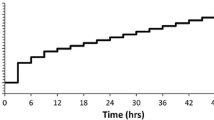
Figure 3 displays the evolution of microhardness of the Al-Ti and Al-Zr alloys upon isothermal aging at 375 °C or 425 °C. Each data point represents a minimum of 20 measurements, with the standard deviation of these measurements indicated by the error bars. At both temperatures, the Al-Ti alloys exhibit a negligible age hardening response after extended aging times up to 3,200 hours (19 weeks). This lack of strengthening contrasts with the significant precipitation hardening response of the Al-Zr alloy, which commences at times as short as 0.1 hours (6 minutes) at 425 °C and achieves peak strength within 25 hours at both 375 °C and 425 °C. As described subsequently, the pronounced strengthening is due to precipitation of small (<10 nm) coherent Al3Zr (L12) precipitates; no evidence for precipitation of a similar Al3Ti phase is observed. The as-cast hardness values of Al-0.22Ti and Al-0.19Zr are both 242 MPa, approximately 20 MPa greater than that of Al-0.18Ti. This moderate strength increase is probably attributable to solid solution strengthening from dissolved Ti or Zr in the as-cast alloys.[48,49] While there is a refinement in the as-cast grain size with increasing solute concentration (Figure 1), Hall–Petch strengthening is not applicable because the scale of the microhardness indent (approximately 125 μm across) is small as compared with the grain size.
3.3 Electron Microscopy
The origin of the precipitation-hardening response indicated in Figure 3 was investigated directly for Al-0.22Ti and Al-0.19Zr using SEM (Figure 4). The dendritic distribution of solute species formed during solidification in both alloys is revealed by preferential etching of the foil’s surface during electropolishing. Figure 4(a) shows the as-cast structure for Al-0.22Ti, where the relief contrast around the dendrite arms is due to variations in solute concentration in solid solution between the dendritic and interdendritic regions of the alloy. The centers of the dendrites are enriched in solute[36,50–52] and are apparently more resistant to electropolishing. Ryum[53] observed similar resistance to electropolishing in the dendritic cells of as-cast specimens of Al-Hf alloys. Figure 4(b) shows the same Al-0.22Ti alloy after aging for 1600 hours at 425 °C. The dendritic cells are delineated as previously, but no evidence for precipitation of Al3Ti within the supersaturated cells is observed, consistent also with the negligible age hardening of the Al-Ti alloys demonstrated in Figure 3. Examination of the same specimen by TEM, both in real space (strain contrast) and reciprocal space (selected area electron diffraction), also provided no evidence for Al3Ti precipitates or any other precipitated phase.
SEM micrographs of electropolished TEM foils. (a) As-cast Al-0.22Ti, showing preferential etching around the solute-enriched dendrites. (b) Al-0.22Ti aged at 425 °C for 1600 h, showing no evidence of precipitation of Al3Ti within the solute-enriched dendrites (black spots are pits from electropolishing). (c) Al-0.19Zr aged at 425 °C for 400 h, showing dendritic distributions of solute similar to panel (a). (d) Magnified view of panel (c), showing Al3Zr dendritic regions with a high number density of small precipitates and interdendritic channels with isolated heterogeneously-nucleated precipitates of larger size
Figures 4(c) and 4(d) show SEM micrographs for Al-0.19Zr aged 400 hours at 425 °C. As with the Ti-containing alloys, dendrites are clearly delineated from the electropolishing process. Unlike the Al-Ti alloys, however, the solute-rich dendrites decompose during aging to form regions with a high number density of homogeneously distributed, nanometer-scale Al3Zr precipitates. Figure 5 shows superlattice dark-field TEM micrographs of the same Al-0.19Zr specimen, exhibiting again precipitate-rich and precipitate-free regions associated with the initial dendritic Zr distribution (Figure 5(a)). Within the center of the dendrites, where the solute supersaturation is largest, the small (<R> = 6.7 ± 1.7 nm) Al3Zr precipitates have the metastable cubic L12 structure and are homogeneously distributed in high number densities. The supersaturation decays with lateral position from the dendrite centers and, correspondingly, the precipitates become progressively larger and occur in smaller number densities (Figures 5(a) and (b)). Figure 5(c) is a complementary bright-field micrograph to that of Figure 5(b), recorded under two-beam conditions. The characteristic Ashby–Brown[54] strain contrast surrounding the Al3Zr (L12) precipitates indicates that they are coherent with α-Al. It is the small, coherent, high number density Al3Zr (L12) precipitates within the dendrites that are responsible for the marked precipitation-hardening response of Al-0.19Zr presented in Figure 3.
TEM micrographs of Al-0.19Zr aged at 425 °C for 400 h. (a) Precipitate-rich dendritic regions separated by a precipitate-free interdendritic channel similar to those in Figs. 4(c) and 4(d). (b) Magnified view of Al3Zr (L12) precipitates near the dendrite periphery. (c) Complementary bright-field image of the region in (b). The strain fields surrounding the precipitates exhibit distinct lines of no-contrast normal to the diffraction vector, g, indicating that the precipitates are coherent with the α-Al solid solution
3.4 Electrical Conductivity
The electrical conductivity of a disordered binary substitutional alloy is a parabolic function of the absolute solute concentration[55] and is therefore a sensitive means of monitoring the decomposition of a supersaturated solid solution during aging. Electrical conductivity measurements were performed on both Al-Ti alloys (Al-0.18Ti and Al-0.22Ti) for extended aging times up to 1600 hours at 425 °C. No detectable change in conductivity was observed, indicating that solute atoms remained dissolved in supersaturated solid solution and did not precipitate as Al3Ti, corroborating the lack of precipitation observed by hardness and electron microscopy.
There was insufficient material to perform electrical conductivity measurements for the present Al-0.19Zr alloy. In a subsequent article,[35] however, we monitor the decomposition of more-dilute Al-0.1 at. pct Zr alloys during isochronal aging, during which a significant increase in electrical conductivity is observed to accompany precipitation of Al3Zr. Moreover, Royset and Ryum[56] recently monitored in detail the decomposition kinetics of dilute Al-0.12 at. pct Sc alloys using a similar eddy current apparatus, indicating that the technique is sensitive enough to detect precipitation of Al3M in dilute alloys.
4 Discussion
The hardness data in Figure 3 demonstrate that the Al-0.19Zr alloy exhibits pronounced precipitation hardening at 375 °C and 425 °C, unlike the Al-Ti alloys with similar solute concentrations. The strong age hardening response of the Zr-containing alloy is due to the precipitation of small (<10 nm) coherent Al3Zr (L12) precipitates, shown by SEM (Figures 4(c) and 4(d)) and TEM (Figure 5). No evidence for precipitation of a similar Al3Ti phase is observed in the Al-Ti alloys by SEM or TEM. Moreover, there is no appreciable change in electrical conductivity after aging Al-0.18Ti or Al-0.22Ti at 425 °C for 1600 hours, which indicates that the Ti concentration in α-Al solid solution is unchanged, confirming the lack of precipitation of Al3Ti.
4.1 Diffusion Kinetics
The measured tracer diffusivity of Ti in α-Al is smaller than that of Zr in α-Al at the aging temperatures employed, and it is conceivable that sluggish diffusion might account for the lack of Al3Ti precipitation. The diffusivities, D, of Ti or Zr in α-Al are given by an Arrhenius relationship:
where Rg is the ideal gas constant, T is the absolute temperature, Q is the activation enthalpy for solute diffusion, and D 0 is the pre-exponential factor. With values Q = 260 and 242 kJ mol−1 and D 0 = 1.12 × 10−1 and 7.28 × 10−2 m2 s−1 for Ti and Zr, respectively,[1] the diffusivity of Ti at 425 °C (3.90 × 10−21 m2 s−1) is comparable to that of Zr at 375 °C (2.26 × 10−21 m2 s−1). The hardness curve in Figure 3(a) demonstrates that the Al-Zr binary alloy reaches near-peak hardness by 6.25 hours of aging at 375 °C, which corresponds to a root-mean-squared (RMS) diffusion distance, \( {\sqrt {4Dt} } \) (where t is the aging time), of 14 nm for Zr atoms. For the slower-diffusing Ti atoms, a similar RMS diffusion distance can be achieved after 115 hours at 375 °C or 4 hours at 425 °C, indicating that Ti atoms diffuse fast enough to support the nucleation and growth of Al3Ti precipitates. Atom-probe tomography studies on Al-Sc-Ti alloys aged at 300 °C [57] and Al-Zr-Ti alloys aged at 375 °C or 425 °C [58] demonstrate that Ti segregates to the Al3(Sc1−x Ti x ) and Al3(Zr1−x Ti x ) precipitates, further evidence that the limited mobility of Ti is not the limiting factor explaining the lack of Al3Ti precipitation.
4.2 Classical Nucleation Theory
The inability to nucleate Al3Ti is a result of an insufficient supersaturation of Ti in solid solution following conventional solidification. Figures 6 and 7 display dilute equilibrium binary phase diagrams for the Al-Ti and Al-Zr systems based on experimental data. From a casting standpoint, a suitable alloying element with Al should produce a low liquidus temperature or, equivalently, exhibit high solubility in the liquid. This liquidus criterion minimizes, for a given alloy composition, the temperature required to form a single-phase melt and also reduces the tendency for primary phase precipitation during solidification.[1] For precipitation strengthening, limited solid solubility is required to maximize the chemical driving force for nucleation as well as the volume fraction of the precipitated phase. As shown in Figures 6 and 7, the disparity in the liquid and solid solubilities of Ti in α-Al is much greater than that of Zr.
The equilibrium phase boundaries in Figures 6 and 7 correspond to the tetragonal-structured trialuminides: D022 for Al3Ti[59] and D023 for Al3Zr.[60] The solubility limits of Ti and Zr in α-Al, in equilibrium with their respective metastable L12 structure Al3M trialuminides, have been evaluated employing ab initio calculations by Liu et al.,[61,62] who show that the ratio of the solid solubilities of the stable and metastable phases may be written as
where \( C^{{{\text{Al}}_{{\text{3}}} {\text{Ti}}}}_{{{\text{ratio}}}} \) and \( C^{{{\text{Al}}_{{\text{3}}} {\text{Zr}}}}_{{{\text{ratio}}}} \) are the ratios of the solid solubility of Al3Ti (L12) and Al3Zr (L12) with their respective equilibrium (D022 or D023) solvus curves, and T is the absolute temperature. Using the equilibrium phase boundaries in Figures 6 and 7 as an accurate reference state, metastable L12 solvus boundaries are calculated using Eqs. [2] and are indicated by the dotted lines in Figures 6 and 7.
According to classical nucleation theory,[63–67] the steady-state nucleation current (nucleation rate per unit volume) is given by
where ΔF * is the net reversible work required for the formation of a critical nucleus, which is given by
The steady-state nucleation current, Eq. [3], is thus a function of the interfacial-free energy, σ; the chemical driving force, ΔF ch ; the reduction of ΔF ch by the elastic strain energy, ΔF el ; and the activation enthalpy for diffusion, Q, for diffusion of the solute. As discussed in Sections 1 through 4, the latter three parameters hinder the nucleation of Al3Ti compared with Al3Zr.
4.2.1 Chemical driving force, ΔFch, for nucleation of Al3M
The metastable L12 solvus boundaries in Figures 6 and 7 demonstrate that the solid solubility of Ti in α-Al is significantly greater than that of Zr, which dictates the chemical driving force for homogenous nucleation of metastable L12 Al3M (M = Ti or Zr). Assuming ideal solution behavior (valid for the dilute concentrations discussed), this driving force is proportional to the natural logarithm of the supersaturation ratio, C 0/C α , where C 0 is the concentration (atomic fraction) of M dissolved in solid solution and C α is the solid solubility of M in α-Al given by Eq. [2]. Following Doherty,[65] the chemical driving force per unit volume driving precipitation of the ordered Al3M phase may be estimated as
where \( C_{{{\text{Al}}_{3} {\text{M}}}} = 0.25 \) is the atomic fraction of solute in the ordered trialuminide, and \( V_{{{\text{Al}}_{{\text{3}}} {\text{M}}}} = {\text{N}}_{{\text{a}}} {\left( {a_{{{\text{Al}}_{{\text{3}}} {\text{M}}}} } \right)}^{3} /4 \) is the molar volume of the precipitated phase (Na is Avogadro’s number and \( a_{{{\text{Al}}_{3} {\text{M}}}} = 0.3967 \) and 0.408 nm for Al3Ti (L12) and Al3Zr (L12), respectively [1]).
Figures 6 and 7 indicate that, for a given solute concentration (C 0), the supersaturation driving precipitation of Al3Zr is significantly greater than that for Al3Ti, which partially explains the absence of precipitation of Al3Ti because of the reduced supersaturation ratio C 0/C α in Eq. [5]. The solute concentration C 0, however, is not constant throughout the alloys because of the segregation visible in the SEM micrographs of Figure 4. For both Al-Ti and Al-Zr, the dendrites are supersaturated with respect to the overall bulk alloy composition, C 0, and so in both cases the local supersaturation of solute in the dendrites is greater than the bulk compositions indicated in Table I and Figures 6 and 7.
The degree of this segregation was not measured directly (e.g., by electron microprobe analysis (EPMA)) but may be estimated from the equilibrium solid-liquid partition coefficient, k 0, that quantifies the difference in the compositions of the solid and liquid phases in local equilibrium during solidification.[68–70] Making the standard assumption that both the liquidus and solidus boundaries are straight lines, k 0 may be expressed as the ratio of the solid and liquid compositions at the peritectic temperature, k 0 = C S /C L . From Figures 6 and 7, k 0 = 10 for Ti, and k 0 = 2.5 for Zr; therefore, the first solid to form is predicted to be enriched by a factor of 10 and 2.5 for Ti and Zr, respectively. This is in reasonable agreement with the results of Bolling and colleagues,[36,50] who measured, using EPMA, the concentration of Ti across the dendritic cells in Al-Ti alloys containing up to 0.28 at. pct Ti and observed a five- to sixfold enrichment in Ti at the dendrite centers. Setiukov and Fridlyander[52] similarly observed that the Ti concentration in the central zones of the dendritic cells exceeded its average value by 6 to 8 times in alloys containing 0.02 to 0.17 at. pct Ti. All present Al-Ti and Al-Zr alloys are therefore locally supersaturated well beyond their respective equilibrium solubilities of solute, and so an insufficient supersaturation of solute, ΔF ch , alone cannot account for the absence of Al3Ti precipitates. As indicated in Eq. [4], the influence of elastic strain energy, ΔF el , must also be considered because this reduces the net driving force for nucleation.
4.2.2 Reduction of the driving force by the elastic strain energy, ΔFel
Solid-state reactions involve strain energy because precipitating a second phase, which is fully or partially coherent with the matrix, requires the straining of the lattices in both the matrix and precipitated phases. This elastic strain energy, ΔF el , diminishes the net driving force, Eq. [4], and hence the rate of nucleation, Eq. [3]. Considering only dilatational strains and assuming elastic isotropy, the strain energy per unit volume for a coherent inclusion is given by[64,67]
where μ = 25.4 GPa[71] and ν = 0.345[72] are the shear modulus and Poisson’s ratio of Al at room temperature. The average lattice parameter mismatches at room temperature, δ, of both the tetragonal (D022 or D023) and metastable cubic (L12) phases are significantly greater for Al3Ti (δ = 2.04 pct and 5.36 pct for L12 and D022, respectively) than for Al3Zr (δ = 0.75 pct and 2.89 pct for L12 and D023, respectively).[1] Thus, the elastic strain energy preventing nucleation of Al3Ti (L12) is more than 7 times greater than that of Al3Zr (L12). The lattice parameter mismatch δ, and thus the elastic strain energy is, however, expected to decrease somewhat at higher temperatures, owing to differences in thermal expansion between the α-Al matrix and the Al3M precipitates.[73,74] Similarly, the shear modulus at higher temperature is somewhat reduced.
4.2.3 Critical solute concentration, C0, to overcome ΔFel
Compared with Al3Zr (L12), nucleation of Al3Ti (L12) is hindered by the following: (1) a reduced chemical driving force (ΔF ch ) for a given C 0; and (2) a greater elastic strain energy opposing nucleation (ΔF el ). To explain why nucleation of Al3Zr (L12) is observed and that of Al3Ti (L12) is not, consider the relative magnitudes of ΔF ch and ΔF el entering Eq. [4] for both systems. The chemical driving force, ΔF ch (Eq. [5]), is calculated substituting C α = 0.0063 for Al3Ti (L12) and C α = 0.0002 for Al3Zr (L12), using Eq. [2] at 425 °C. Substituting the appropriate values for \( V_{{{\text{Al}}_{3} {\text{M}}}} \), ΔF ch = 151.4 ln (158.7C 0) for Al3Ti (L12), and ΔF ch = 141.9 ln (5000C 0) for Al3Zr (L12). The elastic strain energy, ΔF el (Eq. [6]), is 45 MJ mol−1 and 6 MJ mol−1 for Al3Ti (L12) and Al3Zr (L12), respectively. Equating ΔF ch and ΔF el gives a critical solute concentration necessary to overcome the elastic strain energy barrier associated with precipitate nucleation. For Al-Ti alloys, the critical value of C 0 is 0.84 at. pct Ti, and for Al-Zr, it is 0.021 at. pct Zr.
Table I indicates that the bulk compositions of both Al-Ti alloys studied are less than the critical threshold of 0.84 at. pct Ti, whereas Al-0.19Zr is supersaturated well beyond 0.021 at. pct Zr, thus explaining the observed precipitation of Al3Zr (L12) and the lack thereof for Al3Ti. This explanation is not strictly complete because, as discussed, the alloys are segregated and hence locally supersaturated beyond the bulk compositions in Table I. Nevertheless, it is the combination of a small chemical driving force, ΔF ch , coupled with a large elastic strain energy barrier, ΔF el , in the Al-Ti system, as compared with the Al-Zr system, that explains their disparate precipitation behavior.
4.2.4 Activation enthalpy for solute diffusion, Q
Equation [3] indicates that the nucleation current is strongly dependent on temperature. The solute supersaturation (and, correspondingly, ΔF ch ) increases with decreasing temperature (Eq. [2]), while the solute diffusivity D decreases exponentially (Eq. [1]). These competing influences result in an intermediate temperature for optimum nucleation kinetics.[66] As discussed in Section IV–A, the activation enthalpy for solute diffusion, Q, for Ti in α-Al (260 kJ mol−1) is greater than that of Zr (242 kJ mol−1), which increases the temperature for maximum nucleation current for Al3Ti. As discussed, the value of ΔF ch driving precipitation of Al3Ti is significantly less than that for Al3Zr for a given C 0 (Figures 6 and 7). This reduced ΔF ch is exacerbated by the higher Q for Ti diffusion because at the temperatures where Ti is mobile, the supersaturation is not large enough to effect nucleation of Al3Ti.
4.3 Limitations on Increasing the Solute Concentration
The discussion in Section IV–B indicates that an increased concentration of Ti (C 0) beyond those we studied is required for precipitation of Al3Ti. This, however, is not possible under conventional casting conditions. As demonstrated in Figure 1, the marked grain refinement observed with increasing Ti concentration is due to primary precipitation of Al3Ti in the melt, and increasing the Ti concentration does not increase the amount of Ti retained in α-Al solid solution. The inter-relationships among solidification rates, solute concentrations, and solidified microstructures of the Al-Ti and Al-Zr binary systems have been the subject of prior studies,[19,24,36,75,76] which are summarized in Figure 8. These curves show the measured solidification rate, as a function of solute concentration, necessary to suppress primary precipitation of Al3Ti or Al3Zr, thereby obtaining supersaturated α-Al solid solution. Figure 8 indicates that to achieve a concentration of 0.84 at. pct Ti supersaturated in an homogenous α-Al solid solution, required to just offset the elastic strain energy associated with nucleation of Al3Ti (L12), cooling rates of nearly 5 × 104 °C s−1 (based on the data of Hori et al.[75,76]) are required. To achieve a greater driving force for nucleation, still higher cooling rates are required. Nucleation of Al3Zr (L12) requires a small supersaturation to overcome the elastic strain energy barrier, and the critical composition of 0.021 at. pct Zr is readily achieved for even the slowest cooling rates in Figure 8.
4.4 Comparison with Previous Studies
In contrast to the closely related Al-Zr and Al-Hf systems, the precipitation behavior of Al3Ti in Al-Ti alloys has been debated in the scientific literature. As with Al-Zr and Al-Hf alloys, the decomposition sequence of supersaturated Al-Ti solid solutions has been reported to occur first by the precipitation of a metastable Al3Ti (L12) phase, which then transforms to the equilibrium tetragonal (D022) structure after prolonged aging.[1] Asboll and Ryum,[77] however, observed only precipitation of the equilibrium D022 phase in chill-cast Al-0.34 at. pct Ti alloys aged between 400 °C and 550 °C. They also noted that the decomposition kinetics of Al3Ti were sluggish compared with those of Al alloyed with other elements of the same subgroup, Zr or Hf. Pandey and Suryanarayana[78] also compared the decomposition behavior of rapidly solidified Al alloyed with Ti, Zr, or Hf (Group 4 elements) and noted that a metastable Al3M (L12) ordered phase is observed in Al-Zr and Al-Hf alloys but not in Al-Ti alloys. This result agrees with other studies[8,32,43] reporting only precipitation of the equilibrium D022 phase in Al-Ti alloys aged in the broad temperature range of 200 °C to 600 °C.
Other studies report no precipitation during aging of supersaturated Al-Ti solid solutions. Ohashi et al.[79] found no evidence for precipitation of Al3Ti, using microhardness and electrical resistivity measurements, in Al-Ti alloys containing up to 0.45 at. pct Ti produced by RSP during 100 hours of aging in the range of 400 °C to 640 °C. St. John et al.[31] also observed no solid-state precipitation of Al3Ti when aging an Al-1.1 at. pct Ti alloy for 24 hours at 435 °C; their alloy did, however, contain coarse primary Al3Ti precipitates, indicating that the amount of Ti initially in solid solution was reduced.
Nucleation of coherent Al3Ti (L12) precipitates has been reported only for highly supersaturated alloys produced by nonequilibrium means (RSP). Ohashi and Ichikawa[51] observed Al3Ti (L12) precipitates in alloys containing 0.3 to 1.4 at. pct Ti aged at 400 °C to 500 °C. Muddle and co-workers[4,39,80,81] observed precipitation of coherent Al3Ti (L12) in melt-spun alloys containing 2.9 to 3.5 at. pct Ti aged at 300 °C to 500 °C. Similar supersaturations cannot be achieved by conventional casting (Figures 1 and 8) because Ti precipitates in the liquid as properitectic Al3Ti at moderate cooling rates.
4.5 Common Industrial Uses of Ti and Zr Additions to Al
Perhaps the most illustrative comparison of Al-Ti and Al-Zr alloys is found by considering their primary industrial roles. As discussed, minor additions of Ti are used to refine the grain structure in commercial aluminum alloys, where primary Al3Ti precipitates act as heterogeneous nuclei during solidification of the melt.[19–22] A solid-state counterpart to this grain refinement effect is realized when dilute additions of Zr are added to commercial wrought alloys as recrystallization inhibitors.[82–85] Small (20 to 30 nm) coherent Al3Zr (L12) precipitates, formed during post-solidification aging, are effective barriers to grain-boundary migration. Titanium is thus added to form primary precipitates in the melt, whereas Zr is used to form coherent dispersoids precipitated from supersaturated solid solution. This suggests that (1) Zr, compared with Ti, is easier to retain in solid solution during conventional solidification; and (2) Al3Zr, compared with Al3Ti, is more readily precipitated during post-solidification aging. These considerations indicate that the Al-Zr system is a better candidate than the Al-Ti system for developing a castable, precipitation-strengthened alloy.
5 Conclusions
This investigation has compared the decomposition of supersaturated, conventionally cast Al-0.2 at. pct Ti and Al-0.2 at. pct Zr alloys, upon aging at 375 °C or 425 °C. The Zr-containing alloy exhibits a strong age hardening response after about 24 hours at both temperatures, due to copious precipitation of coherent, nanometer-scale Al3Zr (L12) precipitates within the supersaturated dendritic cells of the alloy. Precipitation of a similar Al3Ti phase in supersaturated dendrites is not observed, even for much longer aging times (up to 3200 hours at 425 °C). Compared with Al3Zr, nucleation of Al3Ti (L12) is hindered by the following: (1) a reduced chemical driving force (ΔF ch ) for nucleation (for a given local alloy composition, C 0); (2) a larger elastic strain energy opposing nucleation (ΔF el ); and (3) a greater activation enthalpy for solute diffusion, Q.
Despite Ti enrichment in dendritic regions, the minimum supersaturation to produce solid-state Al3Ti precipitation cannot be achieved in conventionally solidified binary Al-Ti alloys because increasing the Ti concentration beyond the levels we have studied causes crystallization of primary Al3Ti during solidification and thus does not increase the solute available in solid solution for subsequent precipitation. By contrast, the Al-Zr system is more promising for developing a castable, precipitation-hardened alloy with good coarsening and creep resistance.
Notes
TenuPol is a trademark of Struers A/S, Ballerup, Denmark.
PHILIPS is a trademark of Philips Electronic Instruments Corp., Mahwah, NJ.
LEO is a trademark of Zeiss-Leica, Cambridge, England.
References
K.E. Knipling, D.C. Dunand, D.N. Seidman: Z. Metallkd., 2006, vol. 97, pp. 246–65
H. Jones, W.M. Rainforth: Metall. Mater. Trans. A, 2003, vol. 34A, pp. 419–21
J.F. Nie, S. Sridhara, B.C. Muddle: Metall. Trans. A, 1992, vol. 23A, pp. 3193–205
J.F. Nie, A. Majumdar, B.C. Muddle: Mater. Sci. Eng. A, 1994, vol. 179, pp. 619–24
J.F. Nie, B.C. Muddle: Mater. Sci. Eng. A, 1996, vol. 215, pp. 92–103
W.E. Frazier, M.J. Koczak: in High Strength Powder Metallurgy Aluminum Alloys II, G.J. Hildeman, M.J. Koczak, eds., TMS, Warrendale, PA, 1986, pp. 353–66
J.M. Wu, S.L. Zheng, Z.Z. Li: Mater. Sci. Eng. A, 2000, vol. 289, pp. 246–54
Y. Wang, Z. Zhang, W. Wang, X. Bian: Mater. Sci. Eng. A, 2004, vol. 366, pp. 17–24
G.X. Liang, Z.C. Li, E. Wang: J. Mater. Sci., 1996, vol. 31, pp. 901–04
W.E. Frazier, M.J. Koczak: Scripta Metall., 1987, vol. 21, pp. 129–34
G.S. Murty, M.J. Koczak, W.E. Frazier: Scripta Metall., 1987, vol. 21, pp. 141–46
J.A. Hawk, P.K. Mirchandani, R.C. Benn, H.G.F. Wilsdorf: in Dispersion Strengthened Aluminum Alloys, Y.W. Kim, W.M. Griffith, eds., TMS, Warrendale, PA, 1988, pp. 551–72
J.A. Hawk, K.R. Lawless, H.G.F. Wilsdorf: Scripta Metall., 1989, vol. 23, pp. 119–24
J.A. Hawk, J.K. Briggs, H.G.F. Wilsdorf: in Advances in Powder Metallurgy, T.G. Gasbarre, W.F. Jandeska, eds., MPIF, Princeton, NJ, 1989, pp. 285–99
P.K. Mirchandani, R.C. Benn, K.A. Heck: in Lightweight Alloys for Aerospace Applications, E.W. Lee, E.H. Chia, N.J. Kim, eds., TMS, Warrendale, PA, 1989, pp. 33–58
P.K. Mirchandani, D.O. Gothard, A.I. Kemppinen: in Advances in Powder Metallurgy, T.G. Gasbarre, W.F. Jandeska, eds., MPIF, Princeton, NJ, 1989, pp. 161–73
S.H. Wang, P.W. Kao: Acta Mater., 1998, vol. 46, pp. 2675–82
I.C. Barlow, H. Jones, W.M. Rainforth: Acta Mater., 2001, vol. 49, pp. 1209–24
T. Ohashi, R. Ichikawa: Z. Metallkd., 1973, vol. 64, pp. 517–21
F.A. Crossley, L.F. Mondolfo: Trans. AIME, 1951, vol. 191, pp. 1143–48
D.G. McCartney: Int. Mater. Rev., 1989, vol. 34, pp. 247–60
B.S. Murty, S.A. Kori, M. Chakraborty: Int. Mater. Rev., 2002, vol. 47, pp. 3–29
W. Dahl, W. Gruhl, W.G. Burchard, G. Ibe, C. Dumitrescu: Z. Metallkd., 1977, vol. 68, pp. 121–27
S. Hori, S. Saji, A. Takehara: Proc. 4th Int. Conf. on Rapidly Quenched Metals, T. Masumoto, K. Suzuki, eds., The Japan Institute of Metals, Sendai, Japan, 1981, pp. 1545–48
S. Hori, N. Furushiro: Proc. 4th Int. Conf. on Rapidly Quenched Metals, T. Masumoto, K. Suzuki, eds., The Japan Institute of Metals, Sendai, Japan, 1981, pp. 1525–28
S. Hori, N. Furushiro, W. Fujitani: J. Jpn. Inst. Light Met., 1980, vol. 30, pp. 617–25
A.F. Norman, P. Tsakiropoulos: Int. J. Rapid Solid., 1991, vol. 6, pp. 185–213
W.L. Fink, K.R. van Horn, P.M. Budge: AIMME Trans., 1931, vol. 93, pp. 421–39
H.A.F. El-Halfawy, E.S.K. Menon, M. Sundararaman, P. Mukhopadhyay: Metallography, 1979, vol. 12, pp. 257–62
H.A.F. El-Halfawy: Titanium ‘80–Science and Technology–4th Int. Conf. on Titanium, H. Kimura, O. Izumi, eds., TMS, Warrendale, PA, 1980, pp. 1379–87
D.H. St John, L.M. Hogan: J. Mater. Sci., 1980, vol. 15, pp. 2369–75
K. Venkateswarlu, S.K. Das, M. Chakraborty, B.S. Murty: Mater. Sci. Eng. A, 2003, vol. 351, pp. 237–43
N.F. Levoy, J.B. Vander Sande: Metall. Trans. A, 1989, vol. 20A, pp. 999–1019
P. Malek, M. Janecek, B. Smola: Kov. Mater., 2000, vol. 38, pp. 160–77
K.E. Knipling, D.C. Dunand, and D.N. Seidman: unpublished research, 2007
H.W. Kerr, J. Cisse, G.F. Bolling: Acta Metall., 1974, vol. 22, pp. 677–86
H.W. Kerr, W. Kurz: Int. Mater. Rev., 1996, vol. 41, pp. 129–64
D.H. St. John, L.M. Hogan: J. Mater. Sci., 1982, vol. 17, pp. 2413–18
J.F. Nie, B.C. Muddle: Mater. Sci. Eng. A, 1996, vol. 221, pp. 11–21
B.S. You, W.W. Park: Scripta Mater., 1996, vol. 34, pp. 201–05
T. Ohashi, R. Ichikawa: J. Jpn. Inst. Met., 1970, vol. 34, pp. 604–10
V. Dobatkin, V.I. Elagin, V.M. Federov, and R.M. Sizova: Russ. Metall., 1970, pp. 122–27
W.W. Park, T.H. Kim: J. Kor. Inst. Met., 1985, vol. 3, pp. 11–18
W.W. Park, T.H. Kim: Scripta Metall., 1988, vol. 22, pp. 1709–14
R. Ichikawa, T. Ohashi: J. Jpn. Inst. Light Met., 1968, vol. 18, pp. 314–19
T. Sato, A. Kamio, G.W. Lorimer: Mater. Sci. Forum, 1996, vols. 217–222, pp. 895–900
H. Okamoto: Phase Diagrams of Dilute Binary Alloys, ASM INTERNATIONAL, Materials Park, OH, 2002
E. Babic, E. Girt, R. Krsnik, B. Leontic, M. Ocko, Z. Vucic, I. Zoric: Physica Status Solidi A, 1973, vol. 16, pp. K21–K25
E. Sahin, H. Jones: in Rapidly Quenched Metals II, B. Cantor, ed., The Metals Society, London, 1978, pp. 138–46
J. Cisse, H.W. Kerr, G.F. Bolling: Metall. Trans., 1974, vol. 5, pp. 633–41
T. Ohashi, R. Ichikawa: J. Jpn. Inst. Light Met., 1977, vol. 27, pp. 105–12
O.A. Setiukov, I.N. Fridlyander: Mater. Sci. Forum, 1996, vols. 217–222, pp. 195–200
N. Ryum: J. Mater. Sci., 1975, vol. 10, pp. 2075–81
M.F. Ashby, L.M. Brown: Phil. Mag., 1963, vol. 8, pp. 1083–1102
V.L. Nordheim: Ann. Phys., 1931, vol. 9, pp. 641–78
J. Royset, N. Ryum: Mater. Sci. Eng. A, 2005, vol. 396, pp. 409–22
M.E. van Dalen, D.C. Dunand, D.N. Seidman: Acta Mater., 2005, vol. 53, pp. 4225–35
K.E. Knipling, D.C. Dunand, and D.N. Seidman: Microsc. Microanal., 2006, accepted for publication
J.L. Murray: Alcoa, Alcoa Center, PA, personal communication, 2005
J.L. Murray, A. Peruzzi, J.P. Abriata: J. Phase Equil., 1992, vol. 13, pp. 277–91
J.Z. Liu:, Ph.D. Thesis, Northwestern University, Evanston, IL, 2006
J.Z. Liu, G. Ghosh, A. van de Walle, and M. Asta: Phys. Rev. B, 2007, vol. 75, p. 104117
G.W. Lorimer, R.B. Nicholson: Mechanism of Phase Transformations in Crystalline Solids, Session II, Institute of Metals, London, 1969, pp. 36–42
K.C. Russell: in Phase Transformations, H.I. Aaronson, ed., ASM, Metals Park, OH, 1970, pp. 219–68
R.D. Doherty: in Physical Metallurgy, R.W. Cahn, P. Haasen, eds., Elsevier, Amsterdam, 1983, pp. 933–1030
H.I. Aaronson, F.K. LeGoues: Metall. Trans. A, 1992, vol. 23A, pp. 1915–45
R. Wagner, R. Kampmann, P.W. Voorhees: in Phase Transformations in Materials, G. Kostorz, ed., Wiley-VCH, New York, NY, 2001, pp. 309–407
B. Chalmers: Principles of Solidification, John Wiley & Sons, New York, NY, 1964, pp. 126–28
M.C. Flemings: Solidification Processing, McGraw-Hill, New York, NY, 1974, pp. 31–32
W. Kurz, D.J. Fisher: Fundamentals of Solidification, 4th ed., Trans Tech Publications, Aedermannsdorf, Switzerland, 1998. p. 15
H.J. Frost, M.F. Ashby: Deformation-Mechanism Maps: The Plasticity and Creep of Metals and Ceramics, Pergamon Press, New York, NY, 1982, p. 21
M.A. Meyers, K.K. Chawla: Mechanical Metallurgy: Principles and Applications, Prentice-Hall, Englewood Cliffs, NJ, 1984, p. 58
Y. Harada, D.C. Dunand: Scripta Mater., 2003, vol. 48, pp. 219–22
J. Royset, N. Ryum: Scripta Mater., 2005, vol. 52, pp. 1275–79
S. Hori, H. Tai, Y. Narita: J. Jpn. Inst. Light Met., 1982, vol. 32, pp. 596–603
S. Hori, H. Tai, Y. Narita: in Rapidly Quenched Metals, S. Steeb, H. Warlimont, eds., Elsevier Science Publishers, Wurzburg, 1985, pp. 911–14
K. Asboll, N. Ryum: J. Inst. Met., 1973, vol. 101, pp. 212–14
S.K. Pandey, C. Suryanarayana: Mater. Sci. Eng. A, 1989, vol. 111, pp. 181–87
T. Ohashi, K. Suzuki, R. Ichikawa: Bull. Nagoya Inst. Technol., 1971, vol. 23, pp. 459–65
A. Majumdar, R.H. Mair, B.C. Muddle: in Science and Technology of Rapidly Quenched Alloys, M. Tenhover, W.L. Johnson, L.E. Tanner, eds., MRS, Pittsburgh, PA, 1987, pp. 253–60
J.F. Nie, B.C. Muddle: Mater. Sci. Eng. A, 1996, vol. 221, pp. 22–32
N. Ryum: Acta Metall., 1969, vol. 17, pp. 269–78
M. Sundberg, R. Sundberg, B. Jacobson: Jernkont. Ann., 1971, vol. 155, pp. 1–15
S. Rystad, N. Ryum: Aluminium, 1977, vol. 53, pp. 193–95
H. Westengen, L. Auran, O. Reiso: Aluminium, 1981, vol. 57, pp. 797–803
Acknowledgments
This research was supported by the United States Department of Energy, Basic Sciences Division, under Contract No. DE-FG02-02ER45997. Gratitude is expressed to KB Alloys for providing the Al-Zr master alloy. We are indebted to Dr. J.L. Murray (Alcoa), for providing the most recent and reliable data for the binary Al-Ti and Al-Zr phase diagrams. We also thank Dr. J.Z. Liu and Professor M. Asta (Northwestern University and University of California, Davis), for calculating the metastable L12 solvus curves for Al3Ti and Al3Zr, and Professor M.E. Fine (Northwestern University) for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 15, 2006.
Rights and permissions
About this article
Cite this article
Knipling, K., Dunand, D. & Seidman, D. Nucleation and Precipitation Strengthening in Dilute Al-Ti and Al-Zr Alloys. Metall Mater Trans A 38, 2552–2563 (2007). https://doi.org/10.1007/s11661-007-9283-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-007-9283-6