Abstract
The complement system plays an important role in biological defense as an effector to eliminate microorganisms that invade an organism and it is composed of more than 50 proteins, most of which are produced in the liver. Of these proteins, the mRNA expression of C3 and Cfb is known to be positively regulated by the nuclear receptor HNF4α. To investigate whether HNF4α regulates the complement system, we analyzed the hepatic expression of genes involved in the complement activation pathway and membrane attack complex (MAC) formation within the complement system using liver-specific Hnf4a-null mice (Hnf4aΔHep mice) and tamoxifen-induced liver-specific Hnf4a-null mice (Hnf4af/f;AlbERT2cre mice). We found that hepatic expression of many complement genes including C8a, C8b, C8g, and C9 that are involved in formation of the MAC was markedly decreased in Hnf4aΔHep mice and Hnf4af/f;AlbERT2cre mice. Furthermore, expression of C8A, C8B, and C8G was also decreased in human hepatoma cell lines in which the expression of HNF4α was suppressed, and expression of C8G and C9 was induced in a human immortalized hepatocyte cell line with forced expression of HNF4α. Transactivation of C8g and C9 was dependent on HNF4α expression of HNF4α binding sites, indicating that C8g and C9 are novel target genes of HNF4α. The results suggest that hepatic HNF4α plays an important role in regulation of the complement system, mainly MAC formation.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The major role of the complement system in the innate immune system is removal of microorganisms invading a living body, and the complement system consists of more than 50 serum proteins, cell surface receptors, and regulators (Ricklin et al. 2010; Kemper et al. 2014). When a pathogen invades a living body, the complement system recognizes the pathogen and a chain reaction of a complement cascade is activated, resulting in eventual destruction of the pathogen. The complement system is activated by three independent pathways; the classical pathway triggered by a pathogen-bound antibody, the lectin pathway triggered by pathogen-bound mannose-binding lectin (MBL), and the alternative pathway triggered by spontaneous formation of C3b. Fragments activated by a proteolysis cascade exhibit various biological activities including opsonization of a pathogen, recruitment of inflammatory immune cells, and pathogen lysis (Gialeli et al. 2018; Lopez-Lera et al. 2019). Specific complement components work in complement activation: C1q, C1r, C1s, C4, and C2 for the classical pathway; mannose-binding lection-associated serine protease 1 (MASP1), MASP2, MASP3, C4, and C2 for the lectin pathway; and C3 and factors B and D for the alternative pathway. The three pathways commonly result in C3 activation and are followed by formation of the membrane attack complex (MAC) by C5b-C9. The MAC forms a transmembrane pore on the cell surface of pathogens and aged erythrocytes, and water and ions intrude into the cells from the pore, eventually resulting in cell lysis due to dysfunction of the maintenance of osmotic pressure (Morgan et al. 2017). Thus, inherited deficiencies of the complement proteins are mainly associated with enhanced susceptibility to Neisseria infections due to dysfunctional host defense caused by inactivation of the late complement components consisting of C5b-C9 and with autoimmune diseases such as systemic lupus erythematous (SLE) due to deposits of circulating immune complexes caused by inactivation of the early complement components consisting of three independent pathways (Vignesh et al. 2017; Schroder-Braunstein and Kirschfink 2019). However, excessive complement activation in response to pathogens causes excessive consumption of complement proteins and persistent inflammation. Thus, several complement inhibitors have been identified. For example, GPI-anchored CD59 in host cells inhibits MAC formation between C5b-8 and C9, resulting in protection of own cells including erythrocytes from invasion by complement activation (Meri et al. 1990; Kim and Song 2006). In addition, the complement system has been found to have multiple roles in maintenance of cellular homeostasis including clearance of apoptotic and secondary necrotic cells; in adaptive immunity; and in diseases such as inflammatory diseases, autoimmune disorders, rheumatoid arthritis, asthma, Alzheimer’s disease, and cancers (Gullstrand et al. 2009; Ricklin et al. 2010). In this way, an appropriate balance between complement activators and inhibitors has an important role in body homeostasis to prevent invasion of pathogens and excessive immune reaction.
Many complement proteins circulate in the blood stream and are mainly produced in the liver as well as many serum proteins except immunoglobulin (Vignesh et al. 2017). Expression of liver-enriched genes is mainly regulated by liver-enriched transcription factors including HNF1, HNF3 (FOXA), HNF4α, HNF6 (ONECUT), and C/EBP (Schrem et al. 2004; Lau et al. 2018). Of these, hepatic expression of terminal complement genes including C5, C8a, C8b, C8g, and C9 was shown to be decreased in Hnf1a-null mice and an HNF1α binding site was identified in the promoter region of the C8a gene (Pontoglio et al. 2001). It was also shown that HNF4α positively regulates C3 and Cfb expression (Garnier et al. 1996; Shavva et al. 2013). In addition, hepatic expression of many coagulation factors including F5, 9, 11, 12, and 13b was shown to be decreased in liver-specific Hnf4a-null mice (Hnf4aΔHep mice) and Hnf4a-suppressed mouse primary hepatocytes (Inoue et al. 2006; Safdar et al. 2012). The coagulation system also consists of coagulation factors that are mainly produced in the liver and is an enzymatic cascade as is the complement system and the complement and coagulation systems are intimately connected to each other (Foley 2016), indicating that HNF4α might also be a central regulator in the complement system.
In this study, we investigated whether hepatic HNF4α positively regulates the expression of the complement genes using Hnf4aΔHep mice and tamoxifen-induced liver-specific Hnf4a-null mice (Hnf4af/f;AlbERT2cre). We found that hepatic expression of many complement genes was markedly decreased in Hnf4aΔHep mice and that complement C8g and C9 are novel target genes of hepatic HNF4α. These findings reveal that hepatic HNF4α is an important factor in regulation of the complement system including MAC formation.
Materials and methods
Animal
Liver-specific Hnf4a-null (Hnf4aΔHep) mice were kindly provided by Dr. Frank J Gonzalez (Hayhurst et al. 2001). To generate tamoxifen-induced liver-specific Hnf4a-null (Hnf4af/f;AlbERT2cre) mice (Bonzo et al. 2012), SA+/CreER2 mice were kindly provided by Dr. Pierre Chambon (Schuler et al. 2004). All experiments were performed with 45-d-old male Hnf4a-floxed (Hnf4af/f) and Hnf4aΔHep mice, and 13-wk-old tamoxifen-treated Hnf4af/f and Hnf4af/f;AlbERT2cre mice. Mice were housed in a pathogen-free animal facility under standard 12-h light/12-h dark cycle with ad libitum water and chow. All experiments with mice were carried out under Gunma University Animal Care and Experimentation Committee.
Cell lines
HEK293T (purchased from ATCC in February 2010), HepG2 (purchased from RIKEN Cell Bank in December 2011), and OUMS-29 (Kobayashi et al. 2000) (kindly provided from Dr. Nam-Ho Huh, Okayama University) cells were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (Wako, Osaka, Japan) containing 10% fetal bovine serum (HyClone, Logan, UT) and 100 units/ml penicillin/streptomycin (Wako). All cell lines were confirmed to be mycoplasma-free by nuclear staining with Hoechst 33,342 (Thermo Fisher Scientific, Tokyo, Japan). HEK293T and HepG2 were re-authenticated by STR analysis, and OUMS-29 was unique and not cross-contaminated or misidentified because no matched STR profiles of OUMS-29 were found in the Expasy Profile Database (Supplemental Fig. 1).
RNA extraction, reverse-transcription, and quantitative PCR
Extracted total RNA from cell lines and livers of Hnf4aΔHep and Hnf4af/f mice was transcribed using ReverTraAce qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan). These cDNA were used for quantitative PCR using Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) with the specific primers on a LightCycler 480 system II (Roche, Basel, Switzerland). Levels of mRNA expression were normalized relative to Gapdh mRNA as an internal control using ∆∆Ct method. Nucleotide sequences of the primers are shown in Supplemental Table 1.
Transfection of dicer-substrate siRNA
Dicer-substrate siRNA (DsiRNA; 10 nM) against human HNF4A mRNA and negative control (Integrated DNA Technologies, Tokyo, Japan) were transfected into HepG2 cells with Lipofectamine RNAiMAX (Life Technologies). After 48 h of transfection, total RNA and protein were harvested. Nucleotide sequences for the DsiRNAs duplexes are follows: rArUrGrGrCrCrArArGrArUrUrGrArCrArArCrCrUrGrUrUGC and rGrCrArArCrArGrGrUrUrGrUrCrArArUrCrUrUrGrGrCrCrArUrGrC for HNF4α, and rCrGrUrUrArArUrCrGrCrGrUrArUrArArUrArCrGrCrGrUAT and rArUrArCrGrCrGrUrArUrUrArUrArCrGrCrGrArUrUrArArCrGrArC for negative control.
Western blot
Whole cell lysates from HepG2 cells treated with siRNA against HNF4α and HNF4α-overexpressed OUMS-29 cells were also prepared as described previously (Matsuo et al. 2015). The nuclear extracts, the whole cell lysate, and pull-down samples were diluted with Laemmli sample buffer, incubated at 65 °C for 15 min, fractionated by 10% SDS–polyacrylamide gel electrophoresis. The gels were transferred onto a PVDF membrane (GE Healthcare, Tokyo, Japan). The membrane was incubated for 1 h with PBS containing 0.1% Tween 20 and 5% skim milk, and then incubated for 1 h with anti-HNF4α (Perceus Proteomics, Tokyo, Japan) and anti-γ tubulin antibodies (Sigma). After washing, the membrane was incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology), and the reaction product was visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL).
Construction of luciferase reporter plasmids of the mouse complement promoters
The mouse C8g (-1895/1) and C9 (-1992/-1) promoters and the shorter promoters of these genes from the translation start site were amplified from genomic DNA of mouse liver by PCR and cloned into the luciferase reporter vector, pGL4.11 (Promega, Madison, WI). Mutations were introduced into an HNF4α binding site in the C8g and C9 promoters by overlap and inverse PCR-based mutagenesis, respectively. The following primers were used (the mutated HNF4α binding sites were indicated as capital and bold letters): for C8g promoter, tggacagtggacAGAgacctaggacag and ctgtcctaggtcTCTgtccactgtcca; and for C9 promoter, AAGGAacacttagcctaagccaaca and tccaaccaatggagagtcattgtaa. As a positive control, the mouse Otc promoter was used (Inoue et al. 2002).
Transient transfection and luciferase assays
The mouse Otc, C8g, and C9 promoters cloned into pGL4.11 with pGL4.74 as an internal control and HNF4α expression plasmid were co-transfected into the cell lines using polyethyleneimine Max (PolyScience, Warrington, PA) as a transfection reagent. After 48 h, the cells were washed with phosphate-buffered saline and promoter activities were measured using Dual-Glo Luciferase Assay System (Promega).
Electrophoretic mobility shift assay (EMSA)
EMSA was carried out using LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific) and nuclear extracts from HepG2 cells. The following double-stranded probes were used (mutations in the HNF4α binding site are indicated as capital and bold letters): the HNF4α binding site at -203/-192 in the mouse ornithine transcarbamylase (Otc) promoter (Inoue et al. 2002), the HNF4α binding site at -72/-60 in the mouse C8g promoter (wild type; tggacagtggactctgacctaggacagtg and cactgtcctaggtcagagtccactgtcca, mutant; tggacagtggacAGAgacctaggacagtg and cactgtcctaggtcTCTgtccactgtcca), and the HNF4α binding site at -82/-70 in the mouse C9 promoter (wild type; tccattggttggaccttgacacttagccta and taggctaagtgtcaaggtccaaccaatgga, mutant; tccattggttggaAAGGAacacttagccta and taggctaagtgtTCCTTtccaaccaatgga). Nuclear extracts (3 μg) and the 5′-biotin labeled probes of the HNF4α binding sites for the C9 promoter (wild-type) were added and the reaction mixture incubated on ice for 10 min. For competition experiments, a 50-fold excess of unlabeled probe was added to the reaction mixture and the mixture was incubated on ice for 10 min prior to the addition of the 5′-biotin labeled probe. For supershift analysis, 1 μg each of anti-HNF4α, anti-C/EBPα, and anti-PPARβ antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the reaction mixture, and the mixture was incubated on ice for 10 min after the addition of the 5′-biotin labeled probe. DNA–protein complexes were fractionated by 7% PAGE containing 5% glycerol, and blotted onto a Biodyne B Nylon membrane (Pall, Tokyo, Japan). After washing, DNA–protein complexes were visualized using detection module in the kit on an ImageQuant LAS4000.
Chromatin immunoprecipitation
Chromatin immunoprecipitation using liver samples from Hnf4af/f and Hnf4aΔHep mice was performed according to our previous protocol using anti-HNF4α antibody (Perseus Proteomics, Tokyo, Japan) and normal goat IgG (Santa Cruz Biotechnology) (Morimoto et al. 2017). Purified DNA was amplified by quantitative PCR using ΔΔCt method. Enrichment of HNF4α binding site was normalized to the input samples compared with normal goat IgG antibody. The following primers were used for real-time PCR: mouse C8g promoter containing HNF4α binding site (ctaggatggaccctggcttg and tcacctactgtccgtagcagg), mouse C9 promoter containing HNF4α binding site (tggtttattgcataatgacact and tttgttcataggtgttggctta), mouse Otc promoter containing HNF4α binding site (gaagaggctgggctctgaa and atagagtagggcagggtgcag) as a positive control, and mouse Hmgcs2 gene without HNF4α binding site (gatccctgggactcacaca and gaatgcacatttatggaggtca) as a negative control.
Statistical analysis
All values are expressed as the mean ± standard derivation (S.D.). All data were analyzed by the Mann–Whitney test for significant differences between the mean values of each group.
Results
Hepatic expression of many complement genes is downregulated in liver-specific Hnf4a-null mice
Since HNF4α is a master regulator for maintenance of liver functions and many complement proteins are mainly produced in the liver, the expression profile of hepatic mRNAs encoding complement genes was analyzed in liver-specific Hnf4a-null mice (Hnf4aΔHep mice) and control Hnf4af/f mice by quantitative RT-PCR (Fig. 1A). Hepatic expression of many complement genes including C1r, C1s, C2, C4, C5, C6, C8a, C8b, C8g, and C9 was downregulated in Hnf4aΔHep mice, but no significant difference in expression of C1qa, C1qb, C1qc, and C7 was observed between Hnf4af/f and Hnf4aΔHep mice. A similar tendency in expression was observed between tamoxifen-induced liver-specific Hnf4a-null mice (Hnf4af/f;AlbERT2cre mice) and tamoxifen-treated control mice (Hnf4af/f mice) (Fig. 1B). Expression of C3 was significantly upregulated in Hnf4aΔHep mice but was not different in tamoxifen-treated Hnf4af/f and Hnf4af/f;AlbERT2cre mice. Of these downregulated genes, C5b-C9, which are late complement components, form the membrane attack complex (MAC) that leads to cell death by the formation of channels on the plasma membrane. Expression of C8a, C8b, C8g, and C9 was remarkably decreased in Hnf4aΔHep mice and tamoxifen-treated Hnf4af/f;AlbERT2cre mice. These results indicate that HNF4α is a central regulator of the production of complement components in the liver.
Hepatic expression of complement genes in Hnf4aΔHep and tamoxifen-treated Hnf4af/f;AlbERT2cre mice. Quantitative RT-qPCR for Hnf4a and complement mRNAs from total liver RNA of Hnf4af/f and Hnf4aΔHep mice (A) and tamoxifen-treated Hnf4af/f and Hnf4af/f;AlbERT2cre mice (B) (n = 5 for each genotype). The normalized expression in Hnf4aΔHep and tamoxifen-treated Hnf4af/f;AlbERT2cre mice was presented relative to that in Hnf4af/f and tamoxifen-treated Hnf4af/f mice using Tbp mRNA as an internal control. Data are mean ± S.D. *P < 0.05. **P < 0.01.
Expression of complement genes by HNF4α in human hepatoma cells and immortal human hepatocytes
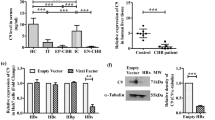
Since hepatic expression of many complement genes was decreased in Hnf4aΔHep mice and tamoxifen-treated Hnf4af/f;AlbERT2cre mice, expression of C1-C9 mRNAs was analyzed in human hepatoma-derived HepG2 cells by siRNA knockdown of HNF4α (Fig. 2A). Expression of C2, C4, C6, C8A, C8B, and C8G was suppressed by inhibition of the expression of HNF4α, and these results were similar to the results in Hnf4aΔHep and tamoxifen-treated Hnf4af/f;AlbERT2cre mice. Expression of C1QA, C1QB, C7, and C9 was not detected in HepG2 cells treated with siRNA of control and HNF4α. Since C1Q and C7 are produced in macrophages and dendritic cells and in endothelial cells, respectively (Langeggen et al. 2000; Lu and Kishore 2017), C1Q and C7 would be produced in extrahepatic cells such as Kuppfer cells and endothelial cells in the liver and would not be target genes of hepatic HNF4α. However, since C9 is mainly produced in hepatocytes, we investigated whether expression of complement genes is induced by HNF4α in highly differentiated immortalized human hepatocytes, OUMS-29 cells (Kobayashi et al. 2000). It was found that the expression of C8G and C9 was significantly induced by HNF4α, but other complement genes were not induced or not detected in HNF4α-overexpressed OUMS-29 cells. Thus, we focused on transcriptional regulation of the C8g and C9 genes by HNF4α.
Expression of complement genes by HNF4α in human hepatoma cells and immortalized human hepatocytes. (A) Western blot analysis of HNF4α and TUBG protein in human hepatoma cell lines, HepG2 cells treated with negative control of siRNA (siCont) and siRNA for HNF4A (siHNF4A) (left). Quantitative RT-PCR from total RNA of HepG2 cells treated with siCont and siHNF4A (right). The normalized expression in siHNF4A-treated cells is presented relative to that in siCont-treated cells using GAPDH mRNA as an internal control. (B) Western blot analysis of HNF4α and TUBG protein in human immortalized human hepatocytes, OUMS-29 cells transfected an empty vector (Empty) and HNF4α expression vector (HNF4A) (left). Quantitative RT-PCR from total RNA of empty vector and HNF4α expression vector-transfected OUMS-29 cells using GAPDH mRNA as an internal control (right). Data are mean ± S.D. *P < 0.05; **P < 0.005 compared to the cells treated with siCont, or the cells transfected the empty vector. ND, not detected.
Direct transactivation of the C8g gene by HNF4α
Expression of C8a, C8b, C8g, and C9 genes, which are components of the MAC, was markedly decreased in Hnf4aΔHep mice and human hepatoma cells treated with siRNA for HNF4α. In addition, expression of C8G and C9 genes was increased by overexpression of HNF4α in immortal human hepatocytes. Thus, promoter analysis was performed to investigate whether HNF4α directly transactivates the C8g and C9 genes. The C8g promoter containing the region at -600/-1 from the translation start site was transactivated by HNF4α (Fig. 3A). The promoter activity of the C8g promoter at -589/-1 was decreased by about half compared to that of the -600/-1 promoter. The promoter activity of the -101/-1 promoter was further decreased and the -44/-1 promoter no longer had promoter activity. Since an HNF4α binding site at -72/-60 and E-box at -595/-590 were predicted in the C8g promoter by the JASPAR database, mutations were introduced into these binding sites. The promoter activity of the -600/-1 promoter that contains mutations in the E-box (-600Mut1) was decreased by half compared to that of the wild-type promoter, and the -600/-1 promoter that contains mutations in the HNF4α binding site was further decreased (-600Mut2). When mutations were introduced into both sites (600Mut1/2), the promoter activity was decreased to the same level as that of the -101/-1 promoter. The promoter activity of the -101/-1 promoter that contains mutations in the predicted HNF4α binding site was decreased to the basal level (-101Mut2), indicating that both the E-box and the HNF4α binding site are critical cis-elements for transactivation of the C8g gene. To determine whether HNF4α can directly bind to the HNF4α binding site in the C8g promoter, an electrophoretic mobility shift assay (EMSA) was performed (Fig. 3B). Nuclear extracts from HepG2 cells bound to the HNF4α binding site (lane 2, lower arrow). This complex was diminished by the addition of excess amounts of unlabeled C8g and Otc competitors that contain functional HNF4α binding sites (lanes 3 and 4) but not the C8g competitor that contains mutations in the HNF4α binding site (lane 5). Furthermore, the complex was supershifted by an anti-HNF4α antibody (lane 6, the upper arrow) but not by an unrelated anti-C/EBPα antibody (lane 7). Chromatin immunoprecipitation (ChIP) analysis using the livers of Hnf4af/f and Hnf4aΔHep mice indicated that HNF4α in Hnf4af/f mice strongly bound to the Otc and C8g promoter regions compared to that in Hnf4aΔHep mouse livers (Fig. 3C), suggesting that HNF4α directly and physiologically binds to the C8g promoter in mouse livers.
Promoter analysis of the mouse C8g gene. (A) Promoter activity of the C8g gene. The C8g promoters from the translation start site were transfected into HepG2 cells. Mutations were introduced into the predicted E box (-595/-590) and HNF4α binding site (-72/-60) in the promoter. (B) Electrophoretic mobility shift assay. Biotin-labelled probe carrying the HNF4α binding site in the C8g promoter was incubated without nuclear extracts (lane 1). Nuclear extracts from HepG2 cells were incubated with biotin-labelled probe carrying the HNF4α binding site in the C8g promoter in the absence (lane 2) or presence of 50-fold excess amounts of the unlabeled C8g and Otc probes (lanes 3 and 4), and the C8g probe that contains mutations in the HNF4α binding site (lane 5). For supershift analysis, anti-HNF4α and anti-C/EBPα antibodies were added, respectively (lanes 6 and 7). Complex between HNF4α and the probe, and supershifted complex are indicated by the lower and upper arrows, respectively. (C) Chromatin immunoprecipitation using the livers of Hnf4af/f and Hnf4aΔHep mice with normal goat IgG and anti-HNF4α antibody. The region containing the HNF4α binding site in the Otc and C8g promoters and the region without an HNF4α binding site in the Hmgcs2 gene were amplified. The data from qPCR was normalized relative to the input and expressed as fold enrichment over data from IgG control. Data are mean ± S.D. *P < 0.05 compared to Hnf4af/f mice.
Direct transactivation of the C9 promoter by HNF4α
Promoter analysis of the mouse C9 gene was also performed in HEK293T cells (Fig. 4A). As a positive control, the mouse Otc promoter containing two HNF4α binding sites was strongly transactivated by HNF4α (Inoue et al. 2002). The C9 promoter at -1992/-1 and that at -91/-1 from the translation start site were also transactivated by HNF4α. However, the activity of the shorter deletion mutant of the C9 promoter at -60/-1 was not induced by HNF4α, indicating that a functional HNF4α binding site might exist between -61 and -91 in the C9 promoter. Since an HNF4α binding site was predicted at -84/-69 in the C9 promoter by the JASPAR database, mutations were introduced into the predicted HNF4α binding site of the -1992/-1 promoter (-1992/Mut). As expected, the promoter activity of the -1992/Mut promoter was not transactivated by HNF4α (Fig. 4A). EMSA showed that nuclear extracts from HepG2 cells bound to the HNF4α binding site (Fig. 4B, lane 2, lower arrow). This complex was diminished by the addition of excess amounts of unlabeled C9 and Otc competitors that contain HNF4α binding site (lanes 3 and 4) but not the C9 competitor that contains mutations in the HNF4α binding site (lane 5). Furthermore, the complex was supershifted by the anti-HNF4α antibody (lane 6, the upper arrow) but not by the anti-PPARβ antibody (lane 7). ChIP using the livers of Hnf4af/f and Hnf4aΔHep mice also indicated that HNF4α in Hnf4af/f mice strongly bound to the promoter region compared to that in Hnf4aΔHep mouse livers (Fig. 4C), suggesting that HNF4α directly and physiologically binds to the C9 promoters in mouse livers.
Promoter analysis of the mouse C9 gene. (A) Promoter activity of the C9 gene. The C9 promoters from the translation start site were co-transfected with an empty vector (Empty), or HNF4α expression vector (+ HNF4α) into HEK293T cells. Mutations were introduced into the predicted HNF4α binding site (-72/-60) in the promoter. (B) Electrophoretic mobility shift assay. Biotin-labelled probe carrying the HNF4α binding site in the C9 promoter was incubated without nuclear extracts (lane 1). Nuclear extracts from HepG2 cells were incubated with biotin-labelled probe carrying the HNF4α binding site in the C9 promoter in the absence (lane 2) or presence of a 50-fold excess amounts of the unlabeled C9 and Otc probes (lanes 3 and 4), and the C9 probe that contains mutations in the HNF4α binding site (lane 5). For supershift analysis, anti-HNF4α and anti-PPARb antibodies were added, respectively (lanes 6 and 7). Complex between HNF4α and the probe and supershifted complex are indicated by the lower and upper arrows, respectively. (C) Chromatin immunoprecipitation using the livers of Hnf4af/f and Hnf4aΔHep mice with normal goat IgG and anti-HNF4α antibody. The region containing the HNF4α binding site in the C9 promoter and the region without an HNF4α binding site in the Hmgcs2 gene were amplified. The data from qPCR was normalized relative to the input and expressed as fold enrichment over data from IgG control. Data are mean ± S.D. *P < 0.05 compared to Hnf4af/f mice.
Discussion
In this study, we found that hepatic expression of many complement genes is decreased in Hnf4aΔHep mice. Promoter activity of the human C3 gene is dependent on HNF4α expression in HepG2 cells (Shavva et al. 2013), but hepatic expression of C3 mRNA was not decreased in Hnf4aΔHep mice and tamoxifen-treated Hnf4af/f;AlbERT2cre mice and in siRNA against HNF4α-treated HepG2 cells. Thus, the C3 gene may not be a direct target of HNF4α. On the other hand, decreased expression of C8a, C8b, and C8g was observed in Hnf4aΔHep mouse livers and tamoxifen-treated Hnf4af/f;AlbERT2cre mice and in siRNA against HNF4α-treated HepG2 cells. Hepatic expression of C9 was significantly decreased in Hnf4aΔHep mice and tamoxifen-treated Hnf4af/f;AlbERT2cre mice, but the expression was not detected in siRNA against HNF4α-treated HepG2 cells, indicating that C9 is produced in normal hepatocytes but is not produced in HepG2 cells. Conversely, the expression of C8A and C8B was not detected, but the expression of C8G and C9 was induced by overexpression of HNF4α in human immortalized hepatocytes.
C8 is composed of three subunits, C8A, C8B, and C8G, and C8A dimerizes with C8G by a disulfide bond (C8A-G), followed by a non-covalent bond with C8B (Steckel et al. 1980; Ng et al. 1987). Hemolytic activity was shown only by C8A and C8B, and addition of C8G to C8A and C8B significantly elevated the activity, indicating that C8G is not essential for hemolytic activity but enhances the activity (Parker and Sodetz 2002). Of C8 deficiency in humans, patients with C8A and C8G deficiencies are abundant in Asians and Africans, but patients with C8B deficiency are more common in Caucasians (Sjoholm 2002). C8 proteins, like other complement proteins, are mainly expressed in the liver, but it was reported that the expression of C8G is elevated in the brains of Alzheimer’s disease mice and patients (Kim et al. 2021). Since treatment with C8G in Alzheimer’s disease model mice attenuated neuroinflammation, C8G plays an important role in protection of the brain from inflammation. Furthermore, there was a positive correlation between circulating C8G protein and metabolic dysfunction-associated steatotic liver disease (MASLD) (Shi et al. 2024). In this study, we showed that C8g is a direct HNF4α target gene, suggesting that the HNF4α-C8G axis might be involved in the pathogenesis of MASLD.
C9 proteins form a transmembrane channel by binding to the C5b-8 complex on the surface of pathogens (Bayly-Jones et al. 2017). C9-deficient mice had impaired antibody-mediated hemolysis and LPS-induced acute shock, indicating that C9 plays a critical role in complement-mediated hemolysis and inflammation activation (Fu et al. 2016). C9 deficiency, which is very rare in Caucasians, is more frequent in Japanese, and some patients exhibit Neisseria infections, but most are asymptomatic (Inai et al. 1989; Grumach and Kirschfink 2014). Moreover, the serum or plasma level of C9 protein was shown to be increased in various cancers including squamous cell lung cancer, gastric cancer, and colorectal cancer (Chong et al. 2010; Murakoshi et al. 2011; Narayanasamy et al. 2011). C9 was also suggested to be associated with other diseases. For instance, an increased urinary ratio of C9 to CD59, which inhibits MAC formation, was associated with tubulointerstitial fibrosis in lupus nephritis patients, and C9 protein was elevated in plasma exosomes of Alzheimer’s disease patients (Cai et al. 2022; Wang et al. 2023). Furthermore, hepatic expression of C9 was decreased in the early stage of NAFLD, with a decreasing trend in the expression with NASH progression (Subudhi et al. 2022). Regarding the regulatory mechanism of C9 expression, expression of HNF4α was inhibited by hepatitis C virus (HCV) infection and the expression of C9 was decreased in plasma of hepatocellular carcinoma (HCC) patients with HCV infection (Aydin et al. 2019; Ferrin et al. 2014). These findings suggested that C9 is possibly regulated by HNF4α, but this study proved that C9 is a novel target gene of HNF4α. In addition, hepatitis B virus-encoded oncogene X protein (HBx) inhibits C9 transcription via inhibition of transcription factor USF-1 function, resulting in inhibition of MAC formation, and dihydro-stilbene gigantol potently alleviates oxidative stress and inflammation in the liver via decreased expression of C9 (Xue et al. 2020; Baidya et al. 2022). Our results provide new insights into the possible involvement of HNF4α in further pathogenesis of various C9-associated diseases.
In summary, our study showed that HNF4α positively regulates the expression of complement genes involved in complement activation and MAC formation and that C8g and C9 are target genes of HNF4α. This study provides insight into the transcriptional mechanism of C8g and C9 by HNF4α, which is expected to lead to a better understanding of the pathogenesis of and therapeutic application for diseases in which these genes have been implicated.
Data availability
All the data presented in the manuscript is provided in the main text and the supplementary file.
References
Aydin Y, Kurt R, Song K, Lin D, Osman H, Youngquist B, Scott JW, Shores NJ, Thevenot P, Cohen A, Dash S (2019) Hepatic stress response in HCV infection promotes STAT3-mediated inhibition of HNF4A-miR-122 feedback loop in liver fibrosis and cancer progression. Cancers (Basel) 11:1407
Baidya A, Khatun M, Mondal RK, Ghosh S, Chakraborty BC, Mallik S, Ahammed SKM, Chowdhury A, Banerjee S, Datta S (2022) Hepatitis B virus suppresses complement C9 synthesis by limiting the availability of transcription factor USF-1 and inhibits formation of membrane attack complex: implications in disease pathogenesis. J Biomed Sci 29:97
Bayly-Jones C, Bubeck D, Dunstone MA (2017) The mystery behind membrane insertion: a review of the complement membrane attack complex. Philos Trans R Soc Lond B Biol Sci 372:20160221
Bonzo JA, Ferry CH, Matsubara T, Kim JH, Gonzalez FJ (2012) Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4alpha in adult mice. J Biol Chem 287:7345–7356
Cai H, Pang Y, Wang Q, Qin W, Wei C, Li Y, Li T, Li F, Wang Q, Li Y, Wei Y, Jia L (2022) Proteomic profiling of circulating plasma exosomes reveals novel biomarkers of Alzheimer’s disease. Alzheimers Res Ther 14:181
Chong PK, Lee H, Loh MC, Choong LY, Lin Q, So JB, Lim KH, Soo RA, Yong WP, Chan SP, Smoot DT, Ashktorab H, Yeoh KG, Lim YP (2010) Upregulation of plasma C9 protein in gastric cancer patients. Proteomics 10:3210–3221
Ferrin G, Ranchal I, Llamoza C, Rodriguez-Peralvarez ML, Romero-Ruiz A, Aguilar-Melero P, Lopez-Cillero P, Briceno J, Muntane J, Montero-Alvarez JL, De la Mata M (2014) Identification of candidate biomarkers for hepatocellular carcinoma in plasma of HCV-infected cirrhotic patients by 2-D DIGE. Liver Int 34:438–446
Foley JH (2016) Examining coagulation-complement crosstalk: complement activation and thrombosis. Thromb Res 141(Suppl 2):S50-54
Fu X, Ju J, Lin Z, Xiao W, Li X, Zhuang B, Zhang T, Ma X, Li X, Ma C, Su W, Wang Y, Qin X, Liang S (2016) Target deletion of complement component 9 attenuates antibody-mediated hemolysis and lipopolysaccharide (LPS)-induced acute shock in mice. Sci Rep 6:30239
Garnier G, Circolo A, Colten HR (1996) Constitutive expression of murine complement factor B gene is regulated by the interaction of its upstream promoter with hepatocyte nuclear factor 4. J Biol Chem 271:30205–30211
Gialeli C, Gungor B, Blom AM (2018) Novel potential inhibitors of complement system and their roles in complement regulation and beyond. Mol Immunol 102:73–83
Grumach AS, Kirschfink M (2014) Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol Immunol 61:110–117
Gullstrand B, Martensson U, Sturfelt G, Bengtsson AA, Truedsson L (2009) Complement classical pathway components are all important in clearance of apoptotic and secondary necrotic cells. Clin Exp Immunol 156:303–311
Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ (2001) Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21:1393–1403
Inai S, Akagaki Y, Moriyama T, Fukumori Y, Yoshimura K, Ohnoki S, Yamaguchi H (1989) Inherited deficiencies of the late-acting complement components other than C9 found among healthy blood donors. Int Arch Allergy Appl Immunol 90:274–279
Inoue Y, Hayhurst GP, Inoue J, Mori M, Gonzalez FJ (2002) Defective ureagenesis in mice carrying a liver-specific disruption of hepatocyte nuclear factor 4alpha (HNF4alpha ). HNF4alpha regulates ornithine transcarbamylase in vivo. J Biol Chem 277:25257–25265
Inoue Y, Peters LL, Yim SH, Inoue J, Gonzalez FJ (2006) Role of hepatocyte nuclear factor 4alpha in control of blood coagulation factor gene expression. J Mol Med (Berl) 84:334–344
Kemper C, Pangburn MK, Fishelson Z (2014) Complement nomenclature 2014. Mol Immunol 61:56–58
Kim DD, Song WC (2006) Membrane complement regulatory proteins. Clin Immunol 118:127–136
Kim JH, Afridi R, Han J, Jung HG, Kim SC, Hwang EM, Shim HS, Ryu H, Choe Y, Hoe HS, Suk K (2021) Gamma subunit of complement component 8 is a neuroinflammation inhibitor. Brain 144:528–552
Kobayashi N, Miyazaki M, Fukaya K, Inoue Y, Sakaguchi M, Noguchi H, Matsumura T, Watanabe T, Totsugawa T, Tanaka N, Namba M (2000) Treatment of surgically induced acute liver failure with transplantation of highly differentiated immortalized human hepatocytes. Cell Transplant 9:733–735
Langeggen H, Pausa M, Johnson E, Casarsa C, Tedesco F (2000) The endothelium is an extrahepatic site of synthesis of the seventh component of the complement system. Clin Exp Immunol 121:69–76
Lau HH, Ng NHJ, Loo LSW, Jasmen JB, Teo AKK (2018) The molecular functions of hepatocyte nuclear factors - In and beyond the liver. J Hepatol 68:1033–1048
Lopez-Lera A, Corvillo F, Nozal P, Regueiro JR, Sanchez-Corral P, Lopez-Trascasa M (2019) Complement as a diagnostic tool in immunopathology. Semin Cell Dev Biol 85:86–97
Lu J, Kishore U (2017) C1 complex: an adaptable proteolytic module for complement and non-complement functions. Front Immunol 8:592
Matsuo S, Ogawa M, Muckenthaler MU, Mizui Y, Sasaki S, Fujimura T, Takizawa M, Ariga N, Ozaki H, Sakaguchi M, Gonzalez FJ, Inoue Y (2015) Hepatocyte nuclear factor 4alpha controls iron metabolism and regulates transferrin receptor 2 in mouse liver. J Biol Chem 290:30855–30865
Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ (1990) Human protectin (CD59), an 18000–20000 MW complement lysis restricting factor, inhibits C5b–8 catalysed insertion of C9 into lipid bilayers. Immunology 71:1–9
Morgan BP, Boyd C, Bubeck D (2017) Molecular cell biology of complement membrane attack. Semin Cell Dev Biol 72:124–132
Morimoto A, Kannari M, Tsuchida Y, Sasaki S, Saito C, Matsuta T, Maeda T, Akiyama M, Nakamura T, Sakaguchi M, Nameki N, Gonzalez FJ, Inoue Y (2017) An HNF4alpha-microRNA-194/192 signaling axis maintains hepatic cell function. J Biol Chem 292:10574–10585
Murakoshi Y, Honda K, Sasazuki S, Ono M, Negishi A, Matsubara J, Sakuma T, Kuwabara H, Nakamori S, Sata N, Nagai H, Ioka T, Okusaka T, Kosuge T, Shimahara M, Yasunami Y, Ino Y, Tsuchida A, Aoki T, Tsugane S, Yamada T (2011) Plasma biomarker discovery and validation for colorectal cancer by quantitative shotgun mass spectrometry and protein microarray. Cancer Sci 102:630–638
Narayanasamy A, Ahn JM, Sung HJ, Kong DH, Ha KS, Lee SY, Cho JY (2011) Fucosylated glycoproteomic approach to identify a complement component 9 associated with squamous cell lung cancer (SQLC). J Proteomics 74:2948–2958
Ng SC, Rao AG, Howard OM, Sodetz JM (1987) The eighth component of human complement: evidence that it is an oligomeric serum protein assembled from products of three different genes. Biochemistry 26:5229–5233
Parker CL, Sodetz JM (2002) Role of the human C8 subunits in complement-mediated bacterial killing: evidence that C8 gamma is not essential. Mol Immunol 39:453–458
Pontoglio M, Pausa M, Doyen A, Viollet B, Yaniv M, Tedesco F (2001) Hepatocyte nuclear factor 1alpha controls the expression of terminal complement genes. J Exp Med 194:1683–1689
Ricklin D, Hajishengallis G, Yang K, Lambris JD (2010) Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797
Safdar H, Cheung KL, Vos HL, Gonzalez FJ, Reitsma PH, Inoue Y, van Vlijmen BJ (2012) Modulation of mouse coagulation gene transcription following acute in vivo delivery of synthetic small interfering RNAs targeting HNF4alpha and C/EBPalpha. PLoS ONE 7:e38104
Schrem H, Klempnauer J, Borlak J (2004) Liver-enriched transcription factors in liver function and development. Part II: the C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol Rev 56:291–330
Schroder-Braunstein J, Kirschfink M (2019) Complement deficiencies and dysregulation: pathophysiological consequences, modern analysis, and clinical management. Mol Immunol 114:299–311
Schuler M, Dierich A, Chambon P, Metzger D (2004) Efficient temporally controlled targeted somatic mutagenesis in hepatocytes of the mouse. Genesis 39:167–172
Shavva VS, Mogilenko DA, Dizhe EB, Oleinikova GN, Perevozchikov AP, Orlov SV (2013) Hepatic nuclear factor 4alpha positively regulates complement C3 expression and does not interfere with TNFalpha-mediated stimulation of C3 expression in HepG2 cells. Gene 524:187–192
Shi Y, Dong H, Sun S, Wu X, Fang J, Zhao J, Han J, Li Z, Wu H, Liu L, Wu W, Tian Y, Yuan G, Fan X, Xu C (2024) Protein-centric omics analysis reveals circulating complements linked to non-viral liver diseases as potential therapeutic targets. Clin Mol Hepatol 30:80–97
Sjoholm AG (2002) Deficiencies of mannan-binding lectin, the alternative pathway, and the late complement components. In N.R. Rose, R.G. Hamilton, Detrick B (eds) Manual of clinical laboratory immunology, 6th ed. ASM Press, Washington, DC, pp. 847–854
Steckel EW, York RG, Monahan JB, Sodetz JM (1980) The eighth component of human complement. Purification and physicochemical characterization of its unusual subunit structure. J Biol Chem 255:11997–12005
Subudhi S, Drescher HK, Dichtel LE, Bartsch LM, Chung RT, Hutter MM, Gee DW, Meireles OR, Witkowski ER, Gelrud L, Masia R, Osganian SA, Gustafson JL, Rwema S, Bredella MA, Bhatia SN, Warren A, Miller KK, Lauer GM, Corey KE (2022) Distinct hepatic gene-expression patterns of NAFLD in patients with obesity. Hepatol Commun 6:77–89
Vignesh P, Rawat A, Sharma M, Singh S (2017) Complement in autoimmune diseases. Clin Chim Acta 465:123–130
Wang S, Broder A, Shao D, Kesarwani V, Boderman B, Aguilan J, Sidoli S, Suzuki M, Greally JM, Saenger YM, Rovin BH, Michelle Kahlenberg J (2023) Urine proteomics link complement activation with interstitial fibrosis/tubular atrophy in lupus nephritis patients. Semin Arthritis Rheum 63:152263
Xue YR, Yao S, Liu Q, Peng ZL, Deng QQ, Liu B, Ma ZH, Wang L, Zhou H, Ye Y, Pan GY (2020) Dihydro-stilbene gigantol relieves CCl(4)-induced hepatic oxidative stress and inflammation in mice via inhibiting C5b–9 formation in the liver. Acta Pharmacol Sin 41:1433–1445
Funding
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in-Aid for Scientific Research, Nos. 16K08728 and 19K07474).
Author information
Authors and Affiliations
Contributions
CI.K.-C: conceptualization, data curation, formal analysis, investigation, validation, writing — original draft. S.Y.: investigation. M.O.: investigation. N.S.: investigation. D.I.: resources, Y.S.: investigation. R.U.: investigation. K.N.: investigation. Y.I.: project administration, writing — original draft, writing — review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kasano-Camones, C.I., Yokota, S., Ohashi, M. et al. Hepatocyte nuclear factor 4α is a critical factor for the production of complement components in the liver. In Vitro Cell.Dev.Biol.-Animal (2024). https://doi.org/10.1007/s11626-024-00972-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11626-024-00972-6