Abstract
Ewing’s sarcoma is an aggressive mesenchymal tumor characterized by the presence of a unique EWSR1-FLI1 translocation. Ewing’s sarcoma primarily occurs in the bone and soft tissues. Cell lines enable researchers to investigate the molecular backgrounds of disease and the significance of genetic alterations in relevant cellular contexts. Here, we report the establishment and characterization of a novel Ewing’s sarcoma cell line following primary Ewing’s sarcoma tumor tissue culture. The established cell line was authenticated by DNA microsatellite short tandem repeat analysis, characterized by in vitro assays, and named NCC-ES1-C1. The NCC-ES1-C1 cell line grew well for 15 mo and was subcultured more than 50 times during this period. Characterization of the cells revealed that they were not adherent and showed floating features. In conclusion, we successfully established a novel Ewing’s sarcoma cell line, NCC-ES1-C1, from primary tumor tissue. The cell line has the characteristic EWSR1-FLI1 gene fusion and exhibits aggressive growth in vitro. Thus, the NCC-ES1-C1 cell line will be a useful tool for investigating the mechanisms of disease and the biological role of the EWSR1-FLI1 fusion gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although Ewing’s sarcoma is rare, it is the second most common primary malignant bone tumor. Ewing’s sarcoma predominantly occurs in children and young adolescents, and accounts for 3% of all childhood malignancies (Rodriguez-Galindo et al. 2007). Ewing’s sarcoma is characterized by the formation of chimeric fusions between the EWS gene and one of the members of the ETS family of transcription factors and 85% of Ewing’s sarcoma cases habor the EWSR1-FLI1 fusion (Delattre et al. 1992; de Álava et al. 2013). The EWSR1-FLI1 fusion product transcriptionally regulates gene expression (Staege et al. 2004), promoting malignant transformation by driving the expression of cancer-associated genes (Bailly et al. 1994; Schwentner et al. 2015). The malignant potential of Ewing’s sarcoma cells depends on the EWSR1-FLI1 fusion oncoprotein (Prieur et al. 2004; Jedlicka 2010; Toomey et al. 2010; Lessnick and Ladanyi 2012; Grohar and Helman 2013), suggesting the possible utility of the EWSR1-FLI1 fusion oncoprotein as a drug target (Uren and Toretsky 2005). Intensive chemotherapy has improved outcomes of patients with Ewing’s sarcoma in the last several decades, and the 5-yr survival rate in patients with localized disease is 70% (Womer et al. 2012). However, treatment options for Ewing’s sarcoma patients with refractory or recurrent tumors are limited (Felgenhauer et al. 2013). Recently, several molecular targeting drugs such as pazopanib (Kasper and Hohenberger 2011; Yamamoto et al. 2014; Alcindor 2015; Attia et al. 2015), figitumumab (Olmos et al. 2010), ganitumab (Juergens et al. 2011; Tap et al. 2012), and regirafenub (clinicaltrials.gov/show/NCT02048371), have been used in the treatment of Ewing’s sarcoma. However, novel therapies using molecular targeting drugs have not yet been developed for Ewing’s sarcoma.
Tissue cultured cells are invaluable tools for pre-clinical research, including examination of the effects of drugs on cell behavior and molecular mechanisms of the tumor suppressive effects of compounds. Currently, a limited number of Ewing’s sarcoma cell lines are available in public cell banks (Bairoch n.d.). Here, we report the establishment of a novel Ewing’s sarcoma cell line. This cell line was established from primary tumor tissues of a 31-yr-old male patient with Ewing’s sarcoma. We characterized the established cell line and used it to examine the efficacy of currently available anti-cancer drugs.
Materials and Methods
Patient background
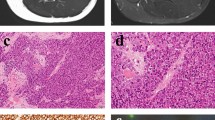
The donor was a 31-yr-old man who had been referred to the National Cancer Center Hospital, Tokyo, Japan, for a bone tumor of the left tibia with progressing knee pain (Fig. 1A, B, C). An incisional biopsy was performed, and fluorescence in situ hybridization revealed FLI1-rearrangement (data not shown). Single pulmonary metastasis and multiple bone metastases were detected by computed tomography (CT) scan and positron emission tomography–CT of the whole body (Fig. 1D). The definitive diagnosis was disseminated Ewing’s sarcoma. The donor patient underwent adjuvant chemotherapy, comprising vincristine, doxorubicin, cyclophosphamide, and actinomycin D, and thigh amputation as local therapy. However, 8 months after the commencement of treatment, the patient died, with multiple visceral, bone, and soft tissue metastases. This study was approved by the ethics committee of the National Cancer Center and written informed consent was obtained from the patient.
Clinical imaging data of the patient with Ewing’s sarcoma. Plain X-ray showing an ill-defined bone lesion of the proximal metaphysis to the center of diaphysis in the left tibia (A). The skeletal tumor was heterogeneous, with iso- to high-intensity signal and over 18 cm long, and an accompanying extraskeletal mass was identified in T2-weighted magnetic resonance imaging, coronal view (B) and axial view (C) of the left lower extremity. Whole body positron emission tomography (D) detected multiple metastases in the lung and spine (arrows) as well as the primary site (arrowheads).
Establishment of a novel ES cell line
Small tumor tissue was cut into small pieces and mechanically dissociated by passing through an 18-gauge (1.02 mm) needle. Cell suspensions were maintained in culture medium DMEM/F12 containing 10% FBS (Gibco, Grand Island, NY) at 37°C in a humidified atmosphere with 5% CO2. Floating and poorly adherent cells were harvested by centrifugation at 1000 rpm/min. The tissue culture medium was exchanged every week. The absence of contaminated mycoplasma was confirmed using e-Myco Mycoplasma PCR Detection Kit (Intron biotechnology, Gyeonggi-do, Korea) according to the manufacture’s instruction.
Authentication and quality control of the established cell line
Genomic DNA was extracted from the tumor tissues or tissue cultured cells using AllPrep DNA/RNA Mini kits (Qiagen, Hilden, Germany). The concentration of DNA was quantified using a NanoDrop 8000 (Thermo Fisher Scientific, Waltham, MA), and the DNA samples were stored at − 80°C until use. Authentication of cells was achieved by examining short tandem repeats (STRs) at 10 loci using STR multiplex assays (GenePrint 10; Promega, Madison, WI) according to the protocol recommended by manufacturer. Genomic DNA (10 ng) was amplified and then examined using a 3500xL Genetic Analyzer (Applied Biosystems, Foster City, CA). Data analysis was performed using GeneMapper 5 software (Applied Biosystems). The STR profiles obtained were compared with those recorded in public cell banks including the American Type Culture Collection, Deutsche Sammlung von Mikroorganismen und Zellkulturen, and the Japanese Collection of Research Bioresources Cell Bank for reference matching.
Fusion transcript
Total RNA was reverse transcribed using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA). PCR amplifications were performed with EWSR1 forward primer F-EWS_22ex7 (5′- TCCTACAGCCAAGCTCCAAGTC-3′) and FLI1 reverse primer R-FLI1_11ex9 (5′- ACTCCCCGTTGGTCCCCTCC-3′) using PlatinumTaq DNA polymerase (Thermo Fisher Scientific, Waltham, MA) (Gamberi et al. 2011). The amplification program consisted of an initial denaturation at 94°C for 2 min and then 40 cycles of denaturation at 94°C for 15 s, annealing at 60°C for 30 s, extension at 68°C for 30 s, and a final extension at 72°C for 5 min using the GeneAmp PCR System 9700 (Applied Biosystems). PCR products were purified with ExoSap-IT (Affimetrix, Santa Clara, CA) and directly sequenced using BigDye v3.1 cycle Sequencing Kit (Applied Biosystems) on the Applied Biosystems 3130xL Genetic Analyzer by Eurofins genomics (Tokyo, Japan). The sequence data were matched against EWSR1 (NM_005243.3) and FLI1 (NM_002017.4) sequences, using BLAST (NIH, Bethesda, MD).
Cell proliferation assay
Cells were seeded in five 96-well culture plates at a density of 1 × 105 cells/well. Cell proliferation was evaluated using the Cell Counting Kit (CCK)-8 kit (Dojindo Molecular Technologies, Kumamoto, Japan), according to the manufacturer’s protocol. In brief, CCK-8 reagent was added to each well after dissociation of aggregated cells by pipetting. The cells were then incubated at 37°C for 2 h. Absorbance at 450 nm was measured using a microplate reader (Bio-Rad, Hercules, CA).
Study of single nucleotide polymorphism (SNP) array
SNP array genotyping was performed using the Infinium OmniExpressExome-8 v1.4 BeadChip (Illumina, San Diego, CA) according to the manufacturer’s instructions. In brief, genomic DNA was extracted from tumor cells and amplified. Amplified DNA then reacted with the array slides in an iScan system (Illumina). Log R ratios and B allele frequencies were calculated using Genome Studio 2011.1 + cnvPartition v3.2.0 (Illumina) and KaryoStudio Data Analysis Software version 1.0 (Illumina). Log R ratios and B allele frequencies represented the normalized signal intensity and the normalized ratio of B allele to the total of both A and B alleles.
Screening anti-cancer drugs for cell growth inhibition
Cells were seeded at a density of 5000 cells/well in a 384-well culture plate in DMEM/F12 medium containing 10% fetal bovine serum, and grown overnight at 37°C in an atmosphere with 5% CO2. One hundred sixty-four low molecular weight chemical compounds, including FDA-approved drugs (Selleck chemicals, Houston, TX), were added using the Bravo Automated liquid handling platform (Agilent technologies, Santa Clara, CA). After 72 h of treatment, living cells were measured by CCK-8. Experiments were performed in duplicate. The anti-cancer drugs used in this study are listed in Supplementary Table 1.
Xenotransplantation of established cell line
NCC-ES1-1C cells (1 × 106) were subcutaneously engrafted into the hind bilateral flanks of 6–10-wk-old female severe immunodeficient NOD.Cg-PrkdcscidIl2rgtm1Sug/Jic (also known as NOD/Shi-scid IL-2Rγnull or NOG) mouse (Central Institute for Experimental Animals, Kanagawa, Japan) with a 25-gauge transplant needle. Tumor formation was visually monitored for 153 d after engraftment. All animal experiments were performed in accordance with the guidelines for Animal Experiments of the National Cancer Center and approved by the Institutional Committee for Ethics of Animal Experimentation.
Results
Detection of EWSR1-FLI1 fusion transcript in the tumor and cell line
We established a cell line, NCC-ES1-C1, from the primary tumor of a patient with Ewing’s sarcoma. The presence of the EWSR1-FLI1 fusion transcripts, unique to Ewing’s sarcoma, in the original tumor tissue and the established cell line was confirmed by RT-PCR and direct Sanger sequencing. Fusion of exon 7 of EWSR1 and exon 8 of FLI1 was consistently observed in both the tumor tissue (Fig. 2A, upper panel) and the cell line (Fig. 2A, lower panel).
Characterization of the NCC-ES1-C1 cell line. NCC-ES1-C1 and tumor mRNA was reverse transcribed, and the EWSR1-Fli1 fusion transcript was sequenced using Sanger sequencing (A, upper panel, original tumor tissue; lower, NCC-ES1-C1 cells). Phase-contrast images of NCC-ES1-C1 cells in suspension cultures (B, C). Cell proliferation was evaluated using the CCK-8 assay (D).
Authentication of the established cell line
STR allele patterns were identical in the original tumor and the cell line (Table 1), and evaluation values higher than 0.7 were not found in public cell banks. Thus, we conclude that the cell line established in the present study is original and had not been previously reported. Based on the guidelines of the International Cell Line Authentication Committee (Capes-Davis et al. 2014; “The International Cell Line Authentication Committee n.d.”), we used PCR to examine the tissue culture medium for mycoplasma contamination. We did not identify DNA fragments unique to mycoplasma (data not shown).
Characterization of NCC-ES1-C1 cells
NCC-ES1-C1 cells exhibited floating and low adherent properties, and showed spheroid formation, even when seeded on conventional tissue culture plates (Fig. 2B, C). The spheroids were highly compact with no distinction between central and peripheral regions and a wide size range. Based on the growth curve, the population doubling time during the logarithmic growth phase was approximately 33 h (Fig. 2D).
Genomic characteristics of tumor tissue and tissue culture cells
An SNP array was used to determine chromosomal gains and losses in the NCC-ES1-C1 cells (Fig. 3). Partially gains were observed at chromosome 11 and 22 on the long arm coincidence of Ewing’s sarcoma harboring with the t(11;22)(q24;q12). Overall, there were few chromosomal gains and losses throughout the entire genome were not excessive.
Xenotransplantation of established cell line
We monitored the tumor formation from subcutaneously engrafted tumor cells for 153 d. However, we did not observe the tumor formation, and concluded that the NCC-ES2-C1 cells did not have a capability to form tumors in mice.
Sensitivity to the treatments with anti-cancer drugs
The sensitivity to anti-cancer drugs clinically available for cancer therapy was assessed in NCC-ES1-C1 cells. Cells were treated with 10 μM of each of 164 anti-cancer drugs (Fig. 4A, Supplementary Table 1), and the cytotoxic effects of anti-cancer drugs were evaluated by a CCK-8 method as described in our previous studies (Kito et al. 2018; Oyama et al. 2018a b, c; Sakumoto et al. 2018). When the cell viability threshold was set to 20%, five drugs, including belinostat, bortezomib, mytomycin C, romidepsin, and sorafenib tosylate, displayed growth-suppressive effects on NCC-ES1-C1 cells. The IC50 values of belinostat, bortezomib, mytomycin C, romidepsin, and sorafenib tosylate were 0.213, 0.002, 0.424, 0.003, and 5.039 μM, respectively (Fig. 4B–F). The IC50 value of pazopanib was considerably high such as 188.8 μM. These results identify these drugs as candidate anti-cancer drugs for Ewing’s sarcoma treatment.
Anti-cancer drug screening in NCC-ES1-C1 cells. (A) NCC-ES1-C1 cells were treated with each of 164 anti-cancer compounds (10 μM) for 72 h. (B–F) Five of the tested drugs inhibited the grown of NCC-ES1-C1 cells when the cell viability threshold was set to 20%. Viability of the cells treated with belinostat, bortezomib, mytomycin C, romidepsin, and sorafenib tosylate anti-cancer drugs. The IC50 value is shown for each anti-cancer drug.
Discussion
Patient-derived cancer models are invaluable tools for cancer research. By investigating phenotypic changes induced by the transfection of genes and treatment with anticancer drugs in these cell lines, we can determine the functions of novel or interesting genes and assess the possible effects of anticancer drugs. Although many Ewing’s sarcoma cell lines are available in cell banks, these cell lines generally lack clinical information about the donor patients. Cell lines with clinical and pathological information will be a useful resource for interpretation of experimental results. Therefore, we have described the details of the donor patient from whom the NCC-ES1-C1 cells were derived (Fig. 1). In contrast, Ewing’s sarcoma cell lines available from public cell banks such as ATCC did not accompany such detailed clinical and pathological information (ATCC, www.atcc.org). Considering the clinical and biological heterogeneity of the tumor and among patients, a single cell line may not be sufficient to establish a correlation between in vitro observations and clinical features. Thus, multiple cell lines from single tumors with the same histological background are required. Ewing’s sarcoma is an extremely rare sarcoma, so conscientious efforts will be required for the establishment of such cell lines.
We confirmed that the EWSR1-FLI1 fusion transcript was preserved in the NCC-ES1-C1 cell line (Fig. 1A). Previous studies in a model system with the EWSR1-FLI1 fusion gene demonstrated that EWSR1-FLI1 gene expression patterns resembled those observed in the original tumor (Moore et al., 2015), probably because EWSR1-FLI1 functions as a transcription regulator. In the present study, gene expression studies were not possible as none of the original tumor remained. However, this hypothesis should be confirmed in our future study when we establish Ewing’s sarcoma cell lines.
Although Ewing’s sarcoma is a solid tumor, NCC-ES1-C1 cells are suspension cells (Fig. 2B, C). (Kodama et al. 1991) previously reported a Ewing’s sarcoma cell line, CADO-ES1, which was established from pleural effusion and partially attached to the tissue culture flask. Anchorage-independent growth is a sign of malignant tumor cells (Santini and Rainaldi 1999), and (Lawlor et al. 2002) reported that, in Ewing’s sarcoma, anchorage-independent spheroids were more closely related to primary tumors with respect to cell morphology, cell-cell junctions, proliferative index, and kinase activation. Therefore, NCC-ES1-C1 cells should be an appropriate model to investigate the malignant characters of tumor cells in Ewing’s sarcoma in vivo.
The NCC-ES1-C1 cell line exhibited continual growth (Fig. 2D), suggesting that these cells can be used in cell-based assays such as those for the anti-proliferative effects of novel compounds and ectopic gene expression studies. We investigated the genomic integrity of the cell line using SNP array. We found that although there was some amplification and deletion of genes, it was not frequently observed in NCC-ES1-C1 cells (Fig. 3). Chromosome instability is a distinctive feature of cancer cells (Snijders et al. 2001). However, the chromosomal constitution of Ewing’s sarcoma is relatively stable, likely because these tumor cells depend on EWS-ETS fusion proteins, and there are few recurrent genetic lesions (Brohl et al. 2014; Crompton et al. 2014; Tirode et al. 2014). Our results are consistent with these reports.
By screening a library of 164 anti-cancer drugs, we found that certain drugs had suppressive effects on growth at relatively low concentrations (Fig. 4, Supplementary Table 1). Specifically, these drugs included belinostat, bortezomib, Mitomycin C, romidepsin, and sorafenib. These anti-cancer drugs have different paharmacological activieis. Belinostat (Plumb et al. 2003) and romidepsin (Ueda et al. 1994) are histone deacetylase inhibitors. Bortezomib is a proteasome inhibitor (Adams and Kauffman 2004). Mitomycin C has an anti-tumor activity by bioreductive alkylation and DNA cross linking (Tomasz 1995). Sorafenib is an inhibitor for multiple kinases such as B-raf, c-KIT, VEGFT, and PDGFR (Wilhelm et al. 2004; Carlomagno et al. 2006). As these drugs have been used for the treatments of other malignancies, their use in the treatment of Ewing’s sarcoma is worth reexamining. The number of patients with Ewing’s sarcoma is awfully limited, making it difficult to perform conventional clinical trials in a practical period of time. The model systems that faithfully reproduce the response to treatments will be helpful for the efficient clinical trials. The anti-cancer drugs whose proliferation-suppressive effects on Ewing’s sarcoma cells are worth investigating by using other patient-derived models such as organoids and xenogrfats for clinical trials.
Conclusion
We established and characterized a novel patient-derived Ewing’s sarcoma cell line, NCC-ES1-C1. NCC-ES1-C1 cells retained the characteristic chimera transcript and genomic features unique to Ewing’s sarcoma. These cells also grew in continuously in suspension, forming spheroid shapes. We demonstrated that a drug library can be screened using NCC-ES1-C1 cells. Thus, we postulate that the NCC-ES1-C1 cell line will be a useful resource in research on Ewing’s sarcoma. In contrast, the original tumor tissue was not remained for global expression study, and the similarity of gene and protein expression between the original tumor cells and NCC-ES1-C1 cells were not evaluated in this study. Moreover, although the clinical data of a donor patient for NCC-ES1-C1 cells are detailed, a single cell line cannot is not enough to establish a meaningful association between the cell line characters and clinical observations. Thus, more number of cell lines should be required to promote the development of therapeutic strategies in Ewing’s sarcoma.
References
Adams J, Kauffman M (2004) Development of the proteasome inhibitor Velcade (Bortezomib). Cancer Investig 22(2):304–311
Alcindor T (2015) Response of refractory Ewing sarcoma to pazopanib. Acta Oncol 54(7):1063–1064. https://doi.org/10.3109/0284186x.2014.971938
Attia S, Okuno SH, Robinson SI, Webber NP, Indelicato DJ, Jones RL et al (2015) Clinical activity of pazopanib in metastatic extraosseous Ewing sarcoma. Rare Tumors 7(2):5992–5988. https://doi.org/10.4081/rt.2015.5992
Bailly RA, Bosselut R, Zucman J, Cormier F, Delattre O, Roussel M, Thomas G, Ghysdael J (1994) DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Mol Cell Biol 14(5):3230–3241
Bairoch, A (n.d.). The Cellosaurus: a cell line knowledge resource
Brohl AS, Solomon DA, Chang W, Wang J, Song Y, Sindiri S et al (2014) The genomic landscape of the Ewing sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genet 10(7):e1004475. https://doi.org/10.1371/journal.pgen.1004475
Capes-Davis A, Dirks W, MacLeod R, Uphoff C (2014) Quality matters: cell lines and their use in research. GIT Lab J Eur 17:12–13
Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, Santoro M (2006) BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst 98(5):326–334. https://doi.org/10.1093/jnci/djj069
Crompton BD, Stewart C, Taylor-Weiner A, Alexe G, Kurek KC, Calicchio ML et al (2014) The genomic landscape of pediatric Ewing sarcoma. Cancer Discov 4(11):1326–1341. https://doi.org/10.1158/2159-8290.cd-13-1037
de Álava E, Lessnick SL, & Sorensen PHB (2013) Ewing sarcoma (C. D. M. Fletcher, J. A. Bridge, P. C. W. Hogendoorn, & F. Mertens Eds.)
Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M et al (1992) Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359(6391):162–165. https://doi.org/10.1038/359162a0
Felgenhauer JL, Nieder ML, Krailo MD, Bernstein ML, Henry DW, Malkin D et al (2013) A pilot study of low-dose anti-angiogenic chemotherapy in combination with standard multiagent chemotherapy for patients with newly diagnosed metastatic Ewing sarcoma family of tumors: a Children’s Oncology Group (COG) phase II study NCT00061893. Pediatr Blood Cancer 60(3):409–414. https://doi.org/10.1002/pbc.24328
Gamberi G, Cocchi S, Benini S, Magagnoli G, Morandi L, Kreshak J et al (2011) Molecular diagnosis in Ewing family tumors: the Rizzoli experience--222 consecutive cases in four years. J Mol Diagn 13(3):313–324. https://doi.org/10.1016/j.jmoldx.2011.01.004
Grohar PJ, Helman LJ (2013) Prospects and challenges for the development of new therapies for Ewing sarcoma. Pharmacol Ther 137(2):216–224. https://doi.org/10.1016/j.pharmthera.2012.10.004
Jedlicka P (2010) Ewing sarcoma, an enigmatic malignancy of likely progenitor cell origin, driven by transcription factor oncogenic fusions. Int J Clin Exp Pathol 3(4):338–347
Juergens H, Daw NC, Geoerger B, Ferrari S, Villarroel M, Aerts I et al (2011) Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol 29(34):4534–4540. https://doi.org/10.1200/jco.2010.33.0670
Kasper B, Hohenberger P (2011) Pazopanib: a promising new agent in the treatment of soft tissue sarcomas. Future Oncol 7(12):1373–1383. https://doi.org/10.2217/fon.11.116
Kito F, Oyama R, Sakumoto M, Takahashi M, Shiozawa K, Qiao Z et al (2018) Establishment and characterization of novel patient-derived osteosarcoma xenograft and cell line. Vitro Cell Dev Biol Anim 54(7):528–536. https://doi.org/10.1007/s11626-018-0274-2
Kodama K, Doi O, Higashiyama M, Mori Y, Horai T, Tateishi R et al (1991) Establishment and characterization of a new Ewing’s sarcoma cell line. Cancer Genet Cytogenet 57(1):19–30
Lawlor ER, Scheel C, Irving J, Sorensen PH (2002) Anchorage-independent multi-cellular spheroids as an in vitro model of growth signaling in Ewing tumors. Oncogene 21(2):307–318. https://doi.org/10.1038/sj.onc.1205053
Lessnick SL, Ladanyi M (2012) Molecular pathogenesis of Ewing sarcoma: new therapeutic and transcriptional targets. Annu Rev Pathol 7:145–159. https://doi.org/10.1146/annurev-pathol-011110-130237
Moore JB t, Loeb DM, Hong KU, Sorensen PH, Triche TJ, Lee DW et al (2015) Epigenetic reprogramming and re-differentiation of a Ewing sarcoma cell line. Front Cell Dev Biol 3:15. https://doi.org/10.3389/fcell.2015.00015
Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, Batzel GN, Yin D, Pritchard-Jones K, Judson I, Worden FP, Gualberto A, Scurr M, de Bono JS, Haluska P (2010) Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol 11(2):129–135. https://doi.org/10.1016/s1470-2045(09)70354-7
Oyama R, Kito F, Sakumoto M, Shiozawa K, Toki S, Endo M et al (2018a) Establishment and proteomic characterization of a novel synovial sarcoma cell line, NCC-SS2-C1. Vitro Cell Dev Biol Anim 54(5):392–399. https://doi.org/10.1007/s11626-018-0237-7
Oyama R, Kito F, Sakumoto M, Shiozawa K, Toki S, Yoshida A et al (2018b) Establishment and proteomic characterization of a novel cell line, NCC-UPS2-C1, derived from a patient with undifferentiated pleomorphic sarcoma. Vitro Cell Dev Biol Anim 54(3):257–263. https://doi.org/10.1007/s11626-018-0229-7
Oyama R, Takahashi M, Kito F, Sakumoto M, Shiozawa K, Qiao Z et al (2018c) Establishment and characterization of patient-derived xenograft and its cell line of primary leiomyosarcoma of bone. Vitro Cell Dev Biol Anim 54(6):458–467. https://doi.org/10.1007/s11626-018-0258-2
Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR, Watkins CJ et al (2003) Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther 2(8):721–728
Prieur A, Tirode F, Cohen P, Delattre O (2004) EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol 24(16):7275–7283. https://doi.org/10.1128/mcb.24.16.7275-7283.2004
Rodriguez-Galindo C, Liu T, Krasin MJ, Wu J, Billups CA, Daw NC et al (2007) Analysis of prognostic factors in Ewing sarcoma family of tumors: review of St. Jude Children’s Research Hospital studies. Cancer 110(2):375–384. https://doi.org/10.1002/cncr.22821
Sakumoto M, Oyama R, Takahashi M, Takai Y, Kito F, Shiozawa K et al (2018) Establishment and proteomic characterization of patient-derived clear cell sarcoma xenografts and cell lines. Vitro Cell Dev Biol Anim 54(2):163–176. https://doi.org/10.1007/s11626-017-0207-5
Santini MT, Rainaldi G (1999) Three-dimensional spheroid model in tumor biology. Pathobiology 67(3):148–157. https://doi.org/10.1159/000028065
Schwentner R, Papamarkou T, Kauer MO, Stathopoulos V, Yang F, Bilke S et al (2015) EWS-FLI1 employs an E2F switch to drive target gene expression. Nucleic Acids Res 43(5):2780–2789. https://doi.org/10.1093/nar/gkv123
Snijders AM, Nowak N, Segraves R, Blackwood S, Brown N, Conroy J et al (2001) Assembly of microarrays for genome-wide measurement of DNA copy number. Nat Genet 29(3):263–264. https://doi.org/10.1038/ng754
Staege MS, Hutter C, Neumann I, Foja S, Hattenhorst UE, Hansen G et al (2004) DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res 64(22):8213–8221. https://doi.org/10.1158/0008-5472.can-03-4059
Tap WD, Demetri G, Barnette P, Desai J, Kavan P, Tozer R et al (2012) Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol 30(15):1849–1856. https://doi.org/10.1200/jco.2011.37.2359
The International Cell Line Authentication Committee. Retrieved from http://standards.atcc.org/kwspub/home/the_international_cell_line_authentication_committee-iclac_/
Tirode F, Surdez D, Ma X, Parker M, Le Deley MC, Bahrami A et al (2014) Genomic landscape of Ewing sarcoma defines an aggressive subtype with co-association of STAG2 and TP53 mutations. Cancer Discov 4(11):1342–1353. https://doi.org/10.1158/2159-8290.cd-14-0622
Tomasz M (1995) Mitomycin C: small, fast and deadly (but very selective). Chem Biol 2(9):575–579
Toomey EC, Schiffman JD, Lessnick SL (2010) Recent advances in the molecular pathogenesis of Ewing's sarcoma. Oncogene 29(32):4504–4516. https://doi.org/10.1038/onc.2010.205
Ueda H, Nakajima H, Hori Y, Fujita T, Nishimura M, Goto T, Okuhara M (1994) FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J Antibiot (Tokyo) 47(3):301–310
Uren A, Toretsky JA (2005) Ewing’s sarcoma oncoprotein EWS-FLI1: the perfect target without a therapeutic agent. Future Oncol 1(4):521–528. https://doi.org/10.2217/14796694.1.4.521
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109. https://doi.org/10.1158/0008-5472.can-04-1443
Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE et al (2012) Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 30(33):4148–4154. https://doi.org/10.1200/jco.2011.41.5703
Yamamoto Y, Nozawa M, Shimizu N, Minami T, Yoshimura K, Uemura H (2014) Pazopanib for recurrent extraosseous Ewing’s sarcoma of the retroperitoneum. Int J Urol 21(11):1183–1184. https://doi.org/10.1111/iju.12546
Acknowledgments
We appreciate Drs. M. Endo, Y. Minami, K. Shimizu, T. Mori, T. Uehara, M. Sugawara, Y. Araki, Ms. R. Nakano, and S. Zenitani, Division of Musculoskeletal Oncology, National Cancer Center Hospital, for sampling tumor-tissue specimens from surgically resected materials. We would also like to thank Editage (www.editage.jp) for English-language editing and their constructive comments on the manuscript.
Funding
This research was supported by the National Cancer Center Research and Development Fund (grant nos. 26-A-3, 26-A-9, and 29-A-2), and by the Fundamental Innovative Oncology Core in the National Cancer Center. We appreciate Dr. H. Sakamoto for SNP array experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Electronic supplementary material
Supplementary Table 1
(XLSX 22 kb)
Rights and permissions
About this article
Cite this article
Oyama, R., Kito, F., Qiao, Z. et al. Establishment of a novel patient-derived Ewing’s sarcoma cell line, NCC-ES1-C1. In Vitro Cell.Dev.Biol.-Animal 54, 770–778 (2018). https://doi.org/10.1007/s11626-018-0302-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-018-0302-2