Abstract
The purposes of this study were to examine the cartilage degradation effects of triamcinolone acetonide (TA) on normal and osteoarthritic (OA) primary canine chondrocytes and cartilage explants and to examine the cartilage degradation effects of TA in combination with low-molecular-weight hyaluronan (LMWHA). To assess the effects of these drugs on cell culture, 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay and real-time PCR were used to measure chondrotoxicity and determine gene expression, respectively. Uronic acid and hydroxyproline remaining in cartilage and histopathology were used to estimate the effects of these drugs on cartilage explants. In chondrocyte cultures, TA reduced chondrocyte viability in a concentration-dependent manner. LMWHA 2.5 mg/ml combined with TA at IC20 (0.09 mg/ml) could increase the viability of normal chondrocytes when compared with TA-treated alone. TA at IC20 induced down-regulation of ACAN and induced up-regulation of ADAMTS5 in canine normal chondrocytes. TA at IC20 (0.11 mg/ml) up-regulated ADAMTS5, MMP2, MMP3, MMP13, and ACAN expression in canine OA chondrocytes. In explant culture, TA at 1.25, 2.5, and 5 mg/ml increased the severity of structural damage, chondrocyte loss and cluster formation, and proteoglycan loss in OA cartilage. LMWHA could decrease the chondrotoxicity of TA at IC20 only in normal chondrocytes, as observed by chondrocyte viability. The combination of LMWHA and TA did not show clearly beneficial effects in all other normal and OA samples. Consequently, using TA alone or in combination with LMWHA in OA cartilage should be of concern because it may lead to cartilage destruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage is a hyaline cartilage which acts as a protector against compression and shear force on the bone (Fox et al. 2009). Articular cartilage is composed of an extracellular matrix (ECM) surrounding specialized resident cells called chondrocytes, which mainly regulate cartilage metabolism (Archer and Francis-West 2003). Hyaluronan is a high-molecular-weight linear polysaccharide that is synthesized by type B synovial cells. It is responsible for the viscoelasticity and lubricating properties of synovial fluid (Laurent et al. 1996).
Osteoarthritis (OA) is a degenerative joint disease that commonly occurs in older humans and animals, especially in dogs in multiple joints. This disease is related to progression of chondrocyte death and cartilage destruction. OA patients are affected by pain, stiffness, lameness, and joint immobility. When an imbalance of proteinase and its inhibitor occurs, ECM degradation begins, leading to greater severity of OA (Dean et al. 1989). In OA, chondrocytes can produce interleukin-1 (IL-1) which degrades the cartilage by stimulating catabolic enzymes such as a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) (Bondeson et al. 2008) and matrix metalloproteinases (MMPs) and other cytokine products (Pelletier et al. 2001), resulting in increased collagen turnover rate and decreased proteoglycan content (Bland and Cooper 1984).
Intra-articular corticosteroids (CSs) are commonly used in joint arthropathy such as inflammatory joint disease, OA, and rheumatoid arthritis (Wernecke et al. 2015). CSs exert a potent anti-inflammatory effect on joints by inhibiting inflammatory cytokines and reducing pain and effusion, but prolonged use of CS may result in negative effects and accelerate OA progression (Evans et al. 2014). Triamcinolone acetonide (TA) is an intermediate-acting synthetic CS suitable for intra-articular injection; its chemical name is 9-fluoro-11β, 16α, 17,21-tetrahydroxy-pregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone. Acetonide esters in TA can delay absorption and extend the duration of action (Soma et al. 2011). Intra-articular (IA) injection of TA has been found to be more effective than other CS for relief of pain in OA joints (Hepper et al. 2009). TA is one of the most popular drugs used for treatment of OA, but adverse effects on both normal articular cartilage (Dechant et al. 2003; Syed et al. 2011) and on some OA joints (Haddad 2000) have been reported.
This gave us the idea to find another drug to combine with TA that would be able to reduce its adverse side effects. Hyaluronan (HA) is one of the most promising candidate drugs because it has been proven to have chondroprotective and disease-modifying effects in OA (Corrado et al. 1995; Listrat et al. 1997; Guidolin et al. 2001; Jubb et al. 2003). HA is also effective for reducing joint pain and stiffness (Goldberg and Buckwalter 2005). It can stimulate proteoglycan and new HA synthesis, reduce proinflammatory cytokines such as IL-1, and degrade enzymes such as MMPs and other proteases. Low-molecular-weight HA (LMWHA), 500–1000 kDa, is widely used in OA joints because it can reduce synovial inflammation, restore synovial fluid properties (Ghosh and Guidolin 2002), suppress chondrocyte apoptosis (Takahashi et al. 2000; Zhou et al. 2008; Barreto et al. 2015), improve mitochondrial function (Grishko et al. 2009), and enhance chondrocyte proliferation (Kawasaki et al. 1999).
Previous studies on the effects of HA combined with other drugs—such as CSs (Doyle et al. 2005; Yates et al. 2006; Schaefer et al. 2009; Siengdee et al. 2015), local anesthesia (Onur et al. 2013; Güngör et al. 2014) and NSAIDs (Euppayo et al. 2015)—have demonstrated that HA can decrease some of the adverse effects of these drugs due to its chondroprotective properties. Therefore, the purpose of this study was to determine the effects of a combination of LMWHA and TA on normal and OA canine chondrocytes and cartilage explants in vitro. The hypothesis was that LMWHA can reduce the chondrotoxicity and cartilage degradation effects of TA in all normal and OA chondrocytes and cartilage explants.
Materials and Methods
Reagents
A commercial specific hyaluronan (HA) fraction of defined molecular chain length (2500–3500 saccharide units, molecular weight 500–750 kDa) (TRB Chemedica, Bangkok, Thailand) was used in this study. The concentration of HA stock solution was 10 mg/ml. Before use, HA was diluted with Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad CA) to 2.5 mg/ml. Triamcinolone acetonide (TA) for IA injection stock solution (10 mg/ml) was obtained from L.B.S. Laboratory (Bangkok, Thailand).
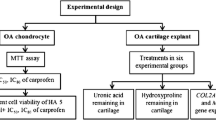
Experimental designs
This research focused on the effects of TA and HA in both canine normal and spontaneous OA on primary canine chondrocytes as well as cartilage explants, in addition to their administration in combination with HA (Fig. 1). The direct effects of the drugs on primary chondrocytes were assessed. After drug treatment for 24 h, 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay and quantitative real-time polymerase chain reaction (qPCR) were used to investigate chondrocyte viability and gene expression, respectively. Cartilage explants were treated with three concentrations of TA, HA, or a combination of the drugs for 14 d. After that, the treated cartilage was assessed for cartilage degradation by measuring uronic acid and hydroxyproline remaining in cartilage. Histopathology was performed using hematoxylin and eosin (H&E) and Safranin O staining.
Experimental design of the study. TA triamcinolone acetonide, HA hyaluronan, MTT 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, OA osteoarthritis, qPCR quantitative real-time polymerase chain reaction, IC 20 inhibitory concentration at 20%, IC 50 inhibitory concentration at 50%, H&E hematoxylin and eosin.
Primary canine chondrocytes cultures
Normal articular chondrocytes and OA articular chondrocytes from normal and OA canine knee and elbow joints were harvested from dog cadavers at the Veterinary Cadaveric Unit, Faculty of Veterinary Medicine, Chiang Mai University, Thailand. After joints were dissected by aseptic technique, normal and OA articular cartilages were chopped into 1–2-mm pieces and placed in separate cell culture disks. Then, 10% collagenase type II (Sigma-Aldrich, St. Louis MO) diluted in DMEM was added to the disks, followed by incubation under conditions of 5% CO2, 37°C, and 70% relative humidity for 21 h (Euppayo et al. 2015). The cartilage disks were cultured in growth medium: DMEM containing 10% fetal bovine serum (FBS; PAA Laboratories, Pasching, Austria) and Gibco® Antibiotic–Antimycotic (100×) solution (Thermo Fisher Scientific, Waltham MA). The growth medium was changed every 3 d until the end of treatment. At 70–80% confluence, cells were trypsinized until passage 3–6.
Canine cartilage explant cultures
Normal cartilage explant cultures from joints without OA lesions and OA cartilage explant cultures from OA canine knee and elbow joints were harvested from dog cadavers (see above). Joints were dissected by aseptic technique. The articular cartilages were then sliced into small pieces (radius ∼3–4 mm2) and incubated in serum-free DMEM supplemented with antibiotic–antimycotic solution (100×) at 37°C under 5% CO2 for 24 h. Three pieces of cartilage, weighing a total of ~30–35 mg, were placed in individual wells in 24-well culture plates, together with 1 ml of serum-free DMEM containing antibiotic–antimycotic reagent, and cultured at 37°C under 5% CO2 (Euppayo et al. 2015).
MTT assay for chondrotoxicity of TA and LMWHA
Normal and OA canine articular chondrocytes were separately trypsinized, seeded in 96-well plates (20,000 cells per well), and cultured in DMEM-free FBS at 37°C under 5% CO2 for 24 h. The cells were then treated with 2-fold dilutions of TA and LMWHA in triplicate, including 0.04, 0.08, 0.16, 0.31, 0.63, 1.25, 2.5, and 5 mg/ml, and incubated under the conditions above for 24 h. The media were replaced with MTT dissolved in DMEM, and incubation was continued at 37°C for 4 h. Dimethyl sulfoxide (DMSO) was added to each well and then shaken for 5 min. Absorbance of the color violet was measured at 540 nm using a microplate reader (Denizot and Lang 1986). The cell viability of chondrocytes treated with the drugs was calculated as follows:
An average inhibitory concentration of 20% (IC20) will result in 80% cell survival, while an average inhibitory concentration of 50% (IC50) will result in 50% survival. Co-treatment with TA (at IC20 and IC50) and HA (2.5 mg/ml) was performed to determine whether the effect of HA could reduce the toxicity of TA, as evaluated by MTT assay.
Gene expression by qPCR
RNA was isolated from conditioned chondrocytes 24 h after incubation using an innuPREP DNA/RNA Mini Kit (Analytik Jena, Jena, Germany) according to the supplementary protocol. Total (DNase-treated) RNA 1.5 μg/μl was reverse transcribed into complementary DNA (cDNA) using 200 U of Tetro reverse transcriptase enzyme (Bioline, Taunton, MA) and 10 mM of oligo (dT) primer in a total reaction volume of 20 μl. Gene expressions of collagen type II alpha 1 (COL2A1), aggrecan (ACAN), interleukin-1β (IL-1β), ADAM metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS5), matrix metalloproteinase-2 (MMP2), matrix metalloproteinase-3 (MMP3), and matrix metalloproteinase-13 (MMP13) were evaluated by comparing with a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), using 1 μl of synthesized cDNA, 5 μl of 2× SensiFAST SYBR® No-ROX Mix (Bioline), and 0.4 μl (each) of 10 μM forward and reverse primers. The relative expression level was calculated using 2−ΔΔCT method (Livak and Schmittgen 2001).
Uronic acid remaining in cartilage explants
The cartilage disks after exposure to either TA, LMWHA, or a combination of TA and LMWHA for 14 d were assessed by colorimetric assay for levels of uronic acid (repeating units of glycosaminoglycans) remaining in cartilage samples (Blumenkrantz and Asboe-Hansen 1973). Briefly, two units of papain were added for digesting the cartilage matrix and incubated at 60°C for 48 h. The papain-digested cartilage was diluted 10-fold with distilled water. After the addition of reagent A (0.025 M Na2B4O7 in concentrated sulfuric acid) to each diluted sample and uronic acid standard using glucuronic acid, they were incubated at 100°C for 15 min in glass test tubes. Subsequently, reagent B (50 mg carbazole in 40 ml absolute ethanol) was added, followed by incubation at 100°C for 15 min. After incubation, uronic acid standards and all samples were added separately into a 96-well plate to measure the absorbance at 540 nm by a microplate reader.
Hydroxyproline remaining in cartilage
Papain-digested cartilage from conditioned cartilage was used for measurement of hydroxyproline level (a major component of collagen) (Kolar 1990). The papain-digested cartilage was treated with HCl and incubated for 24 h at 60°C. Then, NaOH was added to adjust the pH to 7. The samples were diluted 40-fold by adding distilled water. After that, oxidizing solution was added to the samples (at 25°C for 5 min), followed by Ehrlich’s reagent (7.5% dimethylaminobenzaldehyde in propan-2-ol) at 60°C for 45 min. The absorbance of samples and hydroxyproline standard was read at 540 nm by a microplate reader.
Histopathology study
Treated cartilage explants were fixed with 4% paraformaldehyde, dehydrated with ethanol, and embedded in paraffin wax blocks which were cut into 3-μm-thick slices using a rotary microtome. The sections of each sample were stained with H&E to determine the general cartilage structure of the ECM, content damage, and chondrocyte characteristics. Safranin O staining was used to estimate proteoglycan content (Cook et al. 2010). OA in canine cartilage was assessed by veterinarians according to an adapted protocol of the Osteoarthritis Research Society International (OARSI) histopathology initiative recommendations: grading of cartilage structure, chondrocyte pathology, and proteoglycan staining (Cook et al. 2010). Grading of cartilage structure was based on the severity of fissures and erosion of the cartilage layer. Chondrocyte pathology was characterized by the severity of chondrocyte loss and the chondrocyte clusters predominating in each cartilage layer. Proteoglycan staining was assessed by decreased proteoglycan content in cartilage.
Statistical analysis
The data from each experiment (cell viability, gene expression, and uronic acid and hydroxyproline remaining in cartilage) was compared separately in normal and OA groups in terms of means and standard deviation (SD). Data analysis was performed by one-way analysis of variance (ANOVA) and Tukey’s test. SPSS software was used for all statistical analysis. Results with a P value <0.05 were considered statistically significantly different.
Results
Effects of TA with and without HA on cell viability
The effects of TA and HA on normal and OA chondrocyte viability are illustrated in Fig. 2. The percentages of cell viability for TA at the highest concentration of 50% stock solution dilution (5 mg/ml) were 45.73 ± 4.76 and 38.69 ± 6.1% in normal and OA chondrocytes, respectively. The effect of TA on the percentage of cell viability was concentration-dependent in both normal and OA chondrocytes. It was determined that the concentration of TA at IC20 and IC50 was 0.09 and 2.23 mg/ml, respectively, in normal chondrocytes, while in OA chondrocytes, the concentration of TA at IC20 and IC50 was 0.11 and 1.14 mg/ml, respectively. For HA treatment, the highest concentration, 50% stock solution (5 mg/ml), resulted in percentages of cell viability of 94.81 ± 11.77 and 104.38 ± 17.38% in normal and OA chondrocytes, respectively. All concentrations of HA did not affect cell survival, as there was more than 80% cell viability in both normal and OA chondrocytes—not a significant difference when compared with the control groups.
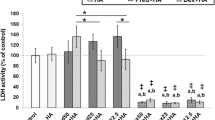
The effects of TA at IC20 and TA at IC50 combined with HA 2.5 mg/ml are shown in Fig. 3. HA treatment alone increased the percentage of cell viability compared with the control group of normal chondrocytes (P < 0.05). The combination of HA and TA at IC20 increased the percentage of cell viability more than treatment with TA at IC20 alone in normal chondrocytes (P < 0.05). In OA chondrocytes, HA alone did not affect chondrocyte viability when compared with the control (P < 0.05). HA combined with TA at IC20 and TA at IC50 did not improve OA chondrocyte viability when compared with TA at each concentration (P < 0.05).
Percentage (mean ± SD) of cell viability in normal and OA primary canine chondrocytes treated for 24 h with TA at average inhibitory concentrations of 20% (IC20) and 50% (IC50), with and without HA 2.5 mg/ml, as determined by MTT assay. Asterisk indicates a significant difference (P < 0.05) compared with the control in normal and OA chondrocyte groups; number sign indicates a significant difference between two treatments in the same chondrocyte group.
Gene expression by qPCR
The relevant gene expressions, including anabolic genes (COL2A1 and ACAN) and catabolic genes (IL-1β, ADAMTS5, MMP2, MMP3, and MMP13), were investigated by qPCR. Before testing the effect of the drugs, gene expressions were performed in non-treated (control) chondrocytes (Fig. 4). The results exhibited a higher level of COL2A1, ACAN, ADAMTS5, MMP2, and MMP13 expression in OA than normal chondrocytes (P < 0.05). Gene expression in treated chondrocytes is shown in Fig. 5. In normal chondrocytes, HA treatment alone could induce the up-regulation of COL2A1 and ADAMTS5 (P < 0.05), whereas the expression of ACAN was significantly down-regulated (P < 0.05) after treatment with TA at IC20. Furthermore, it was found that the simultaneous use of HA and TA at IC20 induced down-regulation of IL-1β, MMP3, and MMP13 expression when compared with TA at IC20 alone, but with no significant difference.
Relative expression (mean ± SD) of COL2A1, ACAN, IL-1β, ADAMTS5, MMP2, MMP3, and MMP13 in normal and OA primary canine chondrocytes treated with TA at an inhibitory concentration of 20% (IC20), with HA (2.5 mg/ml), or in combination. Asterisk indicates a significant difference (P < 0.05) compared with the control in each chondrocyte group; dagger indicates a significant difference between the treatments in a chondrocyte group.
In OA chondrocytes, treatment with TA at IC20 induced up-regulation of ACAN, ADAMTS5, MMP2, and MMP3 expression (P < 0.05). HA induced up-regulation of ADAMTS5, MMP2, and MMP3 expression, in contrast to MMP13. The simultaneous use of HA and TA at IC20 decreased the expression of ACAN, IL-1β, ADAMTS5, MMP2, MMP3, and MMP13 when compared with TA at IC20 alone, but with no significant difference (Fig. 5).
Uronic acid remaining in cartilage
The levels of uronic acid remaining in cartilage explants are represented in Fig. 6. There was no significant difference among experimental groups in both normal and OA cartilage explants. After 14 d incubation, treatment of the cartilage with HA; TA at 1.25, 2.5, and 5 mg/ml; and in combination had no effect on uronic acid remaining in cartilage in both normal and OA groups when compared with the controls (P < 0.05).
Hydroxyproline remaining in cartilage
The amount of hydroxyproline remaining in cartilage explants is shown in Fig. 7. There was no significant difference among the control; HA treatment; TA at 1.25, 2.5, and 5 mg/ml; and a combination of HA and TA in both normal and OA cartilage explants (P < 0.05). These results are similar to uronic acid remaining in cartilage after incubation for 14 d.
Histopathology study
For assessment of cartilage structure, chondrocyte pathology and proteoglycan content, the treated cartilage explants were stained with either H&E or Safranin O, as shown in Figs. 8 and 9, respectively. Most normal cartilage explants had a smooth surface, with all zones intact and with some fissures at the surface; there was no clear difference among experimental groups. Additionally, the pathology of the normal chondrocytes demonstrated normal characteristics, with some partial loss of cells in the superficial zone, and again showing no clear difference among experimental groups (Fig. 8A–H ).
Histopathology using H&E staining of normal and OA canine cartilage explants after treatment with TA (1.25, 2.5, and 5 mg/ml), with and without HA (2.5 mg/ml), for 14 d. The bar indicates 100 μm (100×). (A) control, normal cartilage; (B) TA 1.25 mg/ml, normal cartilage; (C) TA 2.5 mg/ml, normal cartilage; (D) TA 5 mg/ml, normal cartilage; (E) HA, normal cartilage; (F) TA 1.25 mg/ml + HA, normal cartilage; (G) TA 2.5 mg/ml + HA, normal cartilage; (H) TA 5 mg/ml + HA, normal cartilage; (I) control, OA cartilage; (J) TA 1.25 mg/ml, OA cartilage; (K) TA 2.5 mg/ml, OA cartilage; (L) TA 5 mg/ml, OA cartilage; (M) HA, OA cartilage; (N) TA 1.25 mg/ml + HA, OA cartilage; (O) TA 2.5 mg/ml + HA, OA cartilage; (P) TA 5 mg/ml + HA, OA cartilage.
Histopathology using Safranin O staining of normal and OA canine cartilage explants after treatment with TA (1.25, 2.5, and 5 mg/ml), with and without HA (2.5 mg/ml), for 14 d. The bar indicates 100 μm (50×). (A) control, normal cartilage; (B) TA 1.25 mg/ml, normal cartilage; (C) TA 2.5 mg/ml, normal cartilage; (D) TA 5 mg/ml, normal cartilage; (E) HA, normal cartilage; (F) TA 1.25 mg/ml + HA, normal cartilage; (G) TA 2.5 mg/ml + HA, normal cartilage; (H) TA 5 mg/ml + HA, normal cartilage; (I) control, OA cartilage; (J) TA 1.25 mg/ml, OA cartilage; (K) TA 2.5 mg/ml, OA cartilage; (L) TA 5 mg/ml, OA cartilage; (M) HA, OA cartilage; (N) TA 1.25 mg/ml + HA, OA cartilage; (O) TA 2.5 mg/ml + HA, OA cartilage; (P) TA 5 mg/ml + HA, OA cartilage.
In OA cartilage explants (Fig. 8I–P ), the control group presented a smooth surface with multi-focal fissures in the superficial zone. Chondrocyte pathology in the control group showed normal to global loss of cells in the surface zone, with occasional superficial clusters, while the HA group exhibited multi-focal fissures (covering approximately two thirds of the cartilage), erosion in the mid-zone, loss of cells in the surface zone, and occasional superficial clusters (arrow) (Fig. 8M ). TA at 1.25, 2.5, and 5 mg/ml led to various changes in cartilage structure: global surface undulations and fissures and erosion in the superficial to mid-zone (arrow), with unclear differences among the experimental groups. For chondrocyte pathology, TA at 1.25, 2.5, and 5 mg/ml induced global small cell clusters, large cell clusters, and predominant cell loss (Fig. 8J–L ). Combined treatment of TA at 1.25, 2.5, and 5 mg/ml with HA reduced the severity of these effects (Fig. 8N–P ).
Proteoglycan staining of normal cartilage explants with Safranin O revealed a loss of proteoglycan content in the control group in the mid-zone globally (more than two thirds of the cartilage) (arrow), while in the HA group, a loss of proteoglycan in the superficial zone (arrow) was observed (Fig. 9A, E ). HA combined with TA at 5 mg/ml increased proteoglycan content when compared with TA at 5 mg/ml alone (Fig. 9D, H ). In OA cartilage explants, proteoglycan content was decreased in the control group in the mid-zone globally (arrow), and there was some multi-focal decrease of proteoglycan in the deep zone (Fig. 9I ), while in the HA group, a multi-focal decrease of proteoglycan content was observed in the deep zone (arrow) (Fig. 9M ). TA at 5 mg/ml caused a global decrease of proteoglycan content in the mid-zone (arrow), while TA at 5 mg/ml combined with HA resulted in a multi-focal decrease of proteoglycan content in the mid-zone (arrow) (Fig. 9L , P).
Discussion
A number of previous publications chose chondrocytes from both normal and OA joints to assess the variables of their experiments (Héraud et al. 2000; Salter et al. 2002; Fan et al. 2005; Kim et al. 2014). Our study was established to assess the direct effects of TA, HA, and combinations of the two drugs on chondrocytes and cartilage explants under normal conditions, without proinflammatory cytokine involvement, and in spontaneous OA which involves several proinflammatory cytokines that mimic the comparative effects of the IA drugs in normal and OA joints. The reason why primary chondrocytes from the OA joints were chosen for this study is that when OA occurs, proinflammatory cytokines, such as IL-1, tumor necrosis factor α (TNF-α), oncostatin M, IL-17, and IL-18, which are derived from chondrocytes act as catabolic cytokines (Goldring 2000). Therefore, induction of IL-1 or another cytokine alone in chondrocytes may not be a proper representation of induction by cytokines in OA, equivalent to using OA chondrocytes from spontaneous OA joints.
MTT assay was used to evaluate the initial chondrotoxicity of drugs by the percentage of cell viability after drug treatment and by considering the histopathology in cartilage explants. Because histologic assessment is the gold standard for observation of the severity of OA in dogs (Cook et al. 2010), this study used histopathology for grading of chondrocyte pathology to confirm the accuracy of results. In normal chondrocytes, treatment with TA alone can cause chondrocyte death in relation to the size of the dose. At 5 mg/ml, TA reduces the cell viability of normal chondrocytes to about 45%, but it only causes moderate chondrocyte damage in normal cartilage explants when compared with the control group. In OA chondrocytes, TA 5 mg/ml decreases cell viability to about 38% and can increase the severity of chondrocyte pathology in cartilage explants. These different responses in normal and OA chondrocytes may be because chondrocyte apoptosis can occur in OA chondrocytes in response to inducers such as chemical agents and cytokines (Barreto et al. 2015). Moreover, chondrocyte senescence related to OA is induced by biomechanical factors, leading to chondrocyte death (Hwang and Kim 2015). Treatment of OA chondrocytes with TA may lead to higher cell death than in normal chondrocytes at the same concentration of TA.
In this study, LMWHA 2.5 mg/ml was used because a previous study showed its beneficial effects on equine articular chondrocyte pellets (Schaefer et al. 2009). Their results showed that a high concentration of HA (2 mg/ml) could increase glycosaminoglycan (GAG) synthesis and decrease GAG released into culture media more than a lower concentration (0.5 mg/ml). Therefore, this concentration of LMWHA was used in combination with TA at IC20 to assess chondrocyte responses directly by observing cell viability and expression levels of genes involved in cartilaginous matrix degradation. In cartilage explants, the same concentration of LMWHA was used in combination with three concentrations of TA. TAs 1.25 and 2.5 mg/ml are within the recommended dose range for IA in dogs approved by the US Food and Drug Administration (FDA) (1–3 mg). A high dose of TA (5 mg/ml) was used because a previous study reported that it could have a cytotoxic effect on human chondrocytes (Dragoo et al. 2012). A combination of HA together with these various concentrations of TA was tested for their chondroprotective effects in both normal and OA articular cartilages.
This paper is the first report to show the relative gene expression profiles involved in cartilage degradation, including COL2A1, ACAN, IL-1β, ADAMTS5, MMP2, MMP3, and MMP13, comparing normal and OA primary canine chondrocytes. This information could be used as a reference when considering treatment with any drugs, to gauge their effects on normal and OA cells. In this study, canine OA chondrocytes had higher expression levels of COL2A1, ACAN, ADAMTS5, MMP2, and MMP13 compared with normal chondrocytes, similar to the levels in human OA chondrocytes (Bau et al. 2002). This study emphasized the role of proinflammatory cytokines and enzymes in catabolism involved in OA cartilage degradation. The proinflammatory cytokine IL-1β has an important function in catabolic activity by stimulating chondrocytes to secrete MMPs, which damage cartilage structure. MMPs that have been linked to OA are stromelysin, collagenase, and some gelatinases which participate in the proteinase activation cascade (Goldring 2000). We chose to examine ADAMTS5 expression level because this gene may encode ADAMTS5 protein, which is a major aggrecanase of OA and is significantly up-regulated in early-stage OA cartilage in a dog model (Stoker et al. 2006). Moreover, we selected MMP2, MMP3, and MMP13 to represent important genes in each group of MMPs. MMP13 encodes the protein MMP-13, which is involved in the enzymatic degradation of type II collagen in cartilage (Rousseau and Delmas 2007). MMP3 encodes the protein MMP-3, which activates latent collagenase and can destroy proteoglycans; aggrecan; collagen types IV, VII, IX, and XI; and fibronectin (Nagase and Woessner 1999). MMP2 encodes the protein MMP-2, which can damage proteoglycans; collagen types I, IV, V, VII, and X; laminin; elastin; and fibronectin (Nagase and Woessner 1999; Sternlicht and Werb 2001).
In this study, TA induced up-regulation of ACAN, ADAMTS5, MMP2, MMP3, and MMP13 in OA chondrocytes and down-regulated ACAN in normal chondrocytes. TA at 1.25, 2.5, and 5 mg/ml had a detrimental effect on cartilage, according to histopathological observations. These findings are similar to a previous study in which TA decreased GAG synthesis and increased GAG degradation in equine articular cartilage (Dechant et al. 2003). Hence, clinical application of IA TA in OA should be a matter of concern when using a high dose. In contrast, LMWHA could only induce normal chondrocytes, but not OA chondrocytes, to up-regulate COL2A1 expression. This result may be related to the chondroprotective effect of HA by reducing the chondrotoxicity of TA at IC20 in normal chondrocytes; intact type II collagen in the cartilaginous matrix is a key to maintaining chondrocyte survival (Kim et al. 2001). However, some of disadvantages of LMWHA in this study were also noted. LMWHA could induce up-regulation of ADAMTS5 in normal chondrocytes and could induce up-regulation of ADAMTS5, MMP2, and MMP3 expression in OA chondrocytes.
The results of grading OA cartilage structure showed no clear differences from the effect of drug treatment. This may be because (1) OA lesions had occurred before drug treatment or (2) drug treatment of cartilage explants for 14 d mimicked the effects and duration of action of a single-injection dose of IA TA in joints (Dragoo et al. 2012), but it may not have been sufficient to stimulate cartilage repair and regeneration. The reason for this may be because new synthesis of type II collagen, the main structure of cartilage, requires a long period of time (Poole et al. 2001).
The beneficial effects of IA administration of LMWHA have been reported in clinical use in humans and animals (Guidolin et al. 2001; Amiel et al. 2003; Frizziero et al. 2014); it has also shown good results when used in conjunction with IA CSs (Grecomoro et al. 1992; Guidolin et al. 2001) and NSAIDs (Adams et al. 1995). Especially in dogs, IA injection of LMWHA after surgery can improve homeostasis of joints (Nganvongpanit et al. 2013) and delay the progression of patellar cartilage degradation (Wenz et al. 2000). LMWHA is primarily effective in stimulating endogenous HA synthesis in synovial fibroblasts in the synovial membrane (Ghosh and Guidolin 2002) and the secretion of new HA into the synovial fluid to maintain joint homeostasis. Although LMWHA has shown beneficial effects in clinical use, its beneficial effects are still unclear under the conditions of an in vitro study. Therefore, further studies should give precedence to the effects of a combination of HA and TA on chondrocyte apoptosis, using various drug concentrations and molecular weights of HA and a longer incubation time, to compare their effects on both normal and OA joints and to elucidate the pharmacokinetic and signaling pathways involved when using a combination of HA and TA.
Conclusion
In this in vitro study, TA reduced chondrocyte viability in both normal and OA chondrocytes in a concentration-dependent manner; down-regulated the expression of ACAN in canine normal chondrocytes; and up-regulated ADAMTS5, MMP2, MMP3, MMP13, and ACAN in canine OA chondrocytes. Moreover, in cartilage explants, TA increased the severity of cartilage structural damage, chondrocyte loss and cluster formation, and proteoglycan loss in OA cartilage. The addition of LMWHA could decrease the chondrotoxicity of TA at IC20 only in normal chondrocytes, as observed by chondrocyte viability. Otherwise, the combination of LMWHA and TA did not show clearly beneficial effects in any other normal and OA samples. Therefore, using TA alone or in combination with LMWHA for OA conditions should be of concern because it may lead to cartilage destruction.
References
Adams ME, Atkinson MH, Lussier AJ, Schulz JI, Siminovitch KA, Wade JP, Zummer M (1995) The role of viscosupplementation with hylan G-F 20 (Synvisc®) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthr Cartil 3:213–225
Amiel D, Toyoguchi T, Kobayashi K, Bowden K, Amiel ME, Healey RM (2003) Long-term effect of sodium hyaluronate (Hyalgan®) on osteoarthritis progression in a rabbit model. Osteoarthr Cartil 11:636–643
Archer CW, Francis-West P (2003) The chondrocyte. Int J Biochem Cell Biol 35:401–404
Barreto RB, Sadigursky D, de Rezende MU, Hernandez AJ (2015) Effect of hyaluronic acid on chondrocyte apoptosis. Acta Ortop Bras 23:90–93
Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T (2002) Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum 46:2648–2657
Bland JH, Cooper SM (1984) Osteoarthritis: a review of the cell biology involved and evidence for reversibility. Management rationally related to known genesis and pathophysiology. Semin Arthritis Rheum 14:106–133
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bondeson J, Wainwright S, Hughes C, Caterson B (2008) The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol 26:139–145
Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, Lafeber FP (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr Cartil 18:S66–S79
Corrado EM, Peluso GF, Gigliotti S, de Durante C, Palmieri D, Savoia N, Oriani GO, Tajana GF (1995) The effects of intra-articular administration of hyaluronic acid on osteoarthritis of the knee: a clinical study with immunological and biochemical evaluations. Eur J Rheumatol Inflamm 15:47–56
Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr (1989) Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest 84:678–685
Dechant JE, Baxter GM, Frisbie DD, Trotter GW, McIlwraith CW (2003) Effects of dosage titration of methylprednisolone acetate and triamcinolone acetonide on interleukin-1-conditioned equine articular cartilage explants in vitro. Equine Vet J 35:444–450
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–277
Doyle AJ, Stewart AA, Constable PD, Eurell JAC, Freeman DE, Griffon DJ (2005) Effects of sodium hyaluronate and methylprednisolone acetate on proteoglycan synthesis in equine articular cartilage explants. Am J Vet Res 66:48–53
Dragoo JL, Danial CM, Braun HJ, Pouliot MA, Kim HJ (2012) The chondrotoxicity of single-dose corticosteroids. Knee Surg Sports Traumatol Arthrosc 20:1809–1814
Euppayo T, Siengdee P, Buddhachat K, Pradit W, Viriyakhasem N, Chomdej S, Ongchai S, Harada Y, Nganvongpanit K (2015) Effects of low molecular weight hyaluronan combined with carprofen on canine osteoarthritis articular chondrocytes and cartilage explants in vitro. In Vitro Cell Dev Biol Anim 51:857–865
Evans CH, Kraus VB, Setton LA (2014) Progress in intra-articular therapy. Nat Rev Rheumatol 10:11–22
Fan Z, Bau B, Yang H, Soeder S, Aigner T (2005) Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1β. Arthritis Rheum 52:136–143
Fox AJS, Bedi A, Rodeo SA (2009) The basic science of articular cartilage. Sports Health 1:461–468
Frizziero A, Maffulli N, Masiero S, Frizziero L (2014) Six-months pain relief and functional recovery after intra-articular injections with hyaluronic acid (mw 500–730 kDa) in trapeziometacarpal osteoarthritis. Muscles Ligaments Tendons J 4:256–261
Ghosh P, Guidolin D (2002) Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum 32:10–37
Goldberg VM, Buckwalter JA (2005) Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthr Cartil 13:216–224
Goldring MB (2000) The role of the chondrocyte in osteoarthritis. Arthritis Rheum 43:1916–1926
Grecomoro G, Piccione F, Letizia G (1992) Therapeutic synergism between hyaluronic acid and dexamethasone in the intra-articular treatment of osteoarthritis of the knee: a preliminary open study. Curr Med Res Opin 13:49–55
Grishko V, Xu M, Ho R, Mates A, Watson S, Kim JT, Wilson GL, Pearsall AW 4th (2009) Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem 284:9132–9139
Guidolin DD, Ronchetti IP, Lini E, Guerra D, Frizziero L (2001) Morphological analysis of articular cartilage biopsies from a randomized, clinical study comparing the effects of 500–730 kDa sodium hyaluronate (Hyalgan®) and methylprednisolone acetate on primary osteoarthritis of the knee. Osteoarthr Cartil 9:371–381
Güngör İ, Yılmaz A, Ergün MA, Öztürk AM, Kaya K (2014) Does hyaluronic acid decrease the apoptotic effect of bupivacaine? Eklem Hastalık Cerrahisi 25:102–106
Haddad IK (2000) Temporomandibular joint osteoarthrosis. Histopathological study of the effects of intra-articular injection of triamcinolone acetonide. Saudi Med J 21:675–679
Hepper CT, Halvorson JJ, Duncan ST, Gregory AJM, Dunn WR, Spindler KP (2009) The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg 17:638–646
Héraud F, Héraud A, Harmand MF (2000) Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis 59:959–965
Hwang HS, Kim HA (2015) Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci 16:26035–26054
Jubb RW, Piva S, Beinat L, Dacre J, Gishen P (2003) A one-year, randomised, placebo (saline) controlled clinical trial of 500–730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract 57:467–474
Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M (1999) Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol 179:142–148
Kim HA, Suh DI, Song YW (2001) Relationship between chondrocyte apoptosis and matrix depletion in human articular cartilage. J Rheumatol 28:2038–2045
Kim HJ, Braun HJ, Dragoo JL (2014) The effect of resveratrol on normal and osteoarthritic chondrocyte metabolism. Bone Joint Res 3:51–59
Kolar K (1990) Colorimetric determination of hydroxyproline as measure of collagen content in meat and meat products: NMKL collaborative study. J Assoc Off Anal Chem 73:54–57
Laurent TC, Laurent UB, Fraser JRE (1996) The structure and function of hyaluronan: an overview. Immunol Cell Biol 74:A1–A7
Listrat V, Ayral X, Patarnello F, Bonvarlet JP, Simonnet J, Amor B, Dougados M (1997) Arthroscopic evaluation of potential structure modifying activity of hyaluronan (Hyalgan®) in osteoarthritis of the knee. Osteoarthr Cartil 5:153–160
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods 25:402–408
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Nganvongpanit K, Boonsri B, Sripratak T, Markmee P (2013) Effects of one-time and two-time intra-articular injection of hyaluronic acid sodium salt after joint surgery in dogs. J Vet Sci 14:215
Onur TS, Sitron CS, Dang A (2013) Co-administration of hyaluronic acid with local anaesthetics shows lower cytotoxicity than local anaesthetic treatment alone in bovine articular chondrocytes. Bone Joint Res 2:270–275
Pelletier JP, Martel-Pelletier J, Abramson SB (2001) Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 44:1237–1247
Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S (2001) Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res 391:S26–S33
Rousseau JC, Delmas PD (2007) Biological markers in osteoarthritis. Nat Clin Pract Rheumatol 3:346–356
Salter DM, Millward-Sadler SJ, Nuki G, Wright MO (2002) Differential responses of chondrocytes from normal and osteoarthritic human articular cartilage to mechanical stimulation. Biorheology 39:97–108
Schaefer EC, Stewart AA, Durgam SS, Byron CR, Stewart MC (2009) Effects of sodium hyaluronate and triamcinolone acetonide on glucosaminoglycan metabolism in equine articular chondrocytes treated with interleukin-1. Am J Vet Res 70:1494–1501
Siengdee P, Radeerom T, Kuanoon S, Euppayo T, Pradit W, Chomdej S, Ongchai S, Nganvongpanit K (2015) Effects of corticosteroids and their combinations with hyaluronan on the biochemical properties of porcine cartilage explants. BMC Vet Res 11:298
Soma LR, Uboh CE, You Y, Guan F, Boston RC (2011) Pharmacokinetics of intra-articular, intravenous, and intramuscular administration of triamcinolone acetonide and its effect on endogenous plasma hydrocortisone and cortisone concentrations in horses. Am J Vet Res 72:1234–1242
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Stoker AM, Cook JL, Kuroki K, Fox DB (2006) Site-specific analysis of gene expression in early osteoarthritis using the Pond-Nuki model in dogs. J Orthop Surg Res 1, 8. http://doi.org/10.1186/1749-799X-1-8
Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD (2011) Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res 469:2941–2947
Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D (2000) Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol 27:1713–1720
Wenz W, Breusch SJ, Graf J, Stratmann U (2000) Ultrastructural findings after intraarticular application of hyaluronan in a canine model of arthropathy. J Orthop Res 18:604–612
Wernecke C, Braun HJ, Dragoo JL (2015) The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med 3:2325967115581163
Yates AC, Stewart AA, Byron CR, Pondenis HC, Kaufmann KM, Constable PD (2006) Effects of sodium hyaluronate and methylprednisolone acetate on proteoglycan metabolism in equine articular chondrocytes treated with interleukin-1. Am J Vet Res 67:1980–1986
Zhou PH, Liu SQ, Peng H (2008) The effect of hyaluronic acid on IL-1β-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res 26:1643–1648
Acknowledgments
This study was kindly supported by the 50th CMU Anniversary Ph.D. program. The authors are also grateful for additional research funding via a CMU Research Group Grant (2015), Chiang Mai University, Thailand. TE designed and performed the experiments, analyzed primary data, and wrote the manuscript. PS oversaw laboratory work and writing of the manuscript. KP, SC, and SO gave advice on laboratory work and assisted in discussion of the manuscript. KN designed and conducted the overall study and writing of the manuscript. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Euppayo, T., Siengdee, P., Buddhachat, K. et al. In vitro effects of triamcinolone acetonide and in combination with hyaluronan on canine normal and spontaneous osteoarthritis articular cartilage. In Vitro Cell.Dev.Biol.-Animal 52, 723–735 (2016). https://doi.org/10.1007/s11626-016-0022-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-016-0022-4