Abstract
Intra-articular injection with non-steroidal anti-inflammatory drugs (NSAIDs) is used to treat inflammatory joint disease, but the side effects of NSAIDs include chondrotoxicity. Hyaluronan has shown positive effects on chondrocytes by reducing apoptosis and increasing proteoglycan synthesis. The purposes of this study were to evaluate the effects of low molecular weight hyaluronan (low MW HA), carprofen 25 mg/ml, carprofen 12.5 mg/ml, and a combination of HA and carprofen on canine osteoarthritis (OA) articular chondrocytes and a cartilage explant model in terms of cell viability, extracellular matrix remaining, and gene expression after exposure. In chondrocyte culture, MTT assay was used to evaluate the chondrotoxicity of IC50 and IC80 of carprofen with HA. In cartilage explant culture, two kinds of extracellular matrix (uronic acid and collagen) remaining in cartilage were used to evaluate cartilage damage for 14 d after treatment. Expression of COL2A1, AGG, and MMP3 was used to evaluate the synthesis and degradation of the matrix for 7 d after treatment. In chondrocyte culture, low MW HA could preserve OA chondrocyte viability but could not reduce the chondrotoxicity level of carprofen (P < 0.05). In explant culture, low MW HA combined with 12.5 mg/ml carprofen caused less destruction of uronic acid and collagen structure when compared with the control (P < 0.05). Low MW HA caused high expression levels of COL2A1 and AGG in OA cartilage (P < 0.05); HA combined with carprofen resulted in higher COL2A1 and AGG expression levels than carprofen alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA), a degenerative joint disease, is a non-inflammatory arthritis caused by failure of homeostasis of joints. Proteoglycan and type II collagen are degraded at the surface, leading to loss of structural strength of articular cartilage due to aggrecanase and matrix metalloproteinases (MMPs) (Struglics et al. 2006) and decreased type II collagen and aggrecan integrity (Garnero et al. 2002; Jalbă et al. 2011; Henrotin et al. 2013). Therefore, type II collagen and proteoglycan can be used as biochemical markers of early stage canine OA (Matyas et al. 2004).
Hyaluronan, or hyaluronic acid (HA), is a glycosaminoglycan that is a main component in synovial fluid (SF); it possesses viscoelastic properties, and is produced by articular chondrocytes and type B synoviocytes (Archer and Francis-West 2003). The clinical effect of HA is pain relief and disease-modifying activity (Goldberg and Buckwalter 2005). Intra-articular injection of HA has been used for treatment of pain in subjects with severe clinical signs of OA. HA inhibits inflammatory cytokines, MMPs, proteoglycan, and prostaglandin E2 in synoviocytes and chondrocytes, and has been reported to suppress IL-1β-induced MMP production (Wang et al. 2006). HA are capable of suppressing chondrocyte apoptosis (Takahashi et al. 1999) and decreasing iNOS gene expression, resulting in alleviation of nitric acid in the SF of rabbit OA joints (Qiu et al. 2008).
Currently, one of the selective cyclooxygenase-2 inhibitors (selective COX-2 inhibitors), a non-steroidal anti-inflammatory drug (NSAID) called carprofen, is also used for controlling pain, inflammation, and lameness associated with OA (Ding 2002). The advantages of selective COX-2 inhibitors are fewer adverse side effects—e.g., on the gastrointestinal mucosa, renal blood flow, and vascular hemostasis—than from cyclooxygenase-1 inhibitors (COX-1 inhibitors) which can destroy eicosanoid function (Hazewinkel et al. 2008). Many literature reports have shown that carprofen has a strong anti-inflammatory effect which could inhibit catabolism without cytotoxic effects in vitro. In cartilage explants, it was reported that 100 μg/ml carprofen alone could inhibit the release of MMP 1, 3, and 13 in cartilage explants stimulated by interleukin-1β, and that no cell death or cell lysis was found when treated with carprofen (Williams et al. 2013). Moreover, carprofen significantly increased the rate of glycosaminoglycan synthesis and inhibited prostaglandin release (Benton et al. 1997). Carprofen alone also significantly decreased glycosaminoglycan release and elevated proteoglycan synthesis in equine cartilage explants (Armstrong and Lees 1999). Some investigators have used intra-articular injection of NSAIDs for treatment of OA (Uthman et al. 2003) because they have a cytotoxic effect on chondrocytes, causing cell death and suppressing chondrocyte proliferation, but these effects are lower in the case of selective COX-2 inhibitors (Chang et al. 2006).
The purpose of this study was to evaluate the effects of low molecular weight hyaluronan (low MW HA), carprofen, and combination of low MW HA and carprofen on canine OA articular chondrocyte and cartilage explants. MTT assay was used to assess the chondrotoxicity of OA chondrocytes. Uronic acid and hydroxyproline were assessed after treatment with two concentrations of carprofen, with and without HA, for 14 d. Gene expression of some important genes under our explant culture conditions were estimated after 7 d of treatment.
Materials and Methods
Experimental Design.
The experimental design consisted of OA chondrocyte culture and OA cartilage explant culture, as shown in Fig. 1. In OA chondrocyte culture, MTT assay was used to assess the percentage of cell viability after co-treatment with HA and carprofen. In OA cartilage explant culture, samples were divided into six experimental groups, as follows:
-
Control, administered DMEM serum-free media (control)
-
Low molecular weight HA 2.5 mg (HA 2.5 mg)
-
Carprofen 25 mg (CAR 25 mg)
-
Carprofen 25 mg with HA 2.5 mg (CAR 25 mg + HA)
-
Carprofen 12.5 mg (CAR 12.5 mg)
-
Carprofen 12.5 mg with HA 2.5 mg (CAR 12.5 mg + HA)
All six groups were assessed for cartilage damage based on uronic acid and hydroxyproline remaining in cartilage, and gene expression was analyzed by quantitative real-time PCR (qRT-PCR).
Reagents.
Low molecular weight HA 500–730 kDa (TRB Chemedica, Bangkok, Thailand), 10 mg/ml, was diluted with DMEM and used at a concentration of 2.5 mg/ml. Carprofen (Zoetis, Bangkok, Thailand), at a concentration of 50 mg/ml, was serially diluted with DMEM.
Canine OA Primary Chondrocytes.
Canine OA joints with fibrillation and deeper lesions with surrounding damage (Cook et al. 2010) were taken from the Veterinary Cadaveric Unit, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand. The joints were dissected by aseptic technique. The articular cartilage was then sliced into small pieces, approximately 1–2 mm, and incubated in 10% collagenase type II (Gibco, Grand Island, New York, USA) in Dulbecco’s modified Eagle’s medium (DMEM) (Caisson Laboratories, Logan, Utah, USA) for 21 h in conditions of 5% CO2, 37°C and 70% relative humidity. After that, the culture was replaced with growth medium: DMEM supplemented with 10% fetal bovine serum (FBS) (Gibco). Chondrocytes in monolayer culture were incubated in conditions of 5% CO2, 37°C and 70% relative humidity. Growth medium was changed every 3 d until the end of treatment. Trypsinization was done to 70–80% confluence.
Canine OA Cartilage Explant Culture.
Osteoarthritis cartilages were harvested from canine OA joints at the Veterinary Cadaveric Unit, Faculty of Veterinary Medicine, Chiang Mai University. Within 6 h after death, joints were dissected and slices of articular cartilage taken. Articular cartilages of joints had fibrillation, ulceration and some osteophytes. SF was non-viscous. Sliced pieces of cartilage were incubated for 24 h in serum-free DMEM containing 200 U/ml of penicillin and 200 μg/ml of streptomycin (Gibco). After incubation, three pieces of cartilage weighing ∼30–35 mg/well were plated in 24-well plates and cultured with 1 ml of serum-free DMEM before treatment. Cartilage explant cultures were maintained at 5% CO2, 37°C and 70% relative humidity. Samples were divided into six experimental groups; all experiments were performed in triplicate. After treatment for 14 d, cartilage explant samples were collected to measure uronic acid, hydroxyproline remaining in cartilage, and gene expression.
Percentage of Cell Viability by Cytotoxic Assay.
Canine articular chondrocytes in passages 2–4 were collected and measured for viability of cells using MTT assay. Briefly, chondrocytes were grown at a density of 10,000 cells/well in 96-well plates and cultured in growth medium for 24 h. After that, cells were treated with twofold dilutions of carprofen in triplicate, including 25, 12.5, 6.25, 3.12, 1.5, 0.78, 0.39, 0.2, 0.1, and 0.05 mg/ml. After incubation for 24 h, treated cells were washed with PBS and cultured for 4 h in new culture media, i.e., DMEM with 5 mg/ml MTT (3,[4,4-dimethythiazol-2-yl]-2,5-diphenyl-tetrazolium bromide). Then dimethyl sulfoxide (DMSO) 100 μl/well was added (Denizot and Lang 1986) and shaken for 5 min before measuring the absorbance at 540 nm using a microplate reader.
Cell viability of samples was calculated as follows:
The 50% inhibitory concentration (IC50) and 80% inhibitory concentration (IC80) were calculated from a logarithmic graph. The IC50 and IC80 of carprofen combined with 5 mg/ml HA were used to evaluate cell viability by MTT assay.
Uronic acid remaining in cartilage.
Uronic acid (glucuronic acid or iduronic acid) is a component of repeating units in glycosaminoglycan and glycosaminoglycan chains, and is attached to a protein core by covalent bonds to form proteoglycans (Esko et al. 2009). Uronic acid levels remaining in treated cartilage samples were measured by colorimetric assay (Blumenkrantz and Asboe-Hansen 1973). A piece of treated cartilage was immersed in 200 μl of papain 2 units at 60°C for 48 h. Five microliter of papain-digested cartilage culture media was pipetted into 45 μl of distilled water. Sixty microliter of diluted sample and standard (m-hydroxydiphenyl in glucuronic acid lactone) were combined with 300 μl of reagent A (0.025 M Na2B4O7 in concentrated sulfuric acid) at 100°C for 15 min and then cooled down to room temperature. Next, 12 μl of reagent B (50 mg carbazole in 40 ml absolute ethanol) was added and the mixture kept at 100°C for 15 min. Each sample and standard (150 μl/well) was placed in a 96-well plate and the absorbance of the pink color was measured at 540 nm by a microtiter plate reader.
Hydroxyproline Remaining in Cartilage.
A total volume of 100 μl of papain-digested cartilage culture media was combined with 12 N HCl and incubated for 24 h at 60°C, to hydrolysis. The pH was adjusted to pH 7 by adding 6 N NaOH. The samples were then diluted 40-fold by adding distilled water. Fifty microliter of each diluted sample was added to 100 μl of oxidizing solution (50 mM chloramine T) at 25°C for 5 min; then 100 μl of Ehrlich’s reagent (7.5% dimethylaminobenzaldehyde in propan-2-ol) (Kolar 1990) was added at 60°C for 45 min, causing pink color production. The absorbance of the pink color of samples and hydroxyproline standard was read at 540 nm using a microtiter plate reader.
Gene Expression by qRT-PCR.
RNA from each group of cartilage explants treated for 7 d was extracted using an innuPREP DNA/RNA Mini Kit. Then, complementary DNA (cDNA) was synthesized by Tetro reverse transcriptase enzyme; 10 mM oligo (dT) was used in a total reaction volume of 20 μl. Gene expression of collagen type II alpha 1 (COL2A1), aggrecan (AGG), and matrix metalloproteinase-3 (MMP-3) were assessed compared with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, using 2x SensiFAST SYBR® No-ROX Mix 10 μl, 10 μM forward and reverse primer 0.3 μl each, and cDNA 3 μl; the relative expression was calculated using a comparative threshold cycle from the 2−ΔΔCT method (Heid et al. 1996; Livak and Schmittgen 2001).
Statistical Analysis.
Cell viability and relative expression of COL2A1, AGG, and MMP3 genes were compared in terms of means and standard deviation (SD) using one-way analysis of variance (ANOVA). Statistical analysis of uronic acid and hydroxyproline remaining in cartilage was performed by Student’s t test. SPSS software was used for all analyses; a value of P < 0.05 was considered significant.
Results
Cytotoxicity of Carprofen on Canine OA Articular chondrocytes.
Cell viability percentages of cells treated with twofold dilutions of carprofen are shown in Fig. 2. The percentage of cell viability for the lowest concentration of carprofen, 5 mg/ml, was 101.14 ± 14.71%, compared with the control group (non-treated) cell viability percentage of 100 ± 4.23%. Cell viability decreased sharply beginning with a very low concentration of 0.39 mg/ml carprofen (56.88 ± 1.44%), and then decreased gradually with increasing doses, from 1.5 to 25 mg/ml, of carprofen. At the highest concentration of carprofen, 25 mg/ml, cell viability was reduced to approximately 5%. IC50 and IC80, as calculated from the graph plot, were 0.62 and 0.12 mg/ml, respectively.
HA Combined with Carprofen at IC 50 and IC 80 .
The cell viability of chondrocytes was compared among the study groups. The group treated with 5 mg/ml HA alone showed no significant difference from the control (P < 0.05). At both IC50 and IC80 of carprofen alone, cell viability significantly decreased (P < 0.05) compared with the control. Moreover, for treatment with HA combined with IC50 or IC80 of carprofen, there was no significant difference between the groups of carprofen treatment alone, at P < 0.05 (Fig. 3).
Uronic Acid Remaining in Cartilage.
After OA cartilage explant cultures were treated under the various conditions mentioned above, the levels of uronic acid remaining in articular cartilage were measured. The average amounts of uronic acid (one of the repeating units in glycosaminoglycan) remaining in cartilage from each group were as follows: control 0.85 ± 0.04 μg/mg; HA 2.5 mg 0.81 ± 0.06 μg/mg; CAR 25 mg 0.69 ± 0.03 μg/mg; CAR 25 mg + HA 0.66 ± 0.01 μg/mg; CAR 12.5 mg 0.81 ± 0.01 μg/mg; and CAR 12.5 mg + HA 0.87 ± 0.19 μg/mg. There was no significant difference between control and HA 2.5 mg, CAR 25 mg and CAR 25 mg + HA, or CAR 12.5 mg and CAR 12.5 mg + HA. The uronic acid level of CAR 12.5 mg + HA was close to that of control and was significantly higher than CAR 25 mg + HA (P < 0.05), as shown in Fig. 4.
Box plot showing the median amount of uronic acid remaining in canine cartilage explant groups after 14 d of culture under three concentration (25 and 12.5 mg/ml) of carprofen, with or without HA (2.5 mg/ml). Boxes indicate the interquartile range 25th − 75th percentile; the bold line in the box represents the median value, with non-outlier maximum and minimum levels.
Hydroxyproline Remaining in Cartilage.
Hydroxyproline is a marker for collagen production by chondrocytes remaining in cartilage (Fig. 5). Our results showed average hydroxyproline remaining in cartilage of the following: control 0.275 ± 0.018 μg/mg; HA 2.5 mg 0.260 ± 0.021 μg/mg; CAR 25 mg 0.218 ± 0.012 μg/mg; CAR 25 mg + HA 0.212 ± 0.003 μg/mg; CAR 12.5 mg 0.261 ± 0.012 μg/mg; and CAR 12.5 mg + HA 0.274 ± 0.051 μg/mg. Similar to the results for uronic acid remaining in cartilage, there was no significant difference between control and HA 2.5 mg, CAR 25 mg and CAR 25 mg + HA, or CAR 12.5 mg and CAR 12.5 mg + HA. The average amount of hydroxyproline remaining in cartilage for CAR 25 mg and CAR 25 mg + HA was significantly lower compared with control, while CAR 12.5 mg + HA was significantly higher than CAR 25 mg and CAR 25 mg + HA.
Gene Expression.
Relative gene expression of two extracellular matrix genes and one protease gene, COL2A1, AGG, and MMP3, are shown in Fig. 6. Expression level of COL2A1 after treatment with HA 2.5 mg was significantly higher than for CAR 25 mg at P < 0.05. Similarly, the expression of the AGG gene was significantly up-regulated in the HA 2.5 mg-treated group compared with the other groups (P < 0.001). Additionally, the expression level of MMP3 tended to decrease in HA-treated groups compared with control and with groups treated with the same concentration of carprofen alone. Notably, expression levels of COL2A1 and AGG were slightly increased in the groups exposed to carprofen combined with HA compared with groups treated with carprofen alone, while a contrary result was found for the MMP3 gene.
Relative expression of COL2A1, AGG, and MMP3 genes after treatment of six experimental groups for 7 d. The light bars denote the control group and the dark bars denote the treatment groups. * and ** denote P values ≤ 0.05 and ≤ 0.001, respectively. Bold “a” indicates a significant difference compared with all groups. All values are presented as mean ± SEM (n = 3).
Discussion
In chondrocyte culture, MTT assay was used to assess the metabolic activity of live cells to evaluate the direct effects of carprofen, HA, and carprofen combined with HA on chondrocytes in OA joints. The normal concentration of HA in SF in the diarthrodial joint is 0.5–4.0 mg/ml (Laurent and Fraser 1992; Ghosh and Guidolin 2002). In this study, 5 mg/ml HA (50% stock solution) was used to imitate injection of HA into OA joints to reduce chondrotoxicity when combined with carprofen. The results indicated that cell viability tended to have a negative correlation with concentration of carprofen; and at more than 0.09 mg/ml, carprofen could cause chondrocyte death in chondrocyte monolayer. The highest concentration, 25 mg/ml carprofen, caused cell death approximately 18 times more often compared with the control. HA alone significantly preserved chondrocyte survival, a performance nearly comparable to the control group (P < 0.05); this was similar to the results of a previous study showing that HA of molecular weight 500–730 kDa decrease nitric oxide-induced apoptosis of rat chondrocytes by modulation of protein kinase Cα (Peng et al. 2010). However, in this study, HA could not reduce the chondrotoxicity of IC50 and IC80 of carprofen (P < 0.05). Similarly, our preliminary study showed that 2.5 mg/ml HA could not reduce the chondrotoxicity of a 5% stock dilution of carprofen in human articular chondrocytes (P < 0.05) (data unpublished).
In OA cartilage explant culture, measurements of the levels of two matrix markers, uronic acid and collagen, are able to indicate the amount of cartilage damage after treatment. Uronic acid is a component of glycosaminoglycan and proteoglycan in articular cartilage. Collagen structure can be evaluated by hydroxyproline remaining in cartilage because hydroxyproline is a non-proteogenic amino acid, produced by proline hydroxylation, and is a major component in collagen. In this study, the trend of the results of uronic acid remaining in cartilage was similar to those of hydroxyproline remaining in cartilage. HA can preserve uronic acid and collagen structure. Over 14 d of treatment, there was less destruction of uronic acid and collagen structure from CAR 12.5 mg with and without HA when compared with the control. However, both CAR 25 mg and CAR 12.5 mg had severe adverse effects on OA chondrocytes. These effects may be from chondrocytes contact with the drug directly and can cause severe effects on cell death more than in explant culture. Chondrocytes are located in the lacunae and are protected by the surrounding cartilage matrix.
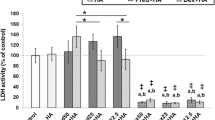
The level of sulfated-glycosaminoglycans, which are released into culture media from damaged cartilage explants, was measured, while lactate dehydrogenase assay (LDH assay) was used to determine the activity of the enzyme lactate dehydrogenase, an indicator of cell apoptosis and cell viability (Terauchi et al. 2003). Both of these methods can be used to evaluate cartilage damage, but unfortunately, we were unable to obtain any results because the dilution of carprofen interfered with the results of colorimetric assays, leading to non-interpretation of results in this experiment. However, this did not interfere with the measurement of uronic acid and hydroxyproline remaining in cartilage. An important limitation, however, is that histopathological evaluation of cartilage expansion (HE staining and Safranin-O staining) was not performed, due to the limited number of cartilage samples. Articular cartilage harvested from OA joints was dramatically lower in volume than that harvested from normal cartilage. Because the amount of cartilage used in this study was very limited, we had to choose the assessment effects of these drugs based on cartilage yield. We decided to measure uronic acid, hydroxyproline remaining in cartilage, and gene expression because the results would be more objective than histopathology.
COL2A1 and AGG are cartilage matrix-specific genes which have important functions in the anabolic pathway of articular cartilage. Type II collagen serves as a major cartilage tissue stabilizer and comprises approximately 90% of articular cartilage (Aigner et al. 2001). The COL2A1 gene provides instructions to produce the pro-alpha 1 (II) chain and encode the alpha-1 chain of type II collagen in the cartilage matrix. Several previous studies have investigated the relationship between COL2A1 gene expression and osteoarthritis (Fukui et al. 2008; Mu et al. 2009; Brew et al. 2010). AGG encodes a large chondroitin sulfate proteoglycan which acts as a core protein that interacts with glycosaminoglycans and HA (Jayasuriya and Chen 2012). The breakdown of proteoglycans and collagen in the cartilage matrix is thought to be primarily due to the action of proteolytic enzymes in the MMP family. MMP-3 can stimulate other MMPs in the family, thus playing an important role in articular cartilage degradation. MMP-3 in both mRNA expression and enzymatic activity has been found to be up-regulated in early OA (Okada et al. 1992; Jayasuriya and Chen 2012).
The present experiment is an in vitro model of grade II/grade III OA from OA joints. Consistent with the biochemical evidence, i.e., matrix marker levels, low MW HA influences the mRNA levels of anabolic and catabolic genes. Of these, two anabolic genes, COL2A1 and AGG, were significantly up-regulated in the HA treatment groups, whereas one of the key catabolic genes, MMP3, which encodes a matrix-degrading enzyme, tended to be down-regulated in the groups exposed to HA. This may be advantageous when combined with carprofen 12.5 mg in the case of severe pain in OA because it induces more COL2A1 and AGG expression but does not destroy the structure of cartilage, especially uronic acid and collagen.
This study chose low molecular weight HA to determine the effects in an in vitro OA model by its properties. In a rabbit OA model, HA injection (in a molecular weight range of 500–1000 kDa) into joints was able to restore the rheological properties of SF because this molecular size can penetrate through synovial tissue to promote synovial fibroblasts to synthesize new molecules of HA (Coleman et al. 2000; Ghosh and Guidolin 2002). Previous in vitro studies showed that low molecular weight HA the positive effects on articular chondrocytes: HA of molecular weight 800 kDa activated proliferation and matrix synthesis in immature rabbit chondrocytes (Kawasaki et al. 1999); HA of molecular weight 500–730 kDa was reduced anti-Fas-induced apoptosis (Lisignoli et al. 2001) and inhibited the inflammation mediator interleukin-1 that is induced by superoxide anions in chondrocytes (Fukuda et al. 1997).
Several previous studies have reported the positive effects of a combination of HA treatment together with NSAID to reduce their toxicity. HA can inhibit NSAID-expedited MMP production followed by inflammatory cytokines in rabbit chondrocytes. HA was also determined to reduce acceleration of MMP production by indomethacin and celecoxib in a rabbit OA model (Hashizume and Mihara 2009). Another study found that high molecular weight HA injection was necessary for treatment of early stage OA to reduce cartilage degeneration by loxoprofen administered orally in a rabbit OA model (Mihara et al. 2007). This study is the first report to assess the effects of a combination of low MW HA and carprofen on canine OA articular cartilage in vitro and to evaluate its potential before use in an animal model. Further study is required concerning the effective dose and duration of carprofen injection when combined with low molecular weight HA in an animal model to relieve pain and inflammation in joints and also to protect chondrocyte viability, while having no adverse effects on cartilage structure.
Conclusions
In an in vitro model of canine osteoarthritis, low MW HA can preserve chondrocyte survival, protect against damage of articular cartilage, and stimulate COL2A1 and AGG expression. In combination with carprofen, low MW HA could not reduce the chondrotoxicity of carprofen. In cartilage explant culture, low MW HA combined with 12.5 mg carprofen did not destroy the cartilage matrix, especially uronic acid and collagen.
References
Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L (2001) Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum 44:2777–2789. doi:10.1002/1529-0131(200112)44:12<2777::AID-ART465>3.0.CO;2-H
Archer CW, Francis-West P (2003) The chondrocyte. Int J Biochem Cell Biol 35:401–404
Armstrong S, Lees P (1999) Effects of R and S enantiomers and a racemic mixture of carprofen on the production and release of proteoglycan and prostaglandin E2 from equine chondrocytes and cartilage explants. Am J Vet Res 60:98–104
Benton HP, Vasseur PB, Broderick-Villa GA, Koolpe M (1997) Effect of carprofen on sulfated glycosaminoglycan metabolism, protein synthesis, and prostaglandin release by cultured osteoarthritic canine chondrocytes. Am J Vet Res 58:286–292
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Brew CJ, Clegg PD, Boot-Handford RP, Andrew JG, Hardingham T (2010) Gene expression in human chondrocytes in late osteoarthritis is changed in both fibrillated and intact cartilage without evidence of generalised chondrocyte hypertrophy. Ann Rheum Dis 69:234–240. doi:10.1136/ard.2008.097139
Chang JK, Wu SC, Wang GJ, Cho MH, Ho ML (2006) Effects of non-steroidal anti-inflammatory drugs on cell proliferation and death in cultured epiphyseal-articular chondrocytes of fetal rats. Toxicology 228:111–123. doi:10.1016/j.tox.2006.08.028
Coleman PJ, Scott D, Mason RM, Levick JR (2000) Role of hyaluronan chain length in buffering interstitial flow across synovium in rabbits. J Physiol 526:425–434
Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, Lafeber FP (2010) The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr Cartil 18:S66–S79. doi:10.1016/j.joca.2010.04.017
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–277
Ding C (2002) Do NSAIDs affect the progression of osteoarthritis? Inflammation 26:139–142. doi:10.1023/A:1015504632021
Esko JD, Kimata K, Lindahl U (2009) Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (eds) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 229–248
Fukuda K, Takayama M, Ueno M, Oh M, Asada S, Kumano F, Tanaka S (1997) Hyaluronic acid inhibits interleukin-1-induced superoxide anion in bovine chondrocytes. Inflamm Res 46:114–117
Fukui N, Ikeda Y, Ohnuki T, Tanaka N, Hikita A, Mitomi H, Ochi T (2008) Regional differences in chondrocyte metabolism in osteoarthritis: a detailed analysis by laser capture microdissection. Arthritis Rheum 58:154–163. doi:10.1002/art.23175
Garnero P, Ayral X, Rousseau JC, Christgau S, Sandell LJ, Dougados M, Delmas PD (2002) Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum 46:2613–2624. doi:10.1002/art.10576
Ghosh P, Guidolin D (2002) Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent. Semin Arthritis Rheum 32:10–37
Goldberg VM, Buckwalter JA (2005) Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthr Cartil 13:216–224. doi:10.1016/j.joca.2004.11.010
Hashizume M, Mihara M (2009) Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthr Cartil 17:1513–1518. doi:10.1016/j.joca.2009.04.018
Hazewinkel HAW, Van den Brom WE, Theyse LFH, Pollmeier M, Hanson PD (2008) Comparison of the effects of firocoxib, carprofen and vedaprofen in a sodium urate crystal induced synovitis model of arthritis in dogs. Res Vet Sci 84:74–79. doi:10.1016/j.rvsc.2007.02.005
Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6(10):986–94
Henrotin Y, Chevalier X, Deberg M, Balblanc JC, Richette P, Mulleman D, Osteoarthritis Group of French Society of Rheumatology (2013) Early decrease of serum biomarkers of type II collagen degradation (Coll2-1) and joint inflammation (Coll2-1 NO2) by hyaluronic acid intra-articular injections in patients with knee osteoarthritis: a research study part of the Biovisco study. J Orthop Res 31:901–907. doi:10.1002/jor.22297
Jalbă BA, Jalbă CS, Vlădoi AD, Gherghina F, Stefan E, Cruce M (2011) Alterations in expression of cartilage-specific genes for aggrecan and collagen type II in osteoarthritis. Rom J Morphol Embryol 52:587–591
Jayasuriya CT, Chen Q (2012) Cartilage extracellular matrix integrity and OA. InTech Open Access. Retrieved from: http://www.intechopen.com/source/pdfs/28932/InTech-Cartilage_extracellular_matrix_integrity_and_oa.pdf
Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M (1999) Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol 179:142–148. doi:10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q
Kolar K (1990) Colorimetric determination of hydroxyproline as measure of collagen content in meat and meat products: NMKL collaborative study. J Assoc Off Anal Chem 73:54–57
Laurent TC, Fraser JR (1992) Hyaluronan. FASEB J 6:2397–2404
Lisignoli G, Grassi F, Zini N, Toneguzzi S, Piacentini A, Guidolin D, Facchini A (2001) Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) involvement. Arthritis Rheum 44:1800–1807. doi:10.1002/1529-0131(200108)44:8<1800::AID-ART317>3.0.CO;2-1
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–8
Matyas JR, Atley L, Ionescu M, Eyre DR, Poole AR (2004) Analysis of cartilage biomarkers in the early phases of canine experimental osteoarthritis. Arthritis Rheum 50:543–552. doi:10.1002/art.20027
Mihara M, Higo S, Uchiyama Y, Tanabe K, Saito K (2007) Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthr Cartil 15:543–549. doi:10.1016/j.joca.2006.11.001
Mu SC, Liu HC, Wu JY, Lee MTM, Chuang HP, Chen LK, Chen YT (2009) A large kindred of early-onset osteoarthritis of the knee and hip: excluding the link to COL2A1 gene. Rheumatology 48:371–374. doi:10.1093/rheumatology/kep010
Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, Bayliss MT, Iwata K, Nagase H (1992) Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest 66:680–690
Peng H, Zhou J, Liu S, Hu Q, Ming J, Qiu B (2010) Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res 59:519–530. doi:10.1007/s00011-010-0156-x
Qiu B, Liu SQ, Peng H (2008) Influence of sodium hyaluronate on iNOS expression in synovium and NO content in synovial fluid of rabbits with traumatic osteoarthritis. Chin J Traumatol 11:293–296
Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS (2006) Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthr Cartil 14:101–113. doi:10.1016/j.joca.2005.07.018
Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D (1999) The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthr Cartil 7:182–190. doi:10.1053/joca.1998.0207
Terauchi R, Takahashi KA, Arai Y, Ikeda T, Ohashi S, Imanishi J, Kubo T (2003) Hsp70 prevents nitric oxide-induced apoptosis in articular chondrocytes. Arthritis Rheum 48:1562–1568. doi:10.1002/art.11040
Uthman I, Raynauld JP, Haraoui B (2003) Intra-articular therapy in osteoarthritis. Postgrad Med J 79:449–453. doi:10.1136/pmj.79.934.449
Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM (2006) High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthr Cartil 14:1237–1247. doi:10.1016/j.joca.2006.05.009
Williams A, Smith JR, Allaway D, Harris P, Liddell S, Mobasheri A (2013) Carprofen inhibits the release of matrix metalloproteinases 1, 3, and 13 in the secretome of an explant model of articular cartilage stimulated with interleukin 1beta. Arthritis Res Ther 15:R223. doi:10.1186/ar4424
Acknowledgments
This study was supported by the 50th CMU Anniversary Ph.D. program and the Faculty of Veterinary Medicine, Chiang Mai University. The authors are also grateful for research funding from the Chiang Mai University (CMU) through the research administration office provides budget to our Excellence Center in Osteology Research and Training Center (ORTC). We gratefully thank the Thailand Excellence Center for tissue engineering and stem cells, Department of Biochemistry, Faculty of Medicine, Chiang Mai University for their kind support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Thippaporn Euppayo and Korakot Nganvongpanit contributed equally to this work.
Rights and permissions
About this article
Cite this article
Euppayo, T., Siengdee, P., Buddhachat, K. et al. Effects of low molecular weight hyaluronan combined with carprofen on canine osteoarthritis articular chondrocytes and cartilage explants in vitro. In Vitro Cell.Dev.Biol.-Animal 51, 857–865 (2015). https://doi.org/10.1007/s11626-015-9908-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9908-9