Abstract
Background
This study aimed to determine the prognostic significance of radiographic sarcopenia (RS) in patients with gallbladder cancer (GBC).
Methods
From March 2001 to December 2013, 158 GBC patients who underwent curative intent surgery were included. The presence of RS was determined by skeletal muscle mass index using abdominal computed tomography.
Results
The 1-, 3-, and 5-year overall survival (OS) rates were 63.6%, 41.9%, and 36.4%, respectively, for patients with RS (n = 88), and 84.3%, 62.6%, and 54.3%, respectively, for those without RS (n = 70) (P = 0.006). Multivariate analysis showed that RS (hazard rate [HR] 1.704, P = 0.024) was a significant prognostic factor for patient survival, as well as disease stage (IV: HR 7.181, P < 0.001), radicality (HR 2.830, P = 0.001), adjuvant therapy (HR 0.537, P = 0.017), and intraoperative blood loss ≥ 1 L (HR 1.851, P = 0.023).
Conclusions
This study showed a significant association between RS and OS in GBC patients. Because RS is the only significant prognostic factor that can be evaluated preoperatively, its assessment would be helpful to provide early preventive therapy allowing the maintenance of muscle mass and patient-tailored treatment based on their physiologic reserves (e.g., skeletal muscle mass).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallbladder cancer (GBC) is the fifth most common malignancy of the gastrointestinal tract and the most common malignancy of the biliary tract.1 Large gallstones (> 3 cm), extensive gallbladder calcification due to chronic cholecystitis (porcelain gallbladder), American Indian and Hispanic descent, chronic bacterial cholangitis (e.g., due to Salmonella typhi, Salmonella paratyphi), and an anomalous pancreaticobiliary duct junction are known risk factors of GBC.2 GBC is commonly accompanied by regional lymph node (LN) involvement and liver invasion. Therefore, extended cholecystectomy, including cholecystectomy, liver parenchyma resection (segments IVb and V), and regional LN dissection could improve surgical outcomes. Although surgical resection is recommended in possible cases, the prognosis is poor.
The most representative effect of aging is a change in body composition, i.e., an increase in the ratio of body fat to body weight and a decrease in lean body mass, especially skeletal muscle mass. In 1989, Rosenberg first described this age-associated skeletal muscle mass reduction as “sarcopenia,” derived from the Greek words “sarx,” which means “muscle” and “penia,” which means “decrease.”3 Skeletal muscle mass decreases by 8% every 10 years after the age of 40 years, and by 15% every 10 years after the age of 70 years.4, 5 In a previous report, sarcopenia was reported to increase the probability of functional limitations, disability, falls, loss of independence, and mortality in elderly patients.6,7,8,9,10
Recently, sarcopenia has received much attention as a prognostic factor in patients with various types of cancers such as colorectal cancer, esophageal cancer, gastric cancer, and hepatocellular carcinoma.11,12,13,14,15,16 However, there have been few reports on the relationship between sarcopenia and postoperative oncologic outcomes in patients with GBC. The primary goal of this study is to elucidate the prognostic value of radiographic sarcopenia (RS), which is determined by computed tomography (CT), in patients with GBC.
Materials and Methods
Patients and Study Design
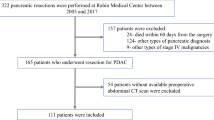
This study included 158 consecutive GBC patients who underwent surgery with curative intent at the National Cancer Center, Korea, from March 2001 to December 2013. Patients with double primary cancer of the gallbladder and other sites (e.g., hilar cholangiocarcinoma, mid/distal common bile duct cancer, pancreatic cancer, ampulla of Vater cancer, duodenal cancer) were excluded from this study, as well as patients with a history of treatment for any other types of cancer within the last 5 years. This study was approved by our Institutional Review Board (NCC2017-0256).
Definition and Surveillance
All the patients underwent CT as part of their presurgical evaluation. Cross-sectional skeletal muscle area (cm2) evaluations were conducted at the level of the third lumbar vertebrae (L3). Two consecutive CT images both showing the vertebral spine at the L3 level were used to measure the muscle area, determined by semiautomatic segmentation. The mean value of the two measurements was regarded as the L3 skeletal muscle area (cm2). The skeletal muscle area (cm2), including the rectus abdominis, external oblique, internal oblique, transverse abdominis, psoas, and paraspinal muscles corresponding to the threshold, ranges between − 30 and + 110 Hounsfield units (Fig. 1).17 The skeletal muscle index (SMI, cm2/m2) was defined as the skeletal muscle area (cm2) at the L3 level divided by the square of the patient’s height (m2). RS was defined as SMI < 52.4 cm2/m2 for male patients and < 38.5 cm2/m2 for female patients.18 All measurements were taken using OsiriX MD version 9.0 (http://www.osirix-viewer.com).
Axial computed tomographic scan at the level of the third lumbar vertebra. Areas of total skeletal muscle (− 30 to + 110 HU) including rectus abdominis, transverse abdominal, external oblique, internal oblique, psoas, and paraspinal muscle were measured. The SMI (cm2/m2) was defined as the area of total skeletal muscle (cm2) divided by the square of the height (m2). HU, Hounsfield unit; SMI, skeletal muscle index
Body mass index (BMI, kg/m2) was defined as the body weight (kg) divided by the square of the height (m2) and BMI values were classified as low (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), or obesity (≥ 25 kg/m2).19 For GBC staging, the 8th edition of the American Joint Committee on Cancer (AJCC) staging system was used.
Recurrence surveillance was performed every 3 or 6 months after discharge using CT or magnetic resonance imaging, and positron emission tomographic-CT scan was performed if necessary. Cancer recurrence was finally confirmed through biopsy in most patients. Patients for whom biopsies were not performed were considered to have recurrent cancer when their serial imaging results clearly indicated cancer progression. Postoperative mortality was defined as death within 30 days after surgery.
Surgical Procedure
In most cases of early GBC (T1), laparoscopic/open cholecystectomy was performed. However, in patients with T1b GBC who were 65 years old or younger and had no severe comorbidity, extended cholecystectomy, including cholecystectomy, resection of segments IVb and V of the liver, and regional lymph node dissection, were considered. In cases of T2 GBC, extended cholecystectomy was performed. Further, in cases of T3/4 GBC, if the invasion of the surrounding organs was suspected, combined resection, including extended right hemihepatectomy, common bile duct resection, pancreaticoduodenectomy, and colectomy was performed along with extended cholecystectomy.
Adjuvant Therapy
Adjuvant therapy was performed as in our previous report.20 Chemoradiation therapy consisted of 45 Gy of radiation in 25 fractions at 1.8 Gy per fraction daily, with concurrent continuous infusions of 5-fluorouracil and leucovorin (intravenous bolus injections of 5-fluorouracil, 400 mg/m2/day and leucovorin, 20 mg/m2/day for 3 days in the first and fifth weeks of radiotherapy).
Statistical Analysis
Continuous variables are given as mean ± standard deviation or median (interquartile range [IQR]) depending on the normality of the distribution. The cut-off values for the continuous variables were defined as the points with the most significant (log-rank test) split using the “maxstat” and “survival” packages of R. Survival was estimated using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional-hazards model was used for multivariate analysis, and a P value of less than 0.05 was considered statistically significant. All calculations were made using the SPSS 24.0 statistical software package (IBM Corporation, Armonk, NY, USA) and R 3.4.3 (https://www.r-project.org).
Results
Patient Characteristics
A total of 158 patients were included in this study, with 88 in the RS group and 70 in the no-RS group (Table 1). The median age was 64.5 years for the RS group and 63.5 years for the no-RS group (P = 0.774). In patients with RS, the proportion of males was higher than in those without RS (n = 58, 65.9% vs. n = 15, 21.4%; P < 0.001). There was a difference in BMI (P < 0.001), neutrophil to lymphocyte ratio (P < 0.003), and serum bilirubin level (P = 0.031) between the two groups. The American Society of Anesthesiologists (ASA) physical status (P = 0.461), Eastern Cooperative Oncology Group (ECOG) performance status (P = 0.841), type of surgery (P = 0.385), operation time (P = 0.107), and red blood cell (RBC) transfusion (P = 0.345) showed no significant differences between the two groups. Nevertheless, significant differences were observed in the Charlson comorbidity index (CCI; P = 0.030) and intraoperative blood loss (P = 0.025). No significant differences in pathologic variables such as tumor size (P = 0.656), tumor cell differentiation (P = 0.424), lymphatic invasion (P = 0.987), perineural invasion (P = 0.363), surgical radicality (P = 0.512), and disease stage ([AJCC, 8th edition] P = 0.475) were observed between the two groups. Adjuvant therapy was more commonly performed in patients without RS (P = 0.045). No significant differences in mortality (P = 0.580) and hospital stay (P = 0.499) were observed between the groups.
Univariate and Multivariate Analyses
Univariate analysis showed that the overall survival (OS) after surgery in patients with GBC was significantly associated with RS (P = 0.008), bilirubin level (P = 0.001), albumin level (P = 0.042), carcinoembryonic antigen (CEA) level (P = 0.043), carcinoembryonic antigen 19-9 (CA19-9) level (P < 0.001), type of surgery (P = 0.016), operation time (P < 0.001), intraoperative blood loss of ≥ 1 L (P < 0.001), RBC transfusion (P = 0.001), lymphatic invasion (P < 0.001), perineural invasion (P < 0.001), surgical radicality (P < 0.001), and disease stage (P < 0.001). Further, recurrence-free survival (RFS) was associated with CEA level (P = 0.010), CA19-9 level (P < 0.001), type of surgery (P = 0.041), operation time (P = 0.004), intraoperative blood loss of ≥ 1 L (P = 0.004), tumor size (P = 0.031), lymphatic invasion (P < 0.001), perineural invasion (P < 0.001), surgical radicality (P < 0.001), and disease stage (P < 0.001) (Table 2).
In multivariate analysis, significant prognostic factors for OS were RS (hazard ratio [HR] 1.704, P = 0.024), intraoperative blood loss of 1 L (HR 1.851, P = 0.023), surgical radicality (HR 2.830, P = 0.001), disease stage (IV: HR 7.181, P < 0.001), and adjuvant therapy (HR 0.537, P = 0.017). In addition, surgical radicality (P = 0.022) and disease stage (P < 0.001) were significant prognostic factors for RFS. The independent predictors for OS and RFS are summarized in Table 3.
Patient Survival and Recurrence
The 1-, 3-, and 5-year OS rates were 63.6%, 41.9%, and 36.4%, respectively, for patients in with RS, and 84.3%, 62.6%, and 54.3%, respectively, for patients without RS (Fig. 2a; P = 0.006). The 1-, 3-, and 5-year RFS rates were 59.8%, 49.0%, and 46.7%, respectively, for patients with RS and 68.1%, 51.8%, and 49.8%, respectively, for patients without RS (Fig. 2b; P = 0.490). In patients with GBC having RS, there was no difference between OS (Fig. 2c; P = 0.552) and RFS (Fig. 2d; P = 0.821) according to obesity status.
Discussion
The concept of sarcopenia was first proposed by Rosenberg in 1989,3 but the worldwide consensus (e.g., European Working Group, International Working Group, and Asian Working Group) began to be established after 2010.21,22,23 According to these, sarcopenia is a condition accompanied by a decrease in the muscle mass and a decrease in the muscle function (strength or performance). However, this retrospective study involved patients from March 2001 to December 2013 and included a significant number of patients without preoperative assessment of muscle function. In addition, in order to evaluate the effect of sarcopenia on the prognosis of patients with GBC, it is appropriate to investigate disease-specific survival rather than overall survival. However, in most cases, there was a legal restriction on the inquiry of the death certificate, making it difficult to identify the specific cause of death. The analysis using RS, which does not reflect muscle function, and overall survival rather than disease-specific survival constrains the interpretation of the results of this study, further highlighting the need for a well-constructed prospective study.
Meanwhile, sarcopenia in patients with cancer has been highlighted over the last decade. Recent studies have reported that sarcopenia has been identified as a poor prognostic factor in patients after several kinds of surgeries such as colectomy, hepatectomy, liver transplantation, esophagectomy, gastrectomy, and pancreatectomy.11,12,13,14,15,16,24,25 However, little is known about the relationship between sarcopenia and postoperative prognosis in GBC patients. Okumura et al. have reported that sarcopenia is a poor prognostic factor for patient survival and cancer recurrence in patients with extrahepatic biliary malignancies, including hilar cholangiocarcinoma, GBC, distal bile duct cancer, and ampulla of Vater cancer.26 However, because the study included only a small number of patients with GBC and different types of cancers with different pathophysiological properties, operation methods/range, and staging systems, it is inappropriate to conclude that the study accurately assessed the effect of sarcopenia on the postoperative outcome of patients with GBC.
In this study, significant prognostic factors for OS in patients with GBC were RS, intraoperative blood loss, surgical radicality, stage, and adjuvant therapy, and significant prognostic factors for RFS were surgical radicality and stage, respectively.
Gospodarowicz et al. classified the prognostic factors for cancer into tumor-related (e.g., tumor pathology, anatomic disease extent, tumor biology), host-related (e.g., age, gender, ethnicity, performance status, comorbidities, immune system), and environment-related (e.g., access to care, health-care policy, access to drugs or technology, choice of treatment, quality of treatment) factors. The study pointed out that the importance of host-related or environment-related factors might be overlooked by attention toward tumor-related factors.27
It is difficult to measure host-related factors, i.e., pretreatment physiologic reserves, among the prognostic factors for cancer. Comorbidity and performance indices, such as the ECOG performance status, ASA physical status, and CCI, which constitute such efforts, do not quantitatively reflect a patient’s preoperative general condition because of their heterogeneities, subjectivity, and imprecision. Therefore, there have been criticisms that they exhibit limitations in predicting prognoses such as patient survival or toxicity after treatment of cancer.28,29,30
Here, RS, one of the host-related factors, should be noted in predicting postoperative survival in GBC patients. In this study, RS, along with stage, surgical radicality, intraoperative blood loss, and adjuvant therapy, was a significant prognostic factor for patient survival. On the other hand, other host-related factors such as age, ECOG performance status, ASA physical status, CCI, and BMI that were routinely collected before surgery were not helpful in predicting prognosis. Thus, the results of our study suggest that skeletal muscle mass may be more useful in assessing the preoperative physiologic reserves of patients with GBC than the conventional comorbidity and performance indices, such as ECOG performance status, ASA physical status, and CCI. Furthermore, among the significant prognostic factors for patient survival, SMI is the only factor that can be evaluated before surgery. Therefore, preoperative identification of patients with RS may be expected to improve prognosis by allowing preemptive treatment for maintaining or increasing muscle mass and may also help establish appropriate treatment strategies for individual patients by predicting preoperative physiologic reserves.31
Meanwhile, aging is associated with an increase in fat mass, particularly visceral fat, as well as skeletal muscle mass reduction. Accumulated adipose tissue secretes hundreds of pro-inflammatory cytokines called “adipokines” (e.g., leptin, adiponectin, apelin, chemerin, and vaspin) that cause chronic systemic inflammation in the body, as well as insulin resistance, atherosclerosis, and neurodegeneration.32,33 This aging-associated obesity works synergistically with sarcopenia, further increasing the risk of metabolic disorders, cardiovascular diseases, and mortality.34
The synergistic effects of sarcopenia and obesity have been reported to cause poor prognosis in patients with cancer.18,35 However, in this study, in patients with GBC having RS, obesity tended to have an even slightly better effect on the prognosis for GBC, although there was no statistical significance (Fig. 2c, d). This observation is referred to as “obesity paradox.”36,37 Obesity paradox was first described in 1999 in overweight and obese patients receiving hemodialysis38 and it has since been found in patients with heart failure and peripheral arterial disease.37,39 According to those studies, obesity, defined as BMI alone, can be misleading because the height-adjusted weight is not a specific indicator of total body fat or abnormal body fat accumulation.40 In other words, obesity means excess body fat, but people with excess lean body mass will have a higher BMI as well.41,42 In addition, the differences in bone density depending on race, sex, and age can affect BMI.
This study has some limitations. First, this study was retrospective and conducted in a single institution. Our results should be confirmed in a larger multicenter prospective cohort. Second, despite our efforts, the complications that correspond to grade 1 or 2 of the Clavien-Dindo classification were difficult to investigate precisely and therefore were not presented in the results. Third, there are legal restrictions on the examination of death certificates of many patients included in this study, which makes it difficult to determine the exact cause of the death, and therefore disease-specific survival could not be considered. Fourth, the semi-automated segmentation function built in OsiriX MD version 9.0 was used to measure the skeletal muscle area and then manually corrected for errors, but the possibility of measurement errors cannot be completely ruled out. Finally, in defining sarcopenia, functional parameters are not sufficiently considered due to the retrospective nature of this study.
Conclusion
This study showed that there was a significant association between RS and GBC patient survival. Furthermore, among the prognostic factors for patient survival, RS is the only factor that can be evaluated before surgery. Evaluation of RS in patients with GBC might be helpful in the development of early prevention strategies to maintain or increase muscle mass, which would improve surgical outcomes and the establishment of appropriate treatment strategies according to the patient’s physiologic reserves.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30.
Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi:https://doi.org/10.2147/clep.s37357.
Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–1s.
Grimby G, Saltin B. The ageing muscle. Clin Physiol. 1983;3(3):209–18.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63.
Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58(11):2055–62. doi:https://doi.org/10.1111/j.1532-5415.2010.03145.x.
Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31(5):652–8. doi:https://doi.org/10.1016/j.clnu.2012.02.007.
Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. Journal of cachexia, sarcopenia and muscle. 2017;8(2):245–50. doi:https://doi.org/10.1002/jcsm.12160.
Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42(2):203–9. doi:https://doi.org/10.1093/ageing/afs194.
Bunout D, de la Maza MP, Barrera G, Leiva L, Hirsch S. Association between sarcopenia and mortality in healthy older people. Australas J Ageing. 2011;30(2):89–92. doi:https://doi.org/10.1111/j.1741-6612.2010.00448.x.
Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewe KW, Hoofwijk AG et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261(2):345–52. doi:https://doi.org/10.1097/sla.0000000000000628.
Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P et al. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2015;261(6):1173–83. doi:https://doi.org/10.1097/sla.0000000000000743.
Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100(11):1523–30. doi:https://doi.org/10.1002/bjs.9258.
Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba-Ssalamah A. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 2016;26(5):1359–67. doi:https://doi.org/10.1007/s00330-015-3963-1.
Tegels JJ, van Vugt JL, Reisinger KW, Hulsewe KW, Hoofwijk AG, Derikx JP et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112(4):403–7. doi:https://doi.org/10.1002/jso.24015.
Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D et al. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg. 2015;19(9):1593–602. doi:https://doi.org/10.1007/s11605-015-2835-y.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. Journal of applied physiology (Bethesda, Md : 1985). 1998;85(1):115–22.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35. doi:https://doi.org/10.1016/s1470-2045(08)70153-0.
Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi:https://doi.org/10.1016/s0140-6736(03)15268-3.
Kim TH, Han SS, Park SJ, Lee WJ, Woo SM, Moon SH et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):e853–9. doi:https://doi.org/10.1016/j.ijrobp.2010.12.019.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi:https://doi.org/10.1093/ageing/afq034.
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. doi:https://doi.org/10.1016/j.jamda.2011.01.003.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi:https://doi.org/10.1016/j.jamda.2013.11.025.
Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A et al. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1,326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J Am Coll Surg. 2016;222(4):397–407.e2. doi:https://doi.org/10.1016/j.jamcollsurg.2015.12.020.
Parkin E, Plumb AA, O'Reilly D, Renehan AG. Body composition and outcome in patients undergoing resection of colorectal liver metastases (Br J Surg 2012; 99: 550-557). Br J Surg. 2012;99(7):1021–2; author reply 2. doi:https://doi.org/10.1002/bjs.8826.
Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Kobayashi A, Iida T et al. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016;159(3):821–33. doi:https://doi.org/10.1016/j.surg.2015.08.047.
Gospodarowicz M, O'Sullivan B. Prognostic factors in cancer. Semin Surg Oncol. 2003;21(1):13–8. doi:https://doi.org/10.1002/ssu.10016.
Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–8. doi:https://doi.org/10.1016/j.jamcollsurg.2010.01.028.
Li JL, Henderson MA, Revenig LM, Sweeney JF, Kooby DA, Maithel SK et al. Frailty and one-year mortality in major intra-abdominal operations. J Surg Res. 2016;203(2):507–12.e1. doi:https://doi.org/10.1016/j.jss.2016.03.007.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi:https://doi.org/10.1016/j.ejca.2015.12.030.
Choi Y, Oh DY, Kim TY, Lee KH, Han SW, Im SA et al. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS One. 2015;10(10):e0139749. doi:https://doi.org/10.1371/journal.pone.0139749.
Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6(1–2):91–101. doi:https://doi.org/10.1002/prca.201100052.
Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. J Physiol. 2009;587(Pt 23):5559–68. doi:https://doi.org/10.1113/jphysiol.2009.179515.
Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi:https://doi.org/10.1097/MCO.0b013e328312c37d.
Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(22):6973–9. doi:https://doi.org/10.1158/1078-0432.ccr-09-1525.
Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–84.
Schmidt DS, Salahudeen AK. Obesity-survival paradox-still a controversy? Seminars in dialysis. 2007;20(6):486–92. doi:https://doi.org/10.1111/j.1525-139X.2007.00349.x.
Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55(4):1560–7. doi:https://doi.org/10.1046/j.1523-1755.1999.00389.x.
Behavioral counseling in primary care to promote a healthy diet: recommendations and rationale. Am Fam Physician. 2003;67(12):2573–6.
Pischon T. Commentary: Use of the body mass index to assess the risk of health outcomes: time to say goodbye? Int J Epidemiol. 2010;39(2):528–9. doi:https://doi.org/10.1093/ije/dyp388.
Salahudeen AK. Obesity and survival on dialysis. Am J Kidney Dis. 2003;41(5):925–32.
Tzamaloukas AH, Murata GH, Hoffman RM, Schmidt DW, Hill JE, Leger A et al. Classification of the degree of obesity by body mass index or by deviation from ideal weight. JPEN J Parenter Enteral Nutr. 2003;27(5):340–8. doi:https://doi.org/10.1177/0148607103027005340.
Author information
Authors and Affiliations
Contributions
-Study concepts and design: ECL, S-JP, SDL, S-SH, and SHK
-Data acquisition and analysis: ECL and SDL
-Manuscript preparation: ECL
-Manuscript review: ECL, S-JP, S-SH, and SHK
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, E.C., Park, SJ., Lee, S.D. et al. Effects of Sarcopenia on Prognosis After Resection of Gallbladder Cancer. J Gastrointest Surg 24, 1082–1091 (2020). https://doi.org/10.1007/s11605-019-04198-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04198-w