Abstract
Background
Resection of certain recurrent malignancies can prolong survival, but resection of recurrent pancreatic ductal adenocarcinoma is typically contraindicated because of poor outcomes.
Methods
All patients from 1992 to 2010 with recurrent pancreatic cancer after intended surgical cure were retrospectively evaluated. Clinicopathologic features were compared from patients who did and did not undergo subsequent reoperation with curative intent to identify factors associated with prolonged survival.
Results
Twenty-one of 426 patients (5 %) with recurrent pancreatic cancer underwent potentially curative reoperation for solitary local-regional (n = 7) or distant (n = 14) recurrence. The median disease-free interval after initial resection among reoperative patients was longer for those with lung or local-regional recurrence (52.4 and 41.1 months, respectively) than for those with liver recurrence (7.6 months, p = 0.006). The median interval between reoperation and second recurrence was longer in patients with lung recurrence (median not reached) than with liver or local-regional recurrence (6 and 9 months, respectively, p = 0.023). Reoperative patients with an initial disease-free interval >20 months had a longer median survival than those who did not (92.3 versus 31.3 months, respectively; p = 0.033).
Conclusion
Patients with a solitary pulmonary recurrence of pancreatic cancer after a prolonged disease-free interval should be considered for reoperation, as they are more likely to benefit from resection versus other sites of solitary recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although advances have been made in the multimodality treatment of pancreatic ductal adenocarcinoma (PDAC), the majority of patients who undergo potentially curative resection develop recurrent locoregional or distant disease at a median of 10–20 months after resection.1–7 Resection of recurrent disease can be curative for selected patients with colorectal cancer, neuroendocrine carcinoma, renal cell carcinoma, and other solid malignancies.8–12 Factors that predict favorable prognosis following surgical management of metastatic disease in such patients can include a longer disease-free interval (DFI), negative surgical margins at the initial resection, and a small burden of disease at the time of recurrence.12–16 Recurrent PDAC, in comparison, has been notoriously difficult to manage surgically as it is typically characterized by aggressive growth, a multifocal pattern of recurrence, and technically unresectable disease.6 Furthermore, even isolated or low-volume recurrence is commonly associated with widespread, radiographically occult micrometastases. Treatment of patients with recurrent PDAC has therefore historically been limited to palliative chemotherapy or best supportive care.
Little is known about the role of surgery for recurrent PDAC. Only anecdotal experiences and small case series have been previously published, and these series reported outcomes of patients diagnosed with a variety of periampullary neoplasms or those with recurrence limited to certain anatomic sites.17–20 We sought to gain a more comprehensive understanding of the outcomes of individuals with isolated recurrent PDAC treated with potentially curative reoperation and hypothesized that reoperation may be appropriate for selected patients with recurrent PDAC. To characterize these patients, we retrospectively evaluated the outcomes of all patients diagnosed at our institution with recurrent PDAC who underwent reoperation with curative intent over an 18-year period. This manuscript presents the largest known series to date of patients who underwent reoperation with curative intent for recurrent local or metastatic PDAC.
Methods
The Institutional Review Board at The University of Texas MD Anderson Cancer Center approved the collection and analysis of data associated with this study. Utilizing a prospectively maintained pancreatic tumor database, we identified all patients with histologically confirmed PDAC who underwent surgical resection with curative intent between January 1, 1992, and December 31, 2010.21 Those with documented recurrent disease were selected for further analysis. Patients from this group who underwent a potentially curative reoperation are the focus of this study. We defined potentially curative reoperation for recurrent PDAC as completion pancreatectomy or tumor bed resection for local recurrence, wedge resection or lobectomy for lung recurrence, or either resection or percutaneous radiofrequency ablation (RFA) for liver recurrence.
Surgical resection of the primary tumor was performed using standardized techniques and was often preceded by chemotherapy and/or chemoradiation.22,23 Following resection, patients underwent routine post-operative surveillance that consisted of a chest X-ray, computed tomography (CT) of the abdomen and pelvis, and biochemical tumor marker assay (CA19-9) every 4 months for the first 2 years, every 6 months for the following 3 years, and annually thereafter. A new lesion on chest X-ray or the lung bases on the abdominal CT, or a rise in CA19-9 level without evidence of intra-abdominal recurrence prompted a dedicated CT of the chest. Recurrence was diagnosed by radiographic evidence of a new, suspicious low-density mass in the region of the resected pancreas, mesenteric root, liver, lung, or other distant sites. Biopsy of suspected recurrence was performed when the diagnosis was equivocal.
The pattern of initial recurrence was categorized as local, regional, or distant. Local recurrence was defined as recurrence isolated to the bed of the pancreatic margin, the pancreatic remnant, or the mesenteric root.1 Tumors suspected as representing a second primary PDAC were reported as a local recurrence. Regional recurrence was defined as cancer in the soft tissue or regional lymph nodes outside the confines of the pancreatic bed, or disease in the peritoneal cavity. Distant recurrence was defined as recurrence in distant organs or lymph nodes. If a patient had more than one site of synchronous recurrence, he or she was included in all applicable categories for statistical purposes. Because of the limited number of patients in each group, individuals with a local or regional recurrence were grouped together and defined as the local-regional recurrence group.
All patients with recurrent PDAC who were considered potential candidates for reoperation were presented at a weekly multidisciplinary pancreatic tumor conference. Historically, patients considered potential candidates for reoperation have included those with a good performance status and a technically operable, solitary site of macroscopic disease, typically following a prolonged disease free interval (DFI).
Statistical analyses were performed using STATA/SE (v12.0) statistical software (Stata Corp. LP, College Station, TX). Summary statistics were used to describe the clinical and demographic characteristics of the study population. Wilcoxon rank-sum test was used to assess differences between continuous variables. Categorical data were compared using Fisher's exact test or Pearson's chi-squared. The “first DFI” was defined as the time interval between the date of resection of the primary tumor and the date of initial recurrence. Censoring occurred at the date of last follow-up if a recurrence or death was not observed. Among patients who underwent potentially curative reoperation for recurrence, the “second DFI” was defined as the time interval between the date of reoperation for the initial recurrence and the date of second recurrence or last follow-up, whichever occurred first. Overall survival (OS) was defined as the time from resection of the primary tumor to death and was censored at the date of last follow-up if death had not occurred. The survival after reoperation was defined as the time interval between the date of reoperation for the initial recurrence and the date of death and was censored at the date of last follow-up if death had not occurred. Univariate Cox proportional hazards regression was used to model the association between each potential prognostic factor and OS or DFI. The Kaplan–Meier product limit method was used to estimate median OS and DFI based on clinicopathologic factors (i.e., patient factors, operative factors, and DFI). A two-sided significance level of 0.05 and a 95 % confidence interval (CI) were used for all statistical analyses.
Results
Patient Demographics and Original Resection Parameters
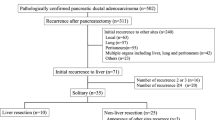
A total of 700 patients underwent potentially curative surgical resection for PDAC between January 1, 1992 and December 31, 2010. Of these patients, 430 (61.4 %) developed recurrent PDAC after resection by the time of last follow-up, 21 of whom were selected for reoperation with curative intent and 405 of whom were not. The remaining four patients were excluded from analysis because of insufficient follow-up data that precluded an accurate determination of disease-free interval and survival. These patients otherwise would have been included in the group not selected for reoperation (Fig. 1). The median follow-up time after surgical resection of the primary tumor of all 426 patients who recurred was 18 months. The median follow-up time for the patients who were selected for reoperation for recurrence was 67 months.
Nineteen patients who underwent reoperation for recurrence had histologically confirmed recurrent PDAC either pre-operatively or on final pathology and two patients, both of whom underwent RFA of the liver, did not. The clinicopathologic profile of the entire cohort including subgroups of those selected and not selected for reoperation is illustrated in Table 1.
Timing and Patterns of Initial Recurrence
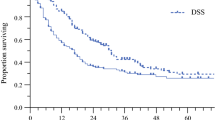
The median first DFI of the 426 evaluable patients who recurred was 8.1 months. The median first DFI of patients who were selected for reoperation was longer than that of patients who were not selected (21.7 versus 7.85 months, respectively; HR 2.91 [CI 1.82–4.65], p < 0.001). Figure 2 demonstrates that among patients selected for reoperation, the median first DFI of patients with lung recurrence was longer than that of patients who recurred in the liver (52.4 vs. 7.6 months, respectively; HR 0.16 [CI 0.04–0.6], p = 0.007). Similarly, the median first DFI of patients with local-regional recurrence was longer than that of patients who recurred in the liver (41.1 vs. 7.6 months, respectively; HR 0.18 [CI 0.05–0.69], p = 0.012).
Median first and second DFI among patients selected for reoperation stratified by pattern of initial recurrence. The recurrence pattern of those selected for reoperation was divided into three categories to elucidate differences in disease-free interval (DFI, displayed in months). The first DFI is defined as the interval from the initial resection of the primary PDAC to first recurrence. The second DFI is defined as the interval from reoperation for recurrence to second recurrence. A statistically significant difference in the first DFI is seen between the lung and locoregional recurrence groups versus the liver recurrence group (p = 0.006). The second DFI for patients with an initial lung recurrence who underwent reoperation was longer than those with a liver or local-regional recurrence (p = 0.023). The median period for the second DFI for the lung recurrence group has not been reached and is reported as the mean interval (asterisk)
The patterns of initial recurrence of all patients in this series are reported in Table 1. The initial recurrence pattern differed between patients who were and were not selected for reoperation as no patient with carcinomatosis or multiple sites of recurrence was selected for reoperation. Among the patients selected for reoperation, the distribution of initial recurrences was 6 (28.6 %) local, 14 (66.7 %) distant, and 1 (4.8 %) regional recurrence as a solitary abdominal wall implant. Distant recurrences in patients selected for reoperation included 6 (42.9 %) cases of liver metastasis, 7 (50 %) cases of lung metastasis, and 1 (7.1 %) case of brain metastasis.
Perioperative Parameters and Recurrence Characteristics of the Reoperation Cohort
The median time between surgical resection of the primary tumor and reoperation for recurrent disease was 27.4 months (range, 10.8–90.3 months). The median duration from the diagnosis of initial recurrence to reoperation was 138 days (range, 4–604 days). During this time, 16 (76.2 %) of 21 patients received chemotherapy and 2 (9.5 %) patients received chemoradiation, all demonstrating stable or decreased disease burden prior to reoperation.
Four (19 %) patients underwent planned curative RFA of a solitary liver metastasis (Table 2). The remaining 17 (81 %) patients underwent a resection of their recurrence which included: lung (n = 7), completion pancreatectomy (n = 5), liver (n = 2), brain (n = 1), local tumor bed recurrence (n = 1), and abdominal wall implant (n = 1). No perioperative deaths occurred after the repeat resections, and 6 (28.6 %) patients went on to receive adjuvant therapy with various regimens.
After reoperation, a total of 14 patients (66.7 %) recurred a second time after a median second DFI of 9 months. There was no statistical difference between the second DFI of patients with liver and local-regional recurrence (6 and 9 months, respectively). The median second DFI of patients after reoperation for lung recurrence (not reached, mean 21 months) was longer than that of patients who underwent reoperation for liver or local-regional recurrence (6 and 9 months, respectively; p = 0.023; Fig. 2).
Survival Data
The 5-year OS rate of the patients selected for reoperation was 52.4 %. The median OS of patients selected for reoperation and those who were not was 81.1 and 18.1 months, respectively (HR 5.33 [CI 3.02–9.4], p < 0.001). Of the 21 patients selected for reoperation, the median survival following the reoperation for recurrent disease was 36 months. Among patients selected for reoperation, the median OS after resection of the primary tumor was significantly longer for patients whom developed an initial lung recurrence (92.3 months) compared to those who developed an initial liver recurrence (32.5 months; HR 0.16 [CI 0.03–0.79], p = 0.024; Fig. 3). Although not statistically significant, the median OS for patients with a local-regional recurrence (79.3 months) was also longer than for those who developed an initial liver recurrence (HR 0.28 [CI 0.07–1.15], p = 0.078; Fig. 3). Patients still alive after reoperation include four who underwent lung resection, three who underwent local-regional resection, and one who underwent liver RFA, with a median follow-up after reoperation of 30.5 months, 14 months, and 1 month, respectively (Table 2).
Kaplan–Meier analysis for DFI and OS between the various study cohorts. The median first DFI for those selected for reoperation versus those who were not selected was 21.7 and 7.85 months, respectively (p < 0.001, a). When those selected for reoperation are sub-categorized by recurrence location, patients with hepatic recurrence had a significantly shorter median first DFI than patients with lung or locoregional recurrence (p = 0.006, b). The median OS after initial resection in those selected for reoperation was significantly shorter in patients with a hepatic recurrence than in those with a lung or locoregional recurrence (p = 0.034, c). Those patients with a median first DFI >20 months and underwent reoperation for recurrence had a significantly longer OS than those with a first DFI <20 months who underwent reoperation (p = 0.033, d)
Among the clinicopathologic factors evaluated, those significantly associated with OS on univariate analysis in the patients selected for reoperation were pattern of initial recurrence, the status of the regional lymph nodes at the initial operation, and the duration of the first DFI. Lymph node positivity at the initial primary tumor resection was associated with a shorter OS (HR 4.59 [CI 1.17–18.04], p = 0.03) but not with a shorter first DFI (HR 2.3 [CI 0.84–6.3], p = 0.1). In addition, utilizing log–rank analysis, patients who underwent reoperation for recurrence with a first DFI of greater than 20 months had a median OS of 92.3 months, which was longer than the median OS of 31.3 months for those reoperative patients who had a first DFI of less than 20 months (HR 0.27 [CI 0.08–0.9], p = 0.033; Fig. 3). The median survival after diagnosis of the second recurrence among the 14 individuals who recurred a second time was 16 months, with no statistical difference in survival duration based on the first site of tumor recurrence.
Discussion
This study presents the largest series to date of patients who underwent potentially curative reoperation for recurrent local-regional or metastatic PDAC following intended curative resection of the primary tumor. Our data suggest that reoperation can be performed safely but demonstrate that reoperation should be considered primarily for individuals who develop a solitary pulmonary recurrence of PDAC after a prolonged initial DFI, as these patients appear most likely to benefit from surgery.
Several small reports regarding re-resection of recurrent PDAC in the lungs or remnant pancreas have been reported previously (Table 3). Arnaoutakis et al.24 reported their experience of selective resection of solitary pulmonary metastases which revealed that the 9 patients who underwent re-resection had a longer OS than the 22 patients who did not (51 versus 23 months, respectively; p = 0.04). They demonstrated that pulmonary metastasectomy can be performed safely as there were no procedure-related complications or perioperative deaths. Lavu et al. 20 retrospectively reviewed 11 patients who underwent reoperative completion pancreatectomy for suspected malignant disease. Of these patients, however, only six were histologically confirmed to be recurrent PDAC. Nevertheless, those with histologically confirmed PDAC had a median survival after re-resection of 32 months with no 30-day post-operative mortality. Finally, Kleeff et al.25 reported resection of recurrent PDAC located either locoregionally or in the lungs in 30 patients. Resection was potentially curative with an R0 or R1 resection in only eight of these individuals, as opposed to an R0 resection in the 17 individuals from the current study who underwent resection for their recurrence. There was no difference in median OS between the R0/R1 resection, R2 resection, and exploration/palliative groups in the Kleeff series. In that series, patients with a first DFI of greater than 9 months appeared to benefit most from re-resection, compared to 20 months in our series. Despite the differences between these two studies in regards to the duration of the first DFI correlating with improved survival, the presence of favorable tumor biology likely played a large role in the ability of patients to have even been considered for reoperation. These reports demonstrate that resection can be performed safely with prolongation of survival in selected patients.
Typically, most patients with PDAC recur at multiple sites or with carcinomatosis, but we have historically considered patients with recurrent PDAC for reoperation who have a good performance status, a technically operable, solitary site of macroscopic disease, and a relatively prolonged DFI. The intent of these general criteria was to select a population of patients with indolent disease for whom reoperation was both possible and likely to be associated with favorable long-term survival. In this study, we confirm that resection of liver recurrence, which typically occur within the first 2 years, is not beneficial.1 However, we also found no data to support the use of surgery for patients with local-regional recurrence, even though the first DFI of such patients may be prolonged. Although patients with local-regional and lung recurrence underwent reoperation following a similarly prolonged first DFI, prolonged disease-free survival after reoperation was only observed among those with lung metastasis as patients with an initial local-regional recurrence tended to re-recur quickly after reoperation. The lung metastasis in each of the patients in our reoperative lung cohort were all unilateral, solitary site of disease although the possibility exists that resection may also be beneficial for multiple, unilateral tumors as has been demonstrated for cancers of other sites.26,27 Finally, in this series, 86 % of patients who underwent reoperation were treated with either chemotherapy or chemoradiation which also served to assess and select for disease biology prior to reoperation.4,22,28 The need to identify, in a prospective manner, patients with recurrent disease who will benefit from surgery supports this use of preoperative therapy prior to reoperation.
Although this study has the inherent limitations of a retrospective study and there is a selection bias in our cohort who underwent reoperation, it is the largest known collection of patients to date who have undergone reoperation specifically for cure of a solitary PDAC recurrence, excluding other periampullary malignancies (Table 3). In contrast to these prior series, the current study evaluated all patients with recurrent pancreatic cancer regardless of metastatic site. Furthermore, all patients reported in this study, except two who had imaging consistent with PDAC recurrence and underwent RFA, had biopsy-proven recurrent PDAC. Finally, reoperation in all cases was potentially curative (either complete macroscopic resection or RFA).
Within this context, we have demonstrated that proper selection of patients with favorable disease biology is critical to long-term survival following reoperation. Although the majority of our patients with a recurrence of PDAC in their liver were treated by RFA, only one of the four patients recurred a second time at the actual RFA site with the remaining three RFA patients and two liver resection patients recurring elsewhere either in the liver or as a malignant pleural effusion, suggesting that additional micrometastatic disease was present at the time of reoperation. To gain further understanding of disease biology and implications for resection of solitary recurrences, paired analyses of reoperative versus non-operative treatment of patients with these recurrences need to be undertaken. Because patients with recurrent or metastatic disease are not typically considered to be operative candidates, it may be difficult to identify patients with a solitary recurrence and prolonged DFI, as they are often relegated to chemotherapy or best supportive care.
Conclusion
We conclude that patients who develop a solitary recurrence of PDAC in the lung after a prolonged DFI should be considered for reoperation. We found that a survival difference was seen with a first DFI of greater than 20 months, and it is imperative that such a prolonged period of observation be used to account for tumor biology and potential future recurrence sites. In addition, individuals who have disease recurrence at non-pulmonary sites are likely to develop subsequent disease recurrence soon after reoperation regardless of their first DFI duration and likely are not appropriate candidates for such a reoperative approach. Further studies are needed to uncover any specific survival advantage gained in patients who undergo resection of recurrent solitary PDAC compared to patients with a solitary recurrence who do not undergo resection. This is especially important for patients with a pulmonary recurrence, as they appear most likely to benefit from such a reoperative approach.
References
Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S, Vauthey JN, Abdalla EK, Lee JE, Pisters PW, Evans DB. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009 Apr;16(4):836–847.
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007 Jan 17;297(3):267–277.
Gastrointestinal Tumor Study Group. Cancer. 1987 Jun 15;59(12):2006–2010.
Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH, Vauthey JN, Lee JE, Pisters PW, Evans DB. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001 Mar;8(2):123–132.
Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, van Cutsem E, van Dekken H, Klinkenbijl JH, Jeekel J. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007 Nov;246(5):734–740.
Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997 Feb;21(2):195–200.
Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE, Pisters PW, Evans DB. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007 Jul;246(1):52–60.
Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991 Jul;110(1):13–29.
Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003 Jan;12(1):165–192, xi.
Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, Campbell KA, Schulick RD, Ahuja N, Wolfgang CL. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008 Nov;15(11):3199–3206.
Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB, Chu CK, Ferrero A, Schulick RD, Choti MA, Mentha G, Strub J, Bauer TW, Adams RB, Aldrighetti L, Capussotti L, Pawlik TM. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010 Dec;17(12):3129–3136.
Musunuru S, Chen H, Rajpal S, Stephani N, McDermott JC, Holen K, Rikkers LF, Weber SM. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg. 2006 Oct;141(10):1000–1004; discussion 1005.
Poultsides GA, Schulick RD, Pawlik TM. Hepatic resection for colorectal metastases: the impact of surgical margin status on outcome. HPB (Oxford). 2010 Feb;12(1):43–49.
Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996 Apr 1;77(7):1254–1262.
Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003 Jul;197(1):29–37.
Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998 Jun;16(6):2261–2266.
Koizumi M, Sata N, Kasahara N, Morishima K, Sasanuma H, Sakuma Y, Shimizu A, Hyodo M, Yasuda Y. Remnant pancreatectomy for recurrent or metachronous pancreatic carcinoma detected by FDG-PET: two case reports. JOP. 2010;11(1):36–40.
Dalla Valle R, Mancini C, Crafa P, Passalacqua R. Pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. JOP. 2006;7(5):473–477.
Miura F, Takada T, Amano H, Yoshida M, Isaka T, Toyota N, Wada K, Takagi K, Kato K. Repeated pancreatectomy after pancreatoduodenectomy. J Gastrointest Surg. 2007 Feb;11(2):179–186.
Lavu H, Nowcid LJ, Klinge MJ, Mahendraraj K, Grenda DR, Sauter PK, Rosato EL, Kennedy EP, Yeo CJ. Reoperative completion pancreatectomy for suspected malignant disease of the pancreas. J Surg Res. 2011 Sep;170(1):89–95.
Hwang RF, Wang H, Lara A, Gomez H, Chang T, Sieffert N, Moon Y, Ram S, Zimmerman S, Lee JH, Pisters PW, Tamm EP, Fleming JB, Abbruzzese JL, Evans DB. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Ann Surg Oncol. 2008 May;15(5):1356–1366.
Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008 Jul 20;26(21):3496–3502.
Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ, Evans DB. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996 May;12(4):373–380.
Arnaoutakis GJ, Rangachari D, Laheru DA, Iacobuzio-Donahue CA, Hruban RH, Herman JM, Edil BH, Pawlik TM, Schulick RD, Cameron JL, Meneshian A, Yang SC, Wolfgang CL. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. J Gastrointest Surg. 2011 Sep;15(9):1611–1617.
Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, Friess H, Buchler MW. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007 Apr;245(4):566–572.
Blackmon SH, Shah N, Roth JA, Correa AM, Vaporciyan AA, Rice DC, Hofstetter W, Walsh GL, Benjamin R, Pollock R, Swisher SG, Mehran R. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg. 2009 Sep;88(3):877–884; discussion 884–875.
Welter S, Jacobs J, Krbek T, Krebs B, Stamatis G. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg. 2007 Jul;84(1):203–210.
Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011 May 15;117(10):2044–2049.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Support: This study was supported by the Khalifa Bin Zayed Al Nahyan Foundation, various donors to the Pancreatic Research Fund at MD Anderson Cancer Center, and the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672. Recipient of the 2012 ASCO Conquer Cancer Foundation Merit Award, American Society of Clinical Oncology.
Rights and permissions
About this article
Cite this article
Thomas, R.M., Truty, M.J., Nogueras-Gonzalez, G.M. et al. Selective Reoperation for Locally Recurrent or Metastatic Pancreatic Ductal Adenocarcinoma Following Primary Pancreatic Resection. J Gastrointest Surg 16, 1696–1704 (2012). https://doi.org/10.1007/s11605-012-1912-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-012-1912-8