Abstract
Purpose
This study aimed to evaluate the clinical significance of surgical resection for liver recurrence in patients with curatively resected pancreatic ductal adenocarcinoma.
Methods
The medical records of patients with a liver recurrence after undergoing curative pancreatectomy for pancreatic ductal adenocarcinoma were retrospectively reviewed. Clinicopathological and prognostic factors were analyzed, as was the clinical impact of surgical resection for liver recurrence.
Results
Overall, 502 patients underwent curative pancreatic ductal adenocarcinoma resection. Of the 311 patients with recurrence after curative pancreatectomy, 71 (23%) had an initial recurrence in the liver, with 35 having solitary recurrence (11%). Patients with solitary, two or three, or more than four recurrences had median overall survival times of 28.5, 18.0, and 12.2 months, respectively (p < 0.001). Surgical indications for liver recurrence in our institution included solitary tumor, good disease control under chemotherapy after recurrence for > 6 months, and sufficient remnant liver function. Ten patients who met our institutional policy inclusion criteria underwent liver resection. Among 35 patients with initially solitary liver recurrence, those who underwent liver resection outlived those who did not (57.6 months vs. 20.1 months, p < 0.001). In multivariate analysis of overall survival, solitary liver recurrence and liver resection were independent favorable prognostic factors in patients with initial liver recurrence.
Conclusion
In selected patients with solitary liver recurrence after curatively resected pancreatic ductal adenocarcinoma, liver resection may be a treatment option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) frequently recurs, even after curative resection [1, 2]. During the first two years, the postoperative recurrence rate is 30–50%, and the prognosis of recurrence after curative resection of PDAC is poor, with a 5-year overall survival (OS) rate of approximately 20% [3, 4]. Systemic chemotherapy is the standard therapy for PDAC recurrence after resection. However, the prognosis of patients with the recurrent disease has gradually improved due to the development of new chemotherapeutic regimens such as 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), and gemcitabine plus nab-paclitaxel (GnP) [5, 6].
Recurrent PDAC is notoriously challenging to surgically manage because it is a technically unresectable disease characterized by aggressive growth and a multifocal recurrence pattern [7]. The liver is one of the most frequent sites of primary recurrence of PDAC [8,9,10]. In addition, the recurrence time is early, and liver recurrence usually results in multiple metastases and a poor prognosis [11]. Therefore, resection of postoperative lung recurrence, which occurs later than other distant recurrences and has a better prognosis, has been carefully recommended [12,13,14]. Nonetheless, resection for liver recurrence remains controversial [15, 16]. Some studies have revealed that resection of liver recurrence may improve prognosis with successful chemotherapy and good disease control [17,18,19]. However, the optimal indications and long-term results have not yet been established. Therefore, this study aimed to evaluate the clinical significance of resection for liver recurrence in patients with curatively resected PDAC.
Material and methods
Study design
We retrospectively reviewed the medical records of patients who underwent curative resection for PDAC at the Department of Surgery of the Graduate School of Biochemical and Health Sciences at Hiroshima University Hospital in Hiroshima, Japan, between May 2002 and March 2020. Furthermore, among these patients, those diagnosed with a recurrence during follow-up imaging were examined. This Institutional Review Board of Hiroshima University (E-1846) approved this study, which was conducted following the principles of the Declaration of Helsinki. In addition, all patients provided written informed consent.
Neoadjuvant therapy
Since 2019, patients with PDAC have been administered neoadjuvant therapy (NAT) in our institution, as are patients with borderline resectable (BR)/locally advanced (LA) PDAC with arterial contacts such as a superior mesenteric artery, hepatic artery, or celiac artery since 2008 [20]. Previously, all patients with pancreatic cancer had received upfront surgery. NAT regimens for patients with resectable PDAC included gemcitabine/S-1 (GS), while those for patients with BR/LA PDAC included GS, gemcitabine/nab-paclitaxel/S-1 (GAS), and FOLFIRINOX. During the study period, no patient received radiation therapy.
Recurrence assessment after pancreatectomy
Every 3–6 months, all patients underwent follow-up blood tests and computed tomography (CT) scans. Patients received adjuvant chemotherapy with surveillance following curative resection of PDAC. Further, when two or more modalities, such as magnetic resonance imaging or positron emission tomography-CT, indicated a recurrence or recurrent lesion at two different time points, recurrence was confirmed. Based on the initial recurrence sites, recurrence patterns were classified into six groups (liver, lung, peritoneal, local, multiple organs, and recurrence at other sites). In addition, recurrence in multiple organs involves two or more sites. Systemic chemotherapy was administered when recurrence was confirmed. The OS, or time between initial treatment and death or last follow-up, was compared for each recurrent organ. The patients received systemic chemotherapy for at least six months when liver recurrence was found. OS was compared between patients who underwent liver resection and those who did not to assess the clinical significance of liver recurrence resection in patients with curatively resected PDAC.
Data collection
The demographic and clinicopathological data of patients included age, sex, initial carbohydrate antigen 19–9 (CA19-9) level, initial tumor size, resectability status according to the National Comprehensive Cancer Network guideline version 1 2021 [16], primary operation, operation time, intraoperative blood loss, lymph node metastasis, adjuvant chemotherapy, and disease-free interval (DFI). In addition, T and overall disease stages were assessed based on the Union for International Cancer Control classification, 8th edition [21], in univariate and multivariate OS analyses of prognostic factors. The survival time after treatment and the cause of death was recorded for patients who died, while the OS time and recurrence status was recorded for those who survived.
Statistical analysis
Data are expressed as medians and interquartile ranges (IQRs) or as absolute values and percentages. The chi-square or Fisher’s exact test was used for the categorical variables, while the Wilcoxon two-sample test was used to compare continuous variables in the univariate analysis. Survival curves were obtained using the Kaplan–Meier method, and the log-rank test was used to compare distributions. The proportional hazard regression model (Cox regression) was used to perform multivariate survival analyses. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. All tests were two-sided, with statistical significance set at p < 0.05. All statistical analyses were performed using the JMP statistical software (version 15.0; SAS Institute, Cary, NC, USA).
Results
Patients
During the study, 502 patients underwent curative resection of PDAC at Hiroshima University. Among these, 311 patients with recurrence following pancreatectomy were enrolled in this study. Based on resectability classifications, resectable tumors were found in 169 patients (54%), BR in 118 patients (38%), and LA in 24 patients (8%). A pancreatoduodenectomy was performed in 212 patients (68%). In addition, 232 patients (75%) had lymph node positivity. Adjuvant chemotherapy was administered to 239 (77%) patients. The median DFI after pancreatectomy was 10.7 months. Regarding recurrence sites, initial recurrence occurred in the liver (n = 71, 23%), local site (n = 63, 20%), lung (n = 57, 18%), peritoneum (n = 55, 18%), and multiple sites (n = 42, 14%) (Fig. 1). Liver resection was performed in ten (14%) out of all patients with initial liver recurrence. The characteristics of patients, which were classified based on the number of initial liver recurrences, revealed that the median DFI from pancreatectomy was significantly longer than that in patients with a solitary liver recurrence than in patients with two or three and > four recurrence groups (11.6 months vs. 8.7 months vs. 4.4 months, p < 0.001) (Table 1).
Overall, 502 patients underwent pathologically curative resection for pancreatic ductal adenocarcinoma. Of the patients with initial solitary liver recurrence (n = 35), ten matched our institutional indication for liver resection, three had other site recurrences, and 22 had multiple liver recurrences after chemotherapy
Liver resection
Thirty-five 35 (49%) of the 71 patients with liver recurrence had solitary tumors. In our institution, indications for liver resection were as follows: (1) solitary tumor and (2) tumor with good control under systemic chemotherapy for ˃ 6 months (decline to normal levels in tumor marker levels and tumor size on CT imaging indicates a more stable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1), and (3) sufficient remnant liver function. Following > 6 months of chemotherapy, ten patients who met the institutional inclusion criteria underwent liver resection (Fig. 1). Furthermore, regarding chemotherapy regimens after recurrence in patients with liver resection, four patients were administered gemcitabine plus S-1, and six were administered GnP. The median duration from initial surgery to liver resection and the interval chemotherapy after recurrence was 28.2 months and 8.6 months, respectively (Table 2).
Partial resection (parenchymal preserving hepatectomy) and left hepatectomy were performed in eight and two patients, respectively. All patients underwent open surgery, and the Pringle maneuver was performed in two patients. The median blood loss was 150 ml, and the median operative time was 175 min. Postoperative complications included two patients with liver abscesses and one patient with bile leakage. Regarding perioperative management, we used fourth-generation cephem antibiotics as perioperative antibiotics 48 h after liver resection, especially for patients who underwent pancreatoduodenectomy. If liver abscess formation is suspected, we perform ultrasonography-guided or CT-guided drainage. Four patients were administered S-1 alone, two were administered gemcitabine plus S-1, one was administered S-1 plus oxaliplatin, and one was administered gemcitabine alone as chemotherapy after liver resection. Re-recurrence occurred in the liver in five patients and multiple sites in two patients. Following liver resection, the median DFI was 12.3 months, while the median survival time (MST) was 21.5 months (Table 2).
Survival analysis
The median follow-up period was 23.8 months (IQR, 13.6–40.3 months). Figure 2a shows the survival curves of patients stratified based on recurrence sites. The MST was 48.3, 29.3, 18.2, and 13.9 months in patients with lung, local, liver, and peritoneum recurrence, respectively (p < 0.001). Patients with recurrence in the liver had a significantly poorer OS than those with recurrence in the lung (p < 0.001). Figure 2b shows the disease-free survival (DFS) from the initial treatment for primary PDAC. The MST was 17.7, 14.9, 8.0, and 7.2 months in patients with lung, local, liver, and peritoneum recurrence, respectively (p < 0.001). Patients with recurrence in the liver had a significantly poorer DFS than those with recurrence in the lung and local site (p < 0.001 and p = 0.005).
a) The median follow-up period was 23.8 months (interquartile range, 13.6–40.3 months). The survival curves of the patients are stratified based on recurrence organs. (b) DFS from the initial treatment for primary PDAC. The MST was 17.7, 14.9, 8.0, and 7.2 months in patients with lung, local, liver, and peritoneum recurrence, respectively (p < 0.001). Abbreviations: DFS, disease free survival; PDAC, pancreatic ductal adenocarcinoma
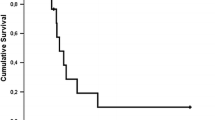
Figure 3a shows the survival curves of patients stratified based on the number of initial liver recurrences. The solitary group had the highest median OS among the three groups (p < 0.001). In addition, among patients with initial solitary liver recurrence, the MST in the liver resection group was significantly better than that in the non-liver resection group (57.6 months vs. 20.1 months, p < 0.001) (Fig. 3b). Adjuvant chemotherapy, the number of initial liver recurrence tumors, and liver resection were all found to be significantly associated with OS in patients with initial liver recurrence. Furthermore, multivariate analysis revealed that multiple tumors (HR, 2.26; 95% CI: 1.30–3.94; p = 0.004) and non-liver resection (HR, 6.90; 95% CI: 2.23–21.3; p < 0.001) were independent risk factors for poor prognosis (Table 3).
a) The survival curves of patients stratified based on the number of initial recurrence tumors of the liver. (b) In patients with an initial solitary liver recurrence, the median survival time from the initial treatment for primary PDAC was significantly better in the liver resection group than in the non-liver resection group (57.6 months vs. 20.1 months, p < 0.001). Abbreviations: PDAC, pancreatic ductal adenocarcinoma
Discussion
This study revealed that the interval from surgery until the appearance of liver recurrence was significantly shorter than that until recurrence in the lung and local site; patients with an initial multiple liver recurrence or multiple organ recurrence had a poorer chance of survival than those with an initial solitary liver recurrence. The early recurrence time characterized the malignant potential of liver recurrence, and patients with liver recurrence were more likely to have multiple metastases, resulting in a poor prognosis. According to the literature, resection for liver recurrence in colorectal cancer is associated with a good prognosis, and the 5-year survival rate following liver resection is reportedly 35–58% [22, 23]. In addition, the OS after resection for oligo-liver recurrence in gastric cancer has been revealed to increase [24, 25]. Similarly, surgical resection for solitary liver recurrence after curative resection of PDAC may have been associated with a relatively good prognosis under optimal surgical indications.
Our patients with liver recurrence had significantly shorter intervals between pancreatectomy and recurrence and shorter OS than those with lung recurrence. Similar findings have been previously reported. Thomas et al. [11] revealed that those with liver recurrence had a shorter median DFI after initial resection (7.6 months) than those with lung or local–regional recurrence (52.4 months and 41.1 months, respectively, p = 0.006). In addition, Wangjam et al. [26] found that patients with liver recurrence had a significantly worse MST from recurrence to death than those with lung recurrence (5.1 months vs. 8.5 months, p = 0.006). Hence, underlying biological features may indicate that PDAC with liver recurrence may have a relatively higher malignant potential than PDAC with lung or local recurrence.
Our study found that patients who underwent resection for liver recurrence under our surgical indications had significantly better OS than those who did not. A few previous studies have revealed that by carefully selecting surgical indications, certain patients may benefit from surgical resection for liver recurrence. For example, Mitsuka et al. [17] reported the following indications for liver resection: (1) liver-only metastasis, (2) approximately three tumors, and (3) no increase in the number of metastases during the 3-month observation period. The MST was significantly better in the liver resection group than in the non-liver resection group after liver recurrence (31 months vs. 7 months, p < 0.001). Schwarz et al. [18] revealed that patients with metachronous oligometastatic disease, defined as 1–3 liver metastases that could be easily resected by atypical or minor liver resection, were eligible for liver resection. Patients who underwent liver resection had a significantly longer median OS than those who did not (36.8 months vs. 9.2 months, p < 0.001). According to some reports, liver oligometastasis of pancreatic cancer is defined as approximately three liver metastasis tumors [27, 28], and oligometastasis may be associated with better survival outcomes than multiple metastases [29, 30]. However, separating oligometastasis into solitary and two or three tumors is necessary for our study. Our institutional indication for liver resection, which included patients with a single solitary liver recurrence after ˃ 6 months of chemotherapy, may have been more stringent than that reported in previous studies. This result is due to the malignant potential of liver recurrence and the fact that patients with initial multiple liver recurrence or recurrence at other sites in this study never improved to solitary liver recurrence. Further, regardless of the small sample size, our relatively strict indication may have resulted in a better prognosis (with an MST of 57.6 months in the liver resection group) than in patients who underwent liver resection in previous studies.
The number of initial liver recurrences was an independent prognostic factor in our multivariate OS analyses of prognostic factors. Among the 35 patients with initial solitary liver recurrence, 25 had recurrence at other sites or multiple liver recurrences during chemotherapy, and 19 were detected within six months after starting systemic chemotherapy. A chemotherapy period of at least six months appears acceptable since prolonged chemotherapy may result in drug intolerance. Therefore, good disease control during systemic chemotherapy is critical for improving OS.
Furthermore, in our institutional indication that residual liver reserve was sufficient, partial liver resection was generally selected with no limitation on the tumor location, unlike previous studies in which minor liver resections, such as partial hepatectomy, were mainly selected [17, 18]. Some patients after pancreatectomy present with liver dysfunction due to malnutrition. Therefore, even when a partial liver resection is performed, the functional capacity of the future remnant liver should be estimated based on data from the indocyanine green test and liver volumetry calculated from CT images as preoperative management; however, these tests were not performed in all patients with liver resection in this study. Eight of the ten patients who underwent surgical resection for liver recurrence received systemic chemotherapy following liver resection, with MST lasting 21.1 months. There are no reports of chemotherapy after liver recurrence resection. Nevertheless, depending on the tolerance of the patients, some chemotherapy may be needed to prevent recurrence.
This study had some limitations. First, it only included patients from a single institution, and the number of patients with liver recurrence who underwent liver resection was small. Hence, further studies using multicenter data or a nationwide database with a larger number of patients are needed to determine the optimal surgical indication for liver recurrence. Second, despite imaging surveillance, every 3–6 months, some patients with multiple organ recurrences were included in our study. The initial recurrences in these patients were challenging to detect, which may have influenced the clinical characteristics or prognosis of each recurrence pattern. Third, patients who underwent surgical resection for liver recurrence were selected without randomization. Therefore, the analyses of these patients included a selection bias. Prospective randomized controlled trials are required to prove the effectiveness of resection for liver recurrence after PDAC radical resection.
Conclusion
Liver recurrence after PDAC radical resection has a poor prognosis. However, liver resection may be a treatment option in selected patients with solitary liver recurrence after curatively resected PDAC.
References
Gleisner AL, Assumpcao L, Cameron JL et al (2007) Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 110:2484–2492. https://doi.org/10.1002/cncr.23074
Shrikhande SV, Kleeff J, Reiser C et al (2007) Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 14:118–127. https://doi.org/10.1245/s10434-006-9131-8
Adam R, Chiche L, Aloia T et al (2006) Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 244:524–535. https://doi.org/10.1097/01.sla.0000239036.46827.5f
Yeo CJ, Cameron JL, Lillemoe KD et al (1995) Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients Ann Surg 221:721–731; discussion 731–733. https://doi.org/10.1097/00000658-199506000-00011
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. https://doi.org/10.1056/NEJMoa1304369
Sperti C, Pasquali C, Piccoli A, Pedrazzoli S (1997) Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 21:195–200. https://doi.org/10.1007/s002689900215
Matsuda Y, Hagio M, Naito Z, Ishiwata T (2012) Clinicopathological features of 30 autopsy cases of pancreatic carcinoma. J Nippon Med Sch 79:459–467. https://doi.org/10.1272/jnms.79.459
Katz MH, Wang H, Fleming JB et al (2009) Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 16:836–847. https://doi.org/10.1245/s10434-008-0295-2
Mierke F, Hempel S, Distler M et al (2016) Impact of Portal Vein Involvement from Pancreatic Cancer on Metastatic Pattern After Surgical Resection. Ann Surg Oncol 23:730–736. https://doi.org/10.1245/s10434-016-5515-6
Thomas RM, Truty MJ, Nogueras-Gonzalez GM et al (2012) Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J Gastrointest Surg 16:1696–1704. https://doi.org/10.1007/s11605-012-1912-8
Zheng B, Ohuchida K, Yan Z, Okumura T, Ohtsuka T, Nakamura M (2017) Primary Recurrence in the Lung is Related to Favorable Prognosis in Patients with Pancreatic Cancer and Postoperative Recurrence. World J Surg 41:2858–2866. https://doi.org/10.1007/s00268-017-4068-6
Lovecek M, Skalicky P, Chudacek J et al (2017) Different clinical presentations of metachronous pulmonary metastases after resection of pancreatic ductal adenocarcinoma: Retrospective study and review of the literature. World J Gastroenterol 23:6420–6428. https://doi.org/10.3748/wjg.v23.i35.6420
Otsuka H, Uemura K, Kondo N et al (2020) Clinical characteristics of initial recurrence in lung after surgical resection for pancreatic ductal adenocarcinoma. Pancreatology 20:1472–1478. https://doi.org/10.1016/j.pan.2020.08.019
Tempero MA, Malafa MP, Al-Hawary M et al (2021) Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 19:439–457. https://doi.org/10.6004/jnccn.2021.0017
Okusaka T, Nakamura M, Yoshida M et al (2020) Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas 49:326–335. https://doi.org/10.1097/mpa.0000000000001513
Mitsuka Y, Yamazaki S, Yoshida N, Yan M, Higaki T, Takayama T (2020) Time interval-based indication for liver resection of metastasis from pancreatic cancer. World J Surg Oncol 18:294. https://doi.org/10.1186/s12957-020-02058-5
Schwarz C, Fitschek F, Primavesi F et al (2020) Metachronous hepatic resection for liver only pancreatic metastases. Surg Oncol 35:169–173. https://doi.org/10.1016/j.suronc.2020.08.005
Tsutsumi C, Abe T, Shinkawa T, Nishihara K, Tamiya S, Nakano T (2020) Long-term survival after hepatectomy for metachronous liver metastasis of pancreatic ductal adenocarcinoma: a case report. Surg Case Rep 6:157. https://doi.org/10.1186/s40792-020-00924-8
Okada K, Uemura K, Kondo N et al (2022) Neoadjuvant therapy contributes to nodal downstaging of pancreatic cancer. Langenbecks Arch Surg 407:623–632. https://doi.org/10.1007/s00423-021-02339-x
Brierley JD, Gospodarowicz MK, Wittekind C (2017) TNM Classification of Malignant Tumors Eighth Edition: Wiley-Blackwell, West Sussex.
Martin LW, Warren RS (2000) Current management of colorectal liver metastases. Surg Oncol Clin N Am 9:853–876; discussion 877–878
Abdalla EK, Vauthey JN, Ellis LM,et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239:818–825; discussion 825–827. https://doi.org/10.1097/01.sla.0000128305.90650.71
Markar SR, Mikhail S, Malietzis G et al (2016) Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg 263:1092–1101. https://doi.org/10.1097/SLA.0000000000001542
Carmona-Bayonas A, Jiménez-Fonseca P et al (2008) Surgery for metastases for esophageal-gastric cancer in the real world: Data from the AGAMENON national registry. Eur J Surg Oncol 44:1191–1198. https://doi.org/10.1016/j.ejso.2018.03.019
Wangjam T, Zhang Z, Zhou XC et al (2015) Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget 6:36903–36910. https://doi.org/10.18632/oncotarget.5054
Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13:8–10. https://doi.org/10.1200/JCO.1995.13.1.8
Wei M, Shi S, Hua J, Xu J, Yu X (2019) Chinese Study Group for Pancreatic C. Simultaneous resection of the primary tumour and liver metastases after conversion chemotherapy versus standard therapy in pancreatic cancer with liver oligometastasis: protocol of a multicentre, prospective, randomised phase III control trial (CSPAC-1). BMJ Open 9:e033452. https://doi.org/10.1136/bmjopen-2019-033452
Yamanaka M, Hayashi M, Yamada S et al (2021) A Possible Definition of Oligometastasis in Pancreatic Cancer and Associated Survival Outcomes. Anticancer Res 41:3933–3940. https://doi.org/10.21873/anticanres.15189
Sakaguchi T, Valente R, Tanaka K, Satoi S, Del Chiaro M (2019) Surgical treatment of metastatic pancreatic ductal adenocarcinoma: A review of current literature. Pancreatology 19:672–680. https://doi.org/10.1016/j.pan.2019.05.466
Acknowledgements
The authors would like to thank Editage (https://www.editage.jp/) for the English language review.
Author information
Authors and Affiliations
Contributions
Study conception and design: Yoshiyuki Shibata, Kenichiro Uemura, Kenjiro Okada, Yoshiaki Murakami, Shinya Takahashi; Acquisition of data: Yoshiyuki Shibata, Tatsuaki Sumiyoshi, Kenjiro Okada, Hiroyuki Otsuka, Masahiro Serikawa, Yasutaka Ishii, Koji Arihiro; Analysis and interpretation of data: Yoshiyuki Shibata, Kenichiro Uemura, Tatsuaki Sumiyoshi, Kenjiro Okada, Hiroyuki Otsuka, Masahiro Serikawa, Yasutaka Ishii; Drafting of manuscript: Yoshiyuki Shibata, Kenichiro Uemura, Kenjiro Okada; Critical revision of manuscript: Yoshiyuki Shibata, Kenichiro Uemura, Kenjiro Okada, Yoshiaki Murakami, Shinya Takahashi.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shibata, Y., Uemura, K., Sumiyoshi, T. et al. Surgical resection for liver recurrence after curative resection of pancreatic ductal adenocarcinoma. Langenbecks Arch Surg 408, 280 (2023). https://doi.org/10.1007/s00423-023-03009-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03009-w