Abstract
Purpose
Our aim was to assess diffusion weighted imaging (DWI) of neuroblastic tumors and whether apparent diffusion coefficient (ADC) value may have a role in discrimination among neuroblastoma, ganglioneuroblastoma and ganglioneuroma.
Material and methods
The DWIs (b = 0–800 s/mm2) of 24 children (13 girls, 11 boys) who were diagnosed neuroblastic tumors on histopathological examination (neuroblastoma = 15, ganglioneuroblastoma = 5, ganglioneuroma = 4) were evaluated retrospectively. The ADC maps were performed by drawing freehand ROI on PACS (Sectra Workstation IDS7, Linköping, Sweden).
Results
We observed a significant decrease in ADC value of neuroblastomas 0.869 ± 0.179 × 10−3 mm2/s compared to ganglioneuroblastomas 0.97 ± 0.203 × 10−3 mm2/s and ganglioneuromas 1.147 ± 0.299 × 10−3 mm2/s (p = 0.026). There was no significant difference in between ganglioneuroblastoma and ganglioneuroma (p = 0.16). In detecting neuroblastomas; the sensitivity, specificity, negative and positive predictive values of ADC were 74, 67, 78.6, 66 % respectively with a cut-off value of 0.93 × 10−3 mm2/s.
Conclusion
Our study stands out as the most comprehensive study with larger sample size on this topic. Moreover, we are able to suggest a cut-off value which can discriminate neuroblastoma from ganglioneuroblastoma and ganglioneuroma. We believe that ADC will evolve to an objective, quantitative measurement in discrimination among malignant and benign neuroblastic tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma, ganglioneuroma, and ganglioneuroblastoma are neuroblastic tumors arising from primordial neural crest cells of sympathetic nervous system [1]. Neuroblastoma is consisted of a mixture of immature neuroblasts and neutrophils whereas ganglioneuroblastoma consisted of mature gangliocytes and immature neuroblasts [2]. Neuroblastoma is the most common extracranial solid childhood malignancy [1–3]. The presence of immature tissue in neuroblastoma and ganglioneuroblastoma indicates malignant behavior. However, ganglioneuroma, arising from mature cells, is a benign tumor. Neuroblastoma and ganglioneuroblastoma are treated by surgical removal, chemotherapy or radiotherapy. They can transform to ganglioneuroma after chemotherapy [3]. The common presentation of neuroblastoma is abdomen in 65 % of the cases [1–3]. The frequency of adrenal localization is higher in older children than infants whereas cervical and thoracic presentation is more common in younger infants [1–3].
Neuroblastic tumors have relatively low signal on T1-W, high signal intensity on T2-W and variably enhancing pattern on MRI. Considering that they have similar signal characteristics on MRI, it is difficult to discriminate subgroups; therefore histopathological examination is a must [3]. DWI can distinguish tissues with similar T1-W and T2-W signals based on their microscopic organization and metabolism [4]. It can provide data regarding cellular organization and metabolism of tissue. Recently, DWI is becoming a favorable method for pediatric oncology cases [5–9].
In this study we aim to assess ADC values of neuroblastic tumors and whether ADC value may have the role in discrimination among neuroblastoma, ganglioneuroma, and ganglioneuroblastoma.
Materials and methods
Informed consents were obtained from all patients’ parents.
Study patients
The DWIs (b = 0–800 s/mm2) of twenty-four children [13 girls, 11 boys; mean age = 3.41 ± 3.41 years (range 10 days–14 years)] who were diagnosed neuroblastic tumors on histopathological examination (neuroblastoma = 15, ganglioneuroblastoma = 5, ganglioneuroma = 4) were included in our study retrospectively. Histopathological examination was obtained after biopsy or surgical removal.
MRI protocol
All patients underwent a routine MRI examination at 1.5 T MR Unit (Aera, Siemens, Erlangen, Germany) with a standard body coil for the initial diagnosis before biopsy or surgical removal. MRI protocol included T1-W, T2-W, STIR and contrast-enhanced T1-W and DWI. Before contrast media injection, DWI (b = 0–800 s/mm2) was obtained in three orthogonal axes (x, y, z) without breath holding in axial plane. Two phase-encoding directions were measured for each orientation (FOV = 320 × 320 mm, matrix = 256 × 256 mm, slice thickness = 4 mm, time = 1 min–24 s).
Imaging evaluation
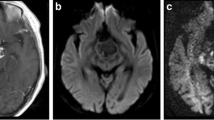
All tumors were solitary masses which did not show any invasion to adjacent solid organ. ADC maps were performed by comparing T1-W, T2-W and contrast-enhanced T1-W images on PACS (Sectra Workstation IDS7, Linköping, Sweden). The DWIs were calculated by drawing freehand ROI (min 10 cm2–max 200 cm2) as large as possible without risking partial volume effects along the boundary of the tumor. Single slice calculations at the level of the largest solid area for each patient were performed and mean ADC value was taken (Fig. 1a, b). Regions of increased diffusion areas including cystic and necrotic spaces which were demonstrated hypointense on T1-W, hyperintense on T2-W and did not show any contrast enhancement on contrast-enhanced images were excluded from ROI. Two pediatric radiologists reviewed all MRI studies of each patient together. ADC values were compared within each group.
Statistical analysis
Mean ADC values were calculated, and independent sample t test was performed to compare ADC values. ROC analysis was done to depict a cut-off value for neuroblastoma by using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). p < 0.05 was significant.
Results
The mean ADC value of all neuroblastic tumors was 0. 936 ± 0.222 × 10−3 mm2/s; (range 0.45–1.391 × 10−3 mm2/s). The mean ADC value of neuroblastoma, ganglioneuroblastoma and ganglioneuroma were 0.869 ± 0.179 × 10−3 mm2/s (range 0.45–1.068 × 10−3 mm2/s); 0.97 ± 0.203 × 10−3 mm2/s (range 0.658–1.18 × 10−3 mm2/s) and 1.147 ± 0.299 × 10−3 mm2/s (range 0.73–1.39 × 10−3 mm2/s) respectively. We observed a significant decrease in ADC value of neuroblastomas compared to ganglioneuroblastomas and ganglioneuromas (p = 0.026). There was no significant difference in between ganglioneuroblastoma and ganglioneuroma (p = 0.16). The overall volume of all tumors was 648 ml (min 202–max 3094 ml). The age difference between neuroblastoma and ganglioneuroblastoma-ganglioneuroma was not statistically significant (p = 0.33). Patients’ information was given in Table 1. In detecting neuroblastomas; the sensitivity, specificity, negative and positive predictive values of ADC calculation were 74, 67, 78.6, 66 % respectively with a cut-off value of 0.93 × 10−3 mm2/s. ROC analysis of neuroblastoma versus ganglioneuroblastoma-ganglioneuroma was shown in Fig. 2. All tumors had high signal intensity on T2-W images compared to paravertebral muscle, and all of them showed intense contrast enhancement. The most common presentation was adrenal gland in 58 % (n = 14) of patients whereas paraaortic region was 25 % (n = 6), the paravertebral region was 16 % (n = 4), the pelvic region was 4 % (n = 1), and the presacral region was 4 % (n = 1).
Discussion
DWI reveals micro-diffusion motion of water molecules within intracellular and extracellular space [3]. Restricted diffusion within tumor appears as high signal intensity on DWI while low signal intensity in ADC maps, representing high cellular density [4]. To the best of our knowledge, there are a limited number of studies comparing ADC values of neuroblastic tumors [5, 10–14]. To date, these tumors can only be distinguished by histolopathological examination. Mc Donald and Demir et al. showed that DWI could be used to detect the chemotherapy response in children with neuroblastoma [12, 13]. Studies dealing with pediatric abdominal tumors showed that ADC might be used to discriminate benign and malignant lesions [10, 11]. Humphries et al. reported a correlation between ADC value and tumor cellularity. In a very recent study, Gahr et al. studied fifteen neuroblastic tumors and they subgrouped them into neuroblastoma and non-neuroblastoma (ganglioneuroma and ganglioneuroblastoma) group and found that neuroblastoma had the lowest ADC values [5]. Our study is in agreement with them and additionally it is the first comprehensive study with 24 patients in the literature.
Gahr et al. found a high ADC value in a neuroblastoma infiltrating the kidney which would be possible that the ADC was not only the tumor but also part of a kidney. They also proposed that ADC calculation could not differentiate neuroblastoma and ganglioneuroma [5]. Our study group consisted of solitary tumors which did not show any infiltration to adjacent solid organ. The diagnostic accuracy of ADC in determining neuroblastoma was found significant with a cut-off value of 0.93 × 10−3 mm2/s; furthermore, there was no significant difference in ADC values of ganglioneuroblastoma and ganglioneuroma in our study. Despite the fact that we had a small sample size in non-neuroblastoma group, these findings might be helpful for radiologists to discriminate neuroblastoma from ganglioneuroblastoma and ganglioneuroma on DWI.
The major limitation of our study is the small number of study patients. We believe that the diagnostic accuracy of ADC in determining neuroblastic tumors could be found higher with more patients in future studies, so ADC calculation may be a comparable method to histopathological examination in predicting the malignancy. Radiolabeled metaiodobenzylguanidine (MIBG) with iodine-123 and laboratory tests (vanillylmandelic acid and homovanillic acid in urine) are powerful methods for staging, screening and follow up of neuroblastoma [15]. However we aim to evaluate the diagnostic accuracy of ADC calculation among neruoblastic tumors in our study. Our MRI examinations are only for the mass characterization by ADC calculation so we did not include the whole body MRI examination. MIBG scan is a whole body scan and should be compared to whole body MRI-DWI which would be an another research topic.
In conclusion, our study stands out as the most comprehensive study with larger sample size on this topic. Moreover, we are able to suggest a cut-off value which can discriminate neuroblastoma from ganglioneuroblastoma and ganglioneuroma. We believe that ADC would evolve to an objective and quantitative measurement in discrimination among malignant and benign neuroblastic tumors.
References
Topcu S, Alper A, Gulhan E, Kocyigit O, Tastepe I, Cetin G. Neurogenic tumours of the mediastinum: a report of 60 cases. Can Respir J. 2000;7:261–5.
Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. RadioGraphics. 2002;22:911–34.
Nakazono T, White CS, Yamasaki F, Yamaguchi K, Egashira R, Irie H, Kudo S. MRI findings of mediastinal neurogenic tumors. AJR Am J Roentgenol. 2011;197:643–52.
Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–35.
Gahr N, Darge K, Hahn G, Kreher BW, von Buiren M, Uhl M. Diffusion-weighted MRI for differentiation of neuroblastoma and ganglioneuroblastoma/ganglioneuroma. Eur J Radiol. 2011;79:443–6.
MacKenzie JD, Gonzalez L, Hernandez A, Ruppert K, Jaramillo D. Diffusion-weighted and diffusion tensor imaging for pediatric musculoskeletal disorders. Pediatr Radiol. 2007;37:781–8.
Abdel Razek AA, Gaballa G, Elhawarey G, Elshafey M, Elhadedy T. Characterization of pediatric head and neck masses with diffusion-weighted MR imaging. Eur Radiol. 2009;19:201–8.
Alibek S, Cavallaro A, Aplas A, Uder M, Staatz G. Diffusion weighted imaging of pediatric and adolescent malignancies with regard to detection and delineation: initial experience. Acad Radiol. 2009;16:866–71.
Abdel Razek AA, Soliman N, Elashery R. Apparent diffusion coefficient values of mediastinal masses in children. Eur J Radiol. 2011;81:1311–4.
Kocaoglu M, Bulakbasi N, Sanal HT, et al. Pediatric abdominal masses: diagnostic accuracy of diffusion weighted MRI. Magn Reson Imaging. 2010;28:629–36.
Uhl M, Altehoefer C, Kontny U, Ilyasov K, Buchert M, Langer M. MRI-diffusion imaging of neuroblastomas: first results and correlation to histology. Eur Radiol. 2002;12:2335–8.
Demir S, Altinkaya N, Kocer NE, Erbay A, Oguzkurt P. Variations in apparent diffusion coefficient values following chemotherapy in pediatric neuroblastoma. Diagn Interv Radiol. 2015;21:184–8.
McDonald K, Sebire NJ, Anderson J, Olsen ØE. Patterns of shift in ADC distributions in abdominal tumours during chemotherapy—feasibility study. Pediatr Radiol. 2011;41:99–106.
Humphries PD, Sebire NJ, Siegel MJ, Olsen ØE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245:848–54.
Boubaker A, Delaloye AB. MIBG scintigraphy for the diagnosis and follow-up of children with neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:388–402.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Serin, H.I., Gorkem, S.B., Doganay, S. et al. Diffusion weighted imaging in differentiating malignant and benign neuroblastic tumors. Jpn J Radiol 34, 620–624 (2016). https://doi.org/10.1007/s11604-016-0565-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-016-0565-z