Abstract
Lithium conducting materials play a major role in developing electrochemical devices. Green materials have gained much attention in order to face an energy crisis and global warming. Many researchers took effort to develop biopolymer electrolyte-based electrochemical devices instead of the synthetic polymer due to its high cost and not being environmentally green. K-carrageenan membranes with different concentrations of lithium bromide (LiBr) have been prepared by a solution casting technique and characterized by XRD, FTIR, DSC, and AC impedance technique. One gram of K-carrageenan with 0.5 wt% of LiBr has the highest conductivity as 3.43 × 10−3 Scm−1 at room temperature, and it has high amorphous nature as per the powder XRD results. FTIR confirms the complex formation between LiBr and K-carrageenan. The shift in glass transition temperature (Tg) of the membrane is observed from the DSC. The highest-conducting polymer electrolyte has a glass transition temperature of 44.55 °C. The DC polarization technique proves that the conductivity is due to ions. Lithium ion–conducting battery has been constructed using the highest-conducting biopolymer electrolyte membrane, and its output voltage is measured.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolytes have gained much attention for preparing proton or lithium-based battery construction as they have many advantages over liquid electrolytes such as light weight, flexibility, compactness, leak proof, and good film-forming property [1, 2]. The biopolymer is of great interest due to its low cost, environment and eco-friendliness, good dimensional membrane, high ionic conductivity, and good mechanical stability [3]. Many researchers show their interest to do research using biopolymers such as starch [4], cellulose [5], chitosan [6], pectin [7, 8], agar [9, 10], and K-carrageenan due to its abundant availability, renewability, and biocompatibility. Sampath et al. [11] reported a lithium ion conductivity of 6.7 × 10−3 Scm−1 for 0.4 wt% of lithium chloride with 1 g of tamarind seed polysaccharide (TSP). Kingslin et al. [12] reported a lithium ion conductivity value of 4.0 × 10−5 Scm−1 for the PVA/PAN blend polymer with 50 wt% of lithium triflate. Kingslin et al. [13] also reported a conductivity of 3.0 × 10−4 Scm−1 for the same PVA/PAN blend polymer with 30 wt% of lithium bromide. A lithium ion conductivity of 2.08 × 10−3 Scm−1 for the composition 50 wt% pectin/50 wt% LiCl was reported by Perumal et al. [7]. Perumal et al. [14] also reported a proton conductivity of 6.83 × 10−4 Scm−1 for the biopolymer K-carrageenan with 0.5 wt% of NH4SCN. Zainuddin et al. [15] reported a proton conductivity value of 3.89 × 10−4 Scm−1 for K-carrageenan with 20 wt% of NH4Br. Karthikeyan et al. [16] reported a proton conductivity of 1.08 × 10−3 Scm−1 for the biopolymer iota-carrageenan (I-C) with 20 wt% of NH4Br. Moniha [17] also found that biopolymer iota-carrageenan (I-C) with 0.4 wt% of NH4NO3 has a proton conductivity of 1.46 × 10−3 Scm−1. Suhaimani [18] reported a protonic conductivity of 2.39 × 10−4 Scm−1 for the K-carrageenan with NH4NO3.
Kappa (K), iota (I), and lambda (λ) are the three commercially available carrageenan. K-carrageenan dissolves in hot water (80 °C). It is also soluble in dimethyl sulfoxide (DMSO) but insoluble in ethanol, acetone, and organic solvents [19]. K-carrageenan belongs to the hydrophilic polysaccharide family, and it is derived from the species of marine red algae. It is used as a thickener, stabilizer, gelling agent, or emulsifier in the food industry [20]. Its application is extended to the pharmaceutical and cosmetic industries [21]. A study on the K-carrageenan biopolymer incorporated with other salts except ammonium salt is rare in literature. “Construction of Lithium ion conducting battery using K-Carrageenan with LiBr has not been done before.” Hence, now an attempt has been made to construct it. Lithium ion conducting biopolymer membranes of 1 g of K-carrageenan with different concentrations from 0 to 0.6 wt% of LiBr have been prepared by the solution casting technique and are characterized by XRD, FTIR, DSC, and AC impedance technique.
Experimental technique
Preparation of polymer electrolyte
K-carrageenan of molecular weight 788.65 g/mol was purchased from Tokyo Chemical Industry, Japan. Lithium bromide (LiBr) of molecular weight 86.85 g/mol was purchased from Merck, Mumbai, India. The raw materials K-carrageenan and lithium bromide are used to prepare biopolymer electrolytes without further purification, and double-distilled water is used as a solvent. A homogeneous solution of K-carrageenan with LiBr has been obtained by continuous stirring of 1 g of K-carrageenan with different concentrations of LiBr (0 wt%, 0.1 wt%, 0.2 wt%, 0.3 wt%, 0.4 wt%, 0.5 wt%, and 0.6 wt%) at temperature of 80 °C. The prepared homogeneous solution is transferred to a polypropylene Petri dish and kept in a hot air oven at 60 °C. A transparent free-standing film is obtained after 2 days. The thickness of the developed polymer membranes is in the range of 0.013–0.026 cm.
Characterization techniques
Structural study
XRD is an efficient, non-destructive technique used to analyze the crystalline/amorphous nature of the biopolymer membrane, using the Rigaku Ultima IV diffractometer (Japan), with Cu-Kα radiation of 1.54060 Å wavelength in a wide 2θ range (angles between 10° and 80°). XRD analysis for the prepared membrane has been undertaken at room temperature (303 K).
Vibrational study
The FTIR measurement has been made with a Shimadzu IRAffinity-1 spectrometer instrument in the wave number range of 400–4000 cm−1 with a resolution of 1 cm−1 at room temperature of 303 K.
Thermal study
The thermal stability of the polymer electrolytes has been studied through DSC analysis using DSC Q20, USA, with a heating rate of 5 °C/min up to 300 °C under nitrogen atmosphere.
Thermogravimetric analysis
Thermo or thermal gravimetric analysis (TGA) is a method of thermal analysis in which the mass of samples is measured over time as the temperature changes. The SDT-Q600 thermogravimetric analyzer (USA) was used for thermal analysis.
Impedance study
The Hioki 3532 LCR meter was used to study the ionic conductivity of the polymer electrolytes in the frequency range 42 Hz to 1 MHz with stainless steel as electrode for measuring conductivity.
Transference number measurement
Transference number measurement by Wagner’s polarization method confirms the nature of the particular charge species. By applying a fixed dc potential of 1.5 V across the cell SS/(K-carrageenan + LiBr)/SS, the current is measured in Wagner’s polarization method to calculate the transference number.
Linear sweep voltammetry study
Bio-Logic Science Instruments VSP-300 multichannel potentiostat/galvanostat (France) was used to study the linear sweep voltammetry (LSV) responses of K-carrageenan with LiBr. A scan rate of 1 mV/s in the potential range of 0–5 V is used.
Lithium ion conducting battery construction
Preparation of anode
For preparing the anode, zinc (metal) powder, ZnSO4·7H2O, and graphite powder were mixed together in the desired proportions (3:1:1) and grinded well. Using a hydraulic press, a thin pellet is formed using the above mixture.
Preparation of cathode
For cathode preparation, PbO2, V2O5, and graphite powder taken in the ratio of 8:2:1 were mixed together and grinded well along with a few drops of biopolymer electrolyte. Then, the mixture was pressed to form a thin pellet using a hydraulic press.
The biopolymer electrolyte sample having the highest conductivity is sandwiched between anode and cathode in order to prepare lithium ion–conducting battery.
Results and discussion
XRD analysis
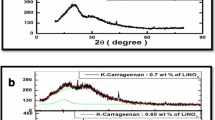
X-ray diffraction (XRD) is an efficient tool used in studying the amorphous/crystalline of the polymer membrane [13]. Figure 1 a shows the XRD patterns of pure K-carrageenan and K-carrageenan doped with lithium bromide at different molar ratios. In the XRD pattern of a sample, if there is a sharp line, it indicates that it is more crystalline in nature. If we get a broad peak in the XRD pattern, it indicates that the sample is amorphous in nature. Two broad peaks at 20° and 31° have been observed for pure K-carrageenan. From Fig. 1a, it is observed that as the concentration of LiBr is increased, the intensity of the peak at 20° and 31° decreases and the broadness of the peaks increases. The decrease in intensity and increase in the broadness of peaks reveal the increase in amorphous nature of the polymer membranes with lithium bromide [14]. This result is in accordance with Hodge et al.’s criteria [22]. From Fig. 1a, it is observed further that K-carrageenan with 0.5 wt% of LiBr has high amorphous nature [23]. Due to the complete dissolution of the salt in the polymer matrix, there are no peaks corresponding to salt up to the polymer complex with 0.5 wt% of LiBr. The XRD pattern of 1 g K-carrageenan with 0.6 wt% of LiBr shows some sharp peaks at 27.86°, 47.56°, 55.61°, and 68.87° which are due to undissociated lithium bromide (JCPDS: 74-1973) present in the membrane. The increase in amorphous nature reduces the energy barrier and causes the segmental motion of the polymer electrolyte [24].
Figure 1 b reveals the deconvoluted XRD patterns, and Table 1 shows the percentage of crystallinity for pure K-carrageenan, pure K-carrageenan with 0.3 wt% of LiBr, pure K-carrageenan with 0.4 wt% of LiBr, pure K-carrageenan with 0.5 wt% of LiBr, and pure K-carrageenan with 0.6 wt% of LiBr samples. The deconvoluted XRD pattern helps to acquire the area under the crystalline and the amorphous peak from which the percentage of crystallinity can be calculated by dividing the area under the crystalline peak by the total area of all the peaks [25]. It is observed that K-carrageenan with 0.5 wt% of LiBr has the lowest crystallinity percentage of 9.3 which reveals that it has high amorphous nature. As the concentration of LiBr increases by up to 0.5 wt%, it is observed from Table 1 that the percentage of the crystallinity decreases from 16.56 to 9.3%. Further increasing the salt concentration, the crystallinity percentage again increases to 9.4%. It is to be noted that there are no big differences in the degree of crystallinity values for the last three ratios (0.4, 0.5, and 0.6). It is well known that if the amorphous nature is maximum, the conductivity is also expected to be maximum [11]. Figure 2 represents the molecular structure of kappa-Carrageenan.
FTIR analysis
An effective tool to study the complex formation between bio-polymer K-carrageenan and lithium bromide (LiBr) salt is the Fourier transform infrared (FTIR) spectroscopy technique. Figure 3 shows the FTIR spectrum of K-carrageenan with different concentrations of LiBr. FTIR peak positions along with their assignments are shown in Table 2.
A broadband has been observed in the region between 3382 and 3385 cm−1. This is due to the stretching vibration that was formed by the hydroxyl group of polysaccharide and water [14]. Pure K-carrageenan has a vibration peak at 3382.82 cm−1 which depicts its contribution to the –OH stretching vibration [14]. Shifting of the peak occurs due to doping LiBr with pure K-carrageenan. The peak observed at 1637.49 cm−1 is assigned to the H–O–H deformation band, and it gets shifted to 1636.72 cm−1, 1639.76 cm−1, 1636.12 cm−1, and 1636.05 cm−1 for 1 g K-carrageenan with 0.3–0.6 wt% of LiBr, respectively [17]. The peak for pure K-carrageenan at 1374.56 cm−1 is due to CH2 in-plane bending [26], and the peak gets shifted to 1370.81 cm−1, 1371.32 cm−1,1370.93 cm−1, and 1372.88 cm−1 for 0.3, 0.4, 0.5, and 0.6 wt% of LiBr with pure K-carrageenan, respectively. The peak formed around 1223–1227 cm−1 for pure K-carrageenan and 0.3 wt%, 0.4 wt%, 0.5 wt%, and 0.6 wt% of LiBr with pure K-carrageenan is because of the sulfate stretching of S–O [14]. The characteristic peak observed at 1125.02 cm−1 is assigned to the C–O stretching for pure K-carrageenan, and this peak has been shifted to 1126.78 cm−1 for all K-carrageenan with different concentrations of LiBr [17]. The characteristic peak at 1064.48 cm−1 reveals its assignment to 3,6-anhydro galactose for pure K-carrageenan, and it shifts to 1065.45 cm−1,1065.47 cm−1, 1066.13 cm−1, and 1066.09 cm−1 for 0.3, 0.4, 0.5, and 0.6 wt% of LiBr with pure K-carrageenan, respectively. The peak formed at 1035 cm−1 for pure K-carrageenan reveals that it is assigned to the combination of the symmetric and asymmetric stretching mode of C–O–C, and there is a shifting of peak at 1038.64 cm−1, 1039.85 cm−1, 1040.18 cm−1, and 1041 cm−1 for 0.3 wt%, 0.4 wt%, 0.5 wt%, and 0.6 wt% of LiBr salt with pure K-carrageenan, respectively. The peak formed around 921 to 923 cm−1 is assigned to the C–O–C of 3,6-anhydro galactose for both pure and LiBr-doped K-carrageenan [14]. The peak observed around 845 cm−1 for pure K-carrageenan and K-carrageenan with lithium bromide is assigned to C–H rocking [27]. The characteristic peak at 698.81 cm−1 is assigned to sulfate on C-4 galactose [17]. The shifting of the peak position and changes in the intensity of peaks confirm the complex formation between polymer and salt.
DSC analysis
Differential scanning calorimetry (DSC) is an efficient tool to detect the glass transition temperature (Tg) of the biopolymer electrolyte. DSC curves for 1 g of pure K-carrageenan and 1 g of pure K-carrageenan with LiBr salt of 0.3 wt%, 0.4 wt%, 0.5 wt%, and 0.6 wt% are depicted in Fig. 4. In the DSC curve, normally a slight step-like transition will be observed. In the figure, it is not possible to show the step-like transition. The center of the step-like transition is taken as a Tg value. Glass transition temperature (Tg) values and melting point temperature values obtained from the DSC curves for biopolymer electrolyte K-carrageenan with LiBr salt are listed in Table 3.
The glass transition temperature (Tg) for pure K-carrageenan is 41.59 °C, and that for 0.3 wt% and 0.4 wt% of LiBr with K-carrageenan is 35.23 °C and 33.36 °C, respectively. The above results agree with the statement that the glass transition temperature (Tg) decreases with the increase in concentration of the dopant to the polymer, due to the plasticizing effect of the salt. 44.55 °C and 45.59 °C are the glass transition temperatures for pure K-carrageenan with 0.5 wt% and 0.6 wt%, respectively, and this value is higher than that of pure K-carrageenan. The increase in glass transition temperature (Tg) with an increase in concentration of 0.5 wt% and 0.6 wt% of LiBr is due to the formation of the transient cross-linkage between polymer chains and Li ions [28]. Normally, Tg decreases by adding salt to the host polymer, but in our present study, the observed Tg values are found to decrease with an increase in the concentration of salt up to 0.4 wt%. Afterwards, an increase in salt concentration leads to an increase in Tg value up to 0.6 wt%. The value of Tg 44.55 °C of the highest-conducting polymer membrane is higher than that of pure K-carrageenan (41.59 °C) but less than that of polymer membrane 1 g K-carrageenan with 0.6 wt% of LiBr. The increase in Tg value may be due to the formation of the transient cross-linkage between K-carrageenan polymer chain segments via the coordinated interaction between oxygen and lithium cations owing to the addition of the high concentration of lithium salt [11]. This effect could also be understood as a result of a reduction in segmental motion caused by an increase in intermolecular coordinations between Li+ cations and the oxygen atoms in the polymer chain. These cross-linkage bonds block the rotation of polymer segments, leading to the hardening of polymer chains and hence intensifying the energy barrier to the segmental movement. Finally, this Li+ and O atom binding decreases the pliability of the polymer backbone [29].
Thermo gravimetric analysis
Thermo or thermal gravimetric analysis (TGA) provides information about physical phenomena, such as phase transitions, absorption, and desorption, as well as chemical phenomena including chemisorptions, thermal decomposition, and solid-gas reactions (e.g., oxidation or reduction).
Figure 5 a shows the TGA of pure K-carrageenan. The figure shows that the weight loss of pure K-carrageenan happened in three different stages. The first stage in the range of 30–191 °C, peaking at 80 °C (17% weight loss), is associated with the loss of water (17%). The second stage occurred in the range of 192–293 °C with maximum decomposition at 223 °C, and it has a weight loss of 25%. The main mass loss is observed in the range of 294–434 °C with a maximum decomposition rate at 350 °C. Weight loss at this stage is 31%. Similarly, three stages of loss have been observed for K-carrageenan by Mahdavinia et al. [30]. A TGA of 0.5 wt% of LiBr-doped K-carrageenan is shown in Fig. 5b. It also exhibits three stages of degradation of mass. The first stage is in the range of 28–178 °C, with maximum decomposition at 68 °C (31% weight loss). The second stage occurred in the range of 179–195 °C with maximum decomposition at 187 °C with a weight loss of 25%. The third stage of decomposition is in the range of 196–446 °C with maximum decomposition rate at 334 °C, and it has a weight loss of 14%.
In the first stage, weight loss for pure K-carrageenan with 0.5 wt% of LiBr is more than that of pure K-carrageenan. The first stage is associated with the loss of water. Weight loss for pure K-carrageenan and pure K-carrageenan with lithium bromide is the same in the second stage. In the third stage, weight loss of pure K-carrageenan with 0.5 wt% of LiBr is better than that of pure K-carrageenan. So pure K-carrageenan and K-carrageenan with lithium bromide have approximately the same thermal stability.
Impedance analysis
The impedance spectroscopic technique is an efficient tool used for characterizing the electrical properties of the material. Figure 6 a shows the Cole-Cole plot for the pure K-carrageenan, and Fig. 6b reveals the Cole-Cole plot for the composition of K-carrageenan with different concentrations of LiBr at room temperature (303 K). In general, there will be an appearance of the semicircle at a higher-frequency region and spikes at a lower-frequency region. The bulk resistance value can be obtained from the low-frequency intercept of the semicircle on the real Z′-axis or the intercept of the spike on the real Z′-axis. If there is only a spike, the interception of the spike at the X-axis of the Cole-Cole plot gives the bulk resistance value. Due to the effect of the electrolyte and roughness of the electrode-electrolyte interface, the low frequency spike is obtained. The Cole-Cole plot for the K-carrageenan with X wt% of LiBr (X = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6) does not have a semicircle which indicates that ionic conductivity is due to mobile ions. The equivalent circuit for the above Cole–Cole plot could be represented by a resistor and constant phase element in series [16]. Ionic conductivity can be calculated using the formula
where
- l:
thickness of the polymer electrolyte
- A:
contact area
- Rb:
bulk resistance
a The Cole-Cole plot of pure K-carrageenan at room temperature (303 K). b The Cole-Cole plot of a 0.1 wt% of LiBr-doped K-carrageenan, b 0.2 wt% of LiBr-doped K-carrageenan, c 0.3 wt% of LiBr-doped K-carrageenan, d 0.4 wt% of LiBr-doped K-carrageenan, e 0.5 wt% of LiBr-doped K-carrageenan, and f 0.6 wt% of LiBr-doped K-carrageenan at room temperature (303 K)
Boukamp’s EQ software program is used to find the bulk resistance (Rb) of the prepared polymer electrolytes [31]. Conductivity is found to increase for pure K-carrageenan with 0.1 to 0.5 wt% of LiBr. Conductivity decreases for pure K-carrageenan with 0.6 wt% of LiBr.
Concentration-dependent conductivity
Variation of conductivity with respect to concentration at room temperature of 303 K is shown in Fig. 7. Ionic conductivity for the pure K-carrageenan is 6.76 × 10−6 Scm−1. Conductivity increases with the addition of LiBr salt with K-carrageenan, and it reaches its highest value of 3.436 × 10−3 Scm−1 for 1 g K-carrageenan with 0.5 wt% of LiBr. The increase in ionic conductivity with an increase in concentration is due to the mobility of charge carriers and an increase in the charge carrier concentration [32]. Further addition of salt to 0.6 wt% of LiBr leads to the decrease in ionic conductivity of 7.798 × 10−4 Scm−1 due to the formation of ion aggregates [33].
Temperature-dependent conductivity
Temperature dependence of lithium conductivity for K-carrageenan doped with various concentrations of LiBr is found at a different temperature ranging from 303 to 343 K. From Fig. 8, a conclusion has been made that the conductivity increases linearly with temperature and K-carrageenan doped with 0.5 wt% of LiBr has the highest conductivity among all. An increase in conductivity with the increase in temperature indicates that the free volume around the polymer chain causes the mobility of ions and polymer segments [34]. Table 4 shows the ionic conductivity values for all the samples at different temperatures.
The conductivity (σ) values obey the following Arrhenius equation:
where
- σ:
ionic conductivity
- σ0:
pre exponential factor
- Ea:
activation energy
- K:
Boltzmann constant
- T:
absolute temperature
Figure 8 shows the Arrhenius plot for pure K-carrageenan and K-carrageenan with 0.1 to 0.6 wt% of LiBr. Activation energy and regression values calculated from the figure and are shown in Table 5.
Variations of conductivity with temperature for the membrane were measured twice, the Arrhenius plot was plotted twice, and activation energy is calculated twice. Activation energy is found to decrease with the increase in salt concentration up to 0.5 wt% of LiBr with K-carrageenan which is nothing but the highest conductivity concentration. The decrease in activation energy with the increase in concentration is due to the amorphous nature of the polymer electrolyte that facilitates the motion of lithium ions in the polymer network [34]. With the further addition of salt, activation energy begins to increase which may be due to an aggregation of ions [35]. As temperature increases, bond rotations in the polymer chain produce a segmental motion due to its faster internal modes. This, in turn, favors inter- and intra-chain ion movements, and as a result, conductivity of the polymer electrolyte becomes high [36].
Conductance spectra analysis
Usually, conductance spectra have three regions in which the low-frequency region represents the space charge polarization at blocking electrodes, the mid-frequency plateau region indicates the DC conductivity, and the high-frequency dispersion region is because of the bulk relaxation process which is formed due to the columbic interaction of the charge carrier and disorder within the structure [37].
Variation of logarithmic ac conductivity with logarithmic angular frequency at room temperature (303 K) is shown in Fig. 9. In this work, low-frequency and mid-frequency plateau regions are only obtained. The dc ionic conductivity values have been obtained by extrapolating the angular frequency–independent region to zero frequency [38]. The conductivity values obtained from the conductance spectra agree with the value obtained from the Cole–Cole plot.
Transference number analysis
The contribution of different ions to total electric current in the polymer electrolyte can be found by the ion transference number. The cationic (t+) and anionic (t−) transport number measurement has been undertaken by the Wagner polarization technique [39]. In this technique, the polymer membrane is sandwiched between two stainless steel electrodes, and the 1.5-V dc potential at ambient temperature is applied. Figure 10 shows the variation of current with respect to time for the highest-conducting polymer membrane 1 g K-carrageenan with 0.5 wt% of LiBr. There is a decrease in initial current with respect to time, and decrease in the current is due to the polarization effect. Constant final current is obtained in a fully depleted situation. The transference number is calculated by the following equation:
where
- t+:
transport number of cation
- t−:
transport number of anion
- Ii:
the initial current
- If:
the final residual current
The diffusion coefficient can be calculated from the values of conductivity and ionic transference number using the following equation.
where
- D:
sum of the diffusion coefficient of cation and anion
- D+:
diffusion coefficient of cation
- D−:
the diffusion coefficient of anion
- k:
Boltzmann constant
- T:
absolute temperature
- σ:
conductivity of the polymer electrolyte
- e:
charge of the electron
- n:
number of molecules cm−3
- n:
N ρ × molar ratio of salt/molecular weight of the salt
- N:
Avogadro number
- ρ:
density of the salt
Ionic mobility was calculated for all the concentration of the samples by the following equations.
where
- μ+:
ionic mobility of cation
- μ−:
ionic mobility of anion
Figure 11 shows the transport parameters of with LiBr. Transference numbers for ions and electrons are 0.9965 and 0.0035, respectively, for the composition of 1 g K-carrageenan with 0.5 wt% of LiBr. The value for the ion is closer to unity, and this suggests that charge transport is mainly due to ions rather than electrons. The diffusion coefficient of the cation (D+) and anion (D−) are 1.35562 × 10−8 and 4.76132 × 10−11, respectively. The cationic value of diffusion is greater than the anionic value of diffusion by three orders. The value of cationic mobility μ+ is 5.1918 × 10−8 greater than that of anionic mobility μ− which is 1.82353 × 10−9 by one order. In both cases, cation possesses a higher value. Measured transference number values are shown in Table 6.
Linear sweep voltammetry studies
For battery application, good electrochemical stability is also required along with high ionic conductivity. The electrochemical stability of K-carrageenan with 0.5 wt% of the LiBr polymer electrolyte was examined by linear sweep voltammetry (LSV) of the cell with a two-electrode system. Figure 12 a and b show the LSV curves for pure K-carrageenan and K-carrageenan with 0.5 wt% of LiBr polymer electrolyte at room temperature. The anodic decomposition limit of the polymer electrolyte is considered as the voltage at which the current flows through the cells [28]. As seen from the plot of current Vs voltage, electrolytes show an electrochemical stability window of 2.4 V and 3.5 V for pure K-carrageenan and with 0.5 wt% of LiBr polymer electrolyte, respectively. Karthikeyan et al. [16] reported the electrochemical stability value of Ι-carrageenan/NH4Br membrane as 2.1 V. Moniha et al. [17] obtained the electrochemical stability window up to 2.47 V for 1 g Ι-carrageenan/0.4 wt% NH4NO3.
Fabrication of lithium ion conducting battery
The highest-conducting polymer membrane (1 g of K-carrageenan with 0.5 wt% of LiBr) is sandwiched between anode and cathode in order to fabricate lithium ion conducting battery.
The theoretical value of oxidation potential of Zn and reduction potential of PbO2 is − 0.7618 V and 1.455 V, respectively, and the overall reaction provides the cell with E0 = 2.2168 V. In this work, an open-circuit voltage (OCV) of 1.64 V is obtained and it is 26% lower than the cell potential which is due to the reduction of ZnSO4·7H2O at the anode [40].
The anode reaction is
The cathode reaction is
The proton produced from the anode side repels Li+ ions in the polymer membrane. This process continues, and the Li+ ions by hopping mechanism reach the cathode side [11]. A similar electrochemical test was conducted and reported by Chithra et al., Sampath et al., Premalatha et al., Perumal et al., Monisha et al., and Kingslin et al. [11, 12, 14, 28, 41, 42], using Zn·ZnSO4 (anode) and PBO2·V2O5 (cathode) for Li+ ion conduction. Chithra et al. reported a voltage of 1.77 V for iota-carrageenan with lithium chloride. Sampath et al. obtained a voltage of 1.57 V for the tamarind seed polysaccharide biopolymer with lithium chloride. An open-circuit voltage of 1.6 V is obtained for Premalatha et al. for tamarind seed polysaccharide complexed with lithium bromide. Perumal et al. doped pectin with lithium chloride and obtained an open-circuit voltage as 1.93 V. Monisha et al. reported a voltage of 1.3 V by complex formation of cellulose acetate (CA) with the ammonium thiocyanate (NH4SCN) salt. Kingslin et al. obtained an open-circuit voltage of 1.69 V for PVA–PAN doped with lithium triflate.
The obtained voltage of 1.64 V is stable up to 48 h, and the battery is allowed to discharge through a constant load of 100 KΩ. During discharging, 0.89 mA current is observed and the cell potential begins to drop from 1.64 to 1.57 V. The initial drop in voltage during discharge is due to the polarization [43], and a dropped voltage of 1.57 V remains constant during discharge for the first 48 h of assembly of the battery. Open-circuit voltage (OCV) was plotted against time and is shown in Fig. 13a. The discharge characteristics of the cell at room temperature are shown in Fig. 13b, and the region in which flat discharge potential occurs is called the plateau region. Figure 14 a shows the battery configuration, and Fig. 14b depicts the open-circuit voltage. Important cell parameters are shown in Table 7.
Conclusion
Lithium ion conducting polymer electrolyte was prepared using the biopolymer K-carrageenan doped with lithium bromide at different molar concentrations of 0 wt%, 0.1 wt%, 0.2 wt%, 0.3 wt%, 0.4 wt%, 0.5 wt%, and 0.6 wt% by the solution casting method. The prepared polymer electrolyte has been characterized by XRD, FTIR, DSC, AC impedance, and electrochemical studies. XRD confirms the amorphous nature due to the addition of LiBr salt at a concentration of 0.5 wt% to 1 g of K-carrageenan. FTIR analysis reveals the complex formation between biopolymer and salt. The glass transition temperature is found to decrease up to 0.4 wt% concentration, and from the highest-conducting sample of 0.5 wt% of LiBr onwards, it increases and it is proved by DSC analysis. AC impedance analysis confirms that K-carrageenan doped with 0.5 wt% of LiBr has the highest conductivity of 3.43 × 10−3 Scm−1 at room temperature. Arrhenius theory was obeyed by the temperature-dependent ionic conductivity of the biopolymer membrane, and the activation energy obtained from the Arrhenius plot is found to be low for the highest-conducting electrolyte. Transference number analysis confirms that the charge transport in the biopolymer membrane is mainly due to the ions. The highest-conducting biopolymer electrolyte is employed to construct the lithium ion battery, and its parameters were reported.
References
Fergus JW (2010) Ceramic and polymeric solid electrolytes for lithium-ion batteries. J Power Sources 195:4554–4569
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state paper like polymer supercapacitors. Nano Lett 10:4025–4403
Rani M, Rudhziah S, Ahmad A, Mohamed N (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymer 6:2371–2385
Ahmad Khair AS, Arof AK (2010) Conductivity studies of starch based polymer electrolytes. Ionics 16:123–129
Selvakumar M, Bhat DK (2008) LiClO4 doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J Appl Polym Sci 110:594–602
Vijayalekshmi V, Khastgir D (2017) Eco-friendly methane sulfonic acid and the sodium salt of dodecyl benzene sulfonic acid doped crosslinked chitosan based green polymer electrolyte membranes for fuel cell applications. J Membr Sci 523:45–59
Perumal P, Christopher Selvin P, Selvasekarapandian S (2018) Characterization of biopolymer pectin with lithium chloride and its applications to electrochemical devices. Ionics 24:3259–3270. https://doi.org/10.1007/s11581-018-2507-5
Vijaya N, Selvasekarapandian S, Sornalatha M, Sujithra KS, Monisha S (2017) Proton-conducting biopolymer electrolytes based on pectin doped with NH4X (X=Cl, Br). Ionics 23:2799–2808
Selvalakshmi S, Vijaya N, Selvasekarapandian S, Premalatha M (2017) Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J Appl Polym Sci 134(15). https://doi.org/10.1002/app.44702
Selvalakshmi S, Mathavan T, Selvasekarapandian S, Premalatha M (2017) Study on NH4I composition effect in agar–agar-based biopolymer electrolyte. Ionics 23(10):2791–2797
Sampath Kumar L, Christopher Selvin P, Selvasekarapandian S, Manjuladevi R, Monisha S, Perumal P (2018) Tamarind seed polysaccharide biopolymer membrane for lithium-ion conducting battery. Ionics 24(12):1–11
Kingslin Mary Genova F, Selvasekarapandian S, Vijaya N, Sivadevi S, Premalatha M, Karthikeyan S (2017) Lithium ion-conducting polymer electrolytes based on PVA–PAN doped with lithium triflate. Ionics 23:2727–2734
Kingslin Mary Genova F, Selvasekarapandian S, Karthikeyan S, Vijaya N, Pradeepa R, Sivadevi S (2015) Study on blend polymer (PVA–PAN) doped with lithium bromide. Polym Sci Ser A 57(6):851–862
Christopher Selvin P, Perumal P, Selvasekarapandian S, Monisha S, Boopathi G, Leena Chandra MV (2018) Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics 24:3535–3542. https://doi.org/10.1007/s11581-018-2521-7
Zainuddin NK, Samsudin AS (2018) Investigation on the effect of NH4Br at transport properties in K-carrageenan based biopolymer electrolytes via structural and electrical analysis. Mater Today Commun 14:199–209
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi G, Aristatil G, Arun A, Madeswaran S (2017) Proton-conducting I-carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23:2775–2780. https://doi.org/10.1007/s11581-016-1901-0
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting K-carrageenan chitosan electrolytes. Funct Mater Lett 1:195–201
Singh R, Polu AR, Bhattacharya B, Rhee H-W, Singh CVP (2016) Perspectives for solid biopolymer electrolytes in dye-sensitized solar cell and battery application. Renew Sust Energ Rev 65:1098–1117
Liang L, Ni R, Yang S, Mao S (2014) Carrageenan and its application in drug delivery. Carbo Hydr Polym 103:1–11
Campo VL, Kowano DF, da Silva DB Jr, Carvalho I (2009) K-carrageenan: biological properties, chemical modifications, and structural analysis- a review. Carbo Hydr Polym 77:167–180
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semi crystalline poly (vinyl alcohol) films. Polymer 37:1371–1376
Nithya S, Selvasekarapandian S, Karthikeyan S, Inbavalli D, Sikkanthar S, Sanjeeviraja C (2014) AC impedance studies on proton conducting PAN-NH4SCN polymer electrolytes. Ionics 20(10):1391–1398
Nithya S, Selvasekarapandian S, Karthikeyan S, Vinoth Pandi D (2015) Effect of propylene carbonate on the ionic conductivity of polyacrylonitrile-based solid polymer electrolytes. J Appl Polym Sci 132(14). https://doi.org/10.1002/app.41743
Hemalatha R, Alagar M, Selvasekarapandian S, Sundaresan B, Moniha V, Boopathi G, Christopher Selvin P (2016) Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acid proline, and NH4Cl and its applications to electrochemical devices. Ionics 25:141–154. https://doi.org/10.1007/s11581-018-2564-9
Boopathi G, Pugalendhi S, Selvasekarapandian S, Premalatha S, Monisha S, Aristatil G (2017) Development of proton conducting biopolymer membrane based on agar–agar for the fuel cell. Ionics 23:2781–2790
Priya SS, Karthika M, Selvasekarapandian S, Manjuladevi R, Monisha (2018) Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics 24:3861–3875. https://doi.org/10.1007/s11581-018-2535-1
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Aristatil G, Mani N, Premalatha M (2017) Investigation of biopolymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47
Nirmala Devi G, Chitra S, Selvasekarapandian S, Premalatha M, Monisha S, Saranya J (2017) Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics 23:3377–3388. https://doi.org/10.1007/s11581-017-2135-5
Mahdavinia GR, Massoudi A, Baghban A, Shokri E (2014) Study of adsorption of cationic dye on magnetic kappa-carrageenan/ PVA nanocomposite hydrogels. J Environ Chem Eng 2:1578–1587
Boukamp BA (1986a) A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20:31–44
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Christopher Selvin P, Mangalam R, Monisha S (2017) Preparation and characterization of blend polymer electrolyte film based on poly (vinyl alcohol)-poly (acrylonitrile)/MgCl2 for energy storage devices. Ionics 24:1083–1095. https://doi.org/10.1007/s11581-017-2273-9
Bhuvaneswari R, Karthikeyan S, Selvasekarapandian S, VinothPandi D, Vijaya N, Araichimani A, Sanjeeviraja C (2014) Preparation and characterization of PVA complexed with amino acid proline. Ionics 21:387–399
Vinoth Pandi D, Selvasekarapandian S, Bhuvaneswari R, Premalatha M, Monisha S, Arunkumar D, Junichi K (2016) Development and characterization of proton conducting polymer electrolyte based on PVA, amino acid glycine and NH4SCN. Solid State Ionics 298:15–22
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Vinoth Pandi D, Selvalakshmi S (2016) Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J Non-Cryst Solids 453:131–140
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, Monisha S (2017) Mg-ion conducting blend polymer electrolyte based on poly (vinyl alcohol) - poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Nurath Unnisa C, Chitra S, Selvasekarapandian S, Monisha S, Nirmala Devi G, Moniha V, Hema M (2018) Development of poly (glycerol suberate) polyester (PGS)–PVA blend polymer electrolytes with NH4SCN and its application. Ionics. https://doi.org/10.1007/s11581-018-2466-x
Ramya CS, Selvasekarapandian S, Hirankumar G, Savitha T, Angelo PC (2008) Investigation on dielectric relaxations of PVP–NH4SCN polymer electrolyte. J Non-Cryst Solids 354:1494–1502
Wagner JB, Wagner C (1957) Electrical conductivity measurements on cuprous halides. J Chem Phys 26:1597–1601
Mishra K, Rai DK (2013) Studies of a plasticized PEO + NH4PF6 proton-conducting polymer electrolyte system and its application in a proton battery. J Korean Phys Soc 62:311–319
Chitra R, Sathya P, Selvasekarapandian S, Monisha S, Moniha V, Meyvel S (2018) Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 25:2147–2157. https://doi.org/10.1007/s11581-018-2687-z
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Selvalakshmi S, Vinoth Pandi D (2017) Tamarind seed polysaccharide (TSP)-based li-ion conducting membranes. Ionics 23(10):2677–2684
Subba Reddy CV, Sharma AK, Narasimha Rao VVR (2003) Conductivity and discharge characteristics of polyblend (PVP +PVA + KIO3) electrolyte. J Power Sources 114(2):338–345
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arockia Mary, I., Selvanayagam, S., Selvasekarapandian, S. et al. Lithium ion conducting membrane based on K-carrageenan complexed with lithium bromide and its electrochemical applications. Ionics 25, 5839–5855 (2019). https://doi.org/10.1007/s11581-019-03150-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03150-x