Abstract
A proton-conducting solid biopolymer electrolyte (SBE) that consists of kappa-carrageenan (K-carrageenan) incorporated with ammonium thiocyanate (NH4SCN) has been prepared by solution-casting technique. The prepared membranes are subjected to XRD, FTIR, and AC impedance analysis to study their structural, optical, and electrical properties of the electrolytes, respectively. The amorphous nature of the membrane has been inferred from the results of XRD analysis. The complexation between salt and host polymer matrix has been confirmed by FTIR. From AC impedance analysis, the polymer incorporated with 0.5% of NH4SCN achieved a maximum ionic conductivity of the order of 6.83 × 10−4 Scm−1. The dielectric spectra and modulus spectra reveal the non-Debye behavior of the polymer membrane. A fuel cell has been constructed using the prepared SBE, and its open circuit voltage has been measured.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolytes (SPEs) are made of polymer host matrix embedded with alkali metal salt in the absence of organic/inorganic liquid solvents, and they have played an important role in solid-state devices [1]. The preparation and characterization of synthetic SPEs such as poly(o-methoxyaniline) (POMA), poly(3-thiophene acetic acid) (PTAA), poly(3,4-ethylenedioxythiophene) (PEDOT), poly(vinyl alcohol) (PVA), and poly(ethylene glycol) (PEG) have been reported by many researchers. However, synthetic SPEs are non-biodegradable, costly, and hazardous and create environmental pollution. To overcome this issue, research has been focused to develop the eco-friendly and biodegradable new proton-conducting solid biopolymer electrolytes (SBEs). SBEs have many advantages such as high ionic conductivity (10−2 to 10−4 Scm−1), wide electrochemical window, simplicity of design, absence of leakage and pollution, resistance to shocks, relative ease of miniaturization, and flexibility compared to other electrolytes [2,3,4]. The biodegradable natural polymer electrolytes such as chitosan [5,6,7], starch [8], gelatin [9], cellulose acetate [10], pectin [11], and agar [12] for various electrochemical applications have been reported.
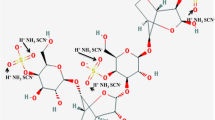
K-Carrageenan has been chosen as a backbone, which is one of natural anionic polymers and non-toxic sulfated polysaccharides, extracted from the red seaweed (algae) from the Rhodophycea family [13]. The structure of K-carrageenan is made up of alternating 3-linked β-d-galactose-4-sulfate and 4-linked 6-anhydro-α-galactopyranose having one negative charge per disaccharide repeating unit. It is widely used as a stabilizer, thickener, emulsifier, or gelling agent in the food industry [14] and is also utilized in pharmaceutical and cosmetic industries [15]. The structure of K-carrageenan is shown in Scheme 1.
Shamsudin et al. [16] prepared the biopolymer electrolytes based on carboxymethyl carrageenan and imidazolium as the ionic liquid. Electrochemical impedance spectroscopy (EIS) reports a highest ionic conductivity of the order of (5.76 ± 0.20) × 10−3 Scm−1 at 298 K with 30 wt% of ionic liquid. The electrochemical stability up to 3.0 V is reported through linear sweep voltammetry (LSV).
SBE has been incorporated with various ammonium salts, which provide a good proton donor to the polymer matrix for conduction such as NH4I, NH4SO3CF3, and NH4NO3 [17]. When ammonium salts dissociated in a solvent, the salt dissociates as NH4+ ion and other anion. In NH4+ ion, three hydrogen atoms were bounded strongly with nitrogen in which the fourth hydrogen ion was very weakly bound to it. The weakly bound hydrogen ion hops under the influence of an electric field [17,18,19].
Shuhaimi et al. [20] reported a polymer blend-salt complex formation from chitosan; K-carrageenan and ammonium nitrate were used as raw materials. The AC impedance analysis reveals a maximum ionic conductivity of 2.39 × 10−4 Scm−1.
Karthikeyan et al. [21] developed the proton-conducting I-carrageenan-based biopolymer electrolyte for fuel cell application. The AC impedance analysis shows the highest ionic conductivity at 1.08 × 10−3 Scm−1 for 20% of NH4Br. The electrochemical stability up to 2.1 V was evaluated by LSV analysis.
Noor et al. [22] have studied ionic conductivity and dielectric properties of carboxymethyl cellulose (CMC) with NH4SCN solid biopolymer electrolytes via solution casting technique. The highest ionic conductivity of 6.48 × 10−5 Scm−1 has been observed with the help of AC impedance analysis.
Studies on proton-conducting solid polymer electrolyte K-carrageenan with ammonium salts are rare in the literature. In this work, an attempt has been made to develop the new proton-conducting SBE through K-carrageenan incorporated with NH4SCN prepared by solution casting technique. The prepared membrane has been characterized by XRD, FTIR, and AC impedance analysis to study the structure, complexation of salt, and conductivity of the membrane.
Experimental
Membranes of K-carrageenan with different mole weight percentages of ammonium thiocyanate (NH4SCN) have been prepared by solution casting technique with distilled water as a solvent. The amount of K-carrageenan is fixed (1 g) for all membranes. Different mole weight percentages of NH4SCN are added to K-carrageenan. The resulting homogenous solution is transferred to Petri disks which are kept at 60 °C and dried to obtain a free-standing film. The amorphous/crystalline nature of the membrane has been studied using X-ray powder diffractometry with Cu Kα radiation (λ = 1.5405 Å) in a wide 2θ range (20 ≤ 2θ ≤ 80). The FTIR spectrum was measured using a Bruker spectrophotometer at room temperature, in the region from 400 to 4000 cm−1 with a resolution of 1 cm−1. The AC impedance measurement is done with the help of a HIOKI 3532 LCR meter in the frequency range from 42 Hz to 1 MHz.
Results and discussion
Structural analysis
The XRD pattern of pure and NH4SCN-incorporated K-carrageenan is shown in Fig. 1. The XRD pattern of pure K-carrageenan exhibits two broad peaks around 2θ = 23° and 32°, respectively. These broad peaks indicate the amorphous nature of the K-carrageenan polymer. The intensity of the broad peaks is increased with addition of 0.4% NH4SCN compared to the K-carrageenan polymer matrix. When the concentration of NH4SCN is increased to 0.5% NH4SCN, the intensity of the peaks at 23° and 32° has decreased along with the increase in the broadness of the peak compared to K-carrageenan with 0.4% NH4SCN. For K-carrageenan with 0.6% NH4SCN, the intensity of the peaks has increased along with reduction in the broadness of the peaks. From the XRD pattern, it is observed that the polymer membrane K-carrageenan with 0.5% NH4SCN is more amorphous in nature and also no noticeable peak corresponding to the NH4SCN salt is observed.
Fourier transform infrared spectroscopy analysis
FTIR spectroscopy is used to probe the complex formation between polymer K-carrageenan and NH4SCN [23]. Figure 2 shows the FTIR spectrum of pure K-carrageenan and K-carrageenan with different concentrations of NH4SCN membrane, and characteristics of peak positions of FTIR spectra are listed along with their assignment in Table 1.
The broad band observed in the region 3000–3500 cm−1 corresponds with –OH stretching vibration formed by the hydroxyl group of polysaccharide and water. The vibration peak at 3319 cm−1 of pure K-carrageenan is attributed to the –OH stretching vibration [24,25,26,27,28,29]. This peak has been shifted to 3290 cm−1 when K-carrageenan has been doped with 0.4% of NH4SCN. Further increase in salt concentration results in the shift in their peak position and split into two sub peaks, the first one around 3396 cm−1 attributed to the –OH stretching mode and another at 3222 cm−1 assigned to N-H stretching vibration, respectively [30]. The band at 2930 cm−1 has been attributed to C-H stretching vibration related to the CH2 and CH3 groups [31] of pure K-carrageenan, and this peak gets shifted to 2927 cm−1 for 0.4% of the NH4SCN-doped membrane. The peak at 1619 cm−1 is assigned to the C=O stretching vibration for the pure membrane. This peak has been shifted and appears at 1617, 1627, and 1630 cm−1 for the 0.4, 0.5, and 0.6% NH4SCN-doped membrane [32].
The vibrational peak around 1428 cm−1 is assigned to the asymmetrical bending of CH2, and another vibration around 1035 cm−1 is assigned to the combination of symmetric and asymmetric stretching modes of C-O-C present in the pure and doped K-carrageenan [33]. The pure membrane exhibits a vibration peak around 1234 cm−1 due to sulfate stretching of the S-O bond (ester sulfate group). This peak has been shifted to 1237, 1235, and 1229 cm−1 for 0.4, 0.5, and 0.6% NH4SCN with the K-carrageenan membrane due to the interaction of salt with the polymer matrix [23, 34]. The peak at 844 cm−1 is assigned to C-O-SO3 stretching in (1-3)-d-galactose vibration of the pure K-carrageenan membrane [26,27,28,29]. This peak has been shifted to 846, 843, and 844 cm−1 for 0.4, 0.5, and 0.6% NH4SCN with the K-carrageenan polymer electrolyte. The peak at 920 cm−1 is assigned to the C-O-C of 3,6-anhydro-o-galactose and the peak around 1159 cm−1 assigned to the C-O stretching band of the C-O-H group. The peak at 700 cm−1 is attributed to sulfate on C-4 of galactose vibration [21].
The new strong absorption peak at 2058 cm−1 has been attributed to the C≡N stretching vibration of SCN− ions [35, 36]. This vibration peak has been present in NH4SCN salt which doped all membranes, indicating the interaction of NH4SCN with the polymer matrix. NH4SCN dissociates into NH4+ ion and SCN− ion. Two of the four hydrogen of NH4+ ions are bound identically; one hydrogen is bound very rigidly and the fourth hydrogen more weakly [30]. The weakly bound hydrogen of NH4+ interacts with the polar group of K-carrageenan polymer matrix, which can be easily dissociated under the influence of an electric field. The observed vibration peak shifts and variation in intensities of the FTIR spectrum of the NH4SCN-doped membrane compared to the FTIR spectrum of pure K-carrageenan suggest complexation of salt with the host polymer matrix [21].
AC impedance analysis
AC impedance spectroscopy is the most elegant and powerful technique for the electrical characterization of solids, in general and electrochemical devices including electrodes as well as electrolytes in particular. This technique is often used to obtain bulk conductivity, with additional information related to the motion of charged particles and the hopping mechanism of mobile charges. The Cole-Cole plots of the pure and NH4SCN-doped K-carrageenan membrane are shown in Fig. 3a, b. The value of bulk resistance (Rb) has been obtained with the help of EQ Software developed by Boukamp [37]. The ionic conductivity of the polymer membrane is calculated by
where l is the thickness of the membrane in centimeters, A is the contact area in square centimeters, and Rb is the bulk resistance of the membrane.
Figure 3a shows the Cole-Cole plot of pure K-carrageenan. It shows a slight arc with tilted spike. The Cole-Cole plots of K-carrageenan with different concentrations of NH4SCN (Fig. 3b) show only a low-frequency tilted spike which may be due to the polarization effect at the electrode/electrolyte interface region [38]. The ionic conductivity of pure K-carrageenan has been found to be 4.81 × 10−6 Scm−1. The conductivity of 0.1, 0.2, and 0.3% NH4SCN-doped membranes is found to be in the order of 10−5 Scm−1, which is one order of magnitude greater than the pure membrane, whereas the 0.4, 0.5, and 0.6% doped polymer electrolytes show conductivity in the order of 10−4 Scm−1 with two orders of magnitude higher compared to the pure membrane. The calculated ionic conductivity values of all prepared membranes are given in Table 2. The increase in concentration of the doped membrane shows the increase in ionic conductivity values up to 0.5% of the NH4SCN sample. As the salt concentration increases, the number of charge carriers increases leading to high ionic conductivity [39]. With further addition of 0.6% NH4SCN salt, conductivity decreases slightly which may be due to aggregation of ions. The re-association of anions and cations is responsible for the existence of salt aggregation in the polymer electrolytes [40]. The aggregation of salt suppresses the number of density of free mobile ions, which leads to a decrease in conductivity [21]. The maximum ionic conductivity of 6.83 × 10−4 Scm−1 is observed for K-carrageenan with 0.5% NH4SCN.
Conductance spectra analysis
The study of the conductivity spectra reveals information about the type, nature of conduction, and hopping dynamics of the charge carriers at different dopant salt concentrations. In general, conductance spectra consist of three well-defined regions such as low-frequency dispersion region, frequency-independent plateau region, and high-frequency dispersion region. The low-frequency region corresponds to the interfacial polarization or electrode polarization effects. The plateau region gives the dc conductivity value of the polymer membrane, and the high-frequency region corresponds to the bulk relaxation phenomenon, which is responsible for the relaxation of mobile ion hopping due to the columbic interaction of charge carrier and disorder within the structure [40]. The variation of logarithmic plots of ac conductivity with angular frequency for different compositions of K-carrageenan/NH4SCN polymer electrolyte is shown in Fig. 4.
Polymer membrane K-carrageenan with 0.4, 0.5, and 0.6% NH4SCN shows similarity in the trend conductance spectrum which consists of two defined regions: (i) low-frequency region and (ii) plateau region. The low-frequency region suggests more and more accumulation of charge carriers at the electrode-electrolyte interface which leads to a decrease in the number of mobile ions and hence decrease in conductivity. The ionic conductivity is sufficient to produce buildup of charges at electrodes which reduces the effective applied field at the low-frequency region. The plateau region characterizes the long-range conduction, which is responsible for the hopping motion of mobile ions [41]. The mid-frequency region, the period of applied field, is short for charging to occur and the ac conductivity is generally taken to assume the frequency-independent value, which is equal to the dc conduction [42]. The conductivity values obtained from the conductance spectrum agree with the value obtained from the Cole-Cole plot.
Dielectric Analysis
Dielectric analysis is an effective non-destructive technique to study electrical properties of a material. The complex permittivity of the polymeric system is defined by the relation
where ε′ is the real part of dielectric permittivity or dielectric constant, and ε″ is the imaginary part of dielectric permittivity or dielectric loss during each cycle of the electric field. The frequency dependence of the dielectric permittivity of pure and NH4SCN embedded K-membrane is shown Fig. 5a, b.
The dielectric constant of the membrane increases with the increase in the concentration of NH4SCN and decreases with the increase in the frequency. The dielectric constant is high at low frequencies due to the electrode or interfacial polarization. The higher dielectric constant at low frequency is attributed to the accumulation of dipoles nearer the electrodes [43].
The 0.5% of NH4SCN incorporated K-carrageenan membrane exhibits a higher dielectric constant which causes higher conductivity of the membrane. The low and saturated dielectric constant at high frequency could be attributed to the lack of ionic response with the direction of the applied electric field. The dielectric analysis also reveals that the prepared membranes are non-Debye in nature [40]. The higher value of dielectric loss (ɛ″) at low frequency is due to the free charge motion and polarization of dipoles at the interface, which resembles the conductivity relaxation [44]. The NH4SCN (0.5%)-embedded KC membrane shows a α-relaxation peak at the high-frequency region which is caused by main chain dipoles [40].
Modulus analysis
Generally, electrical modulus analysis is a convenient and powerful tool to investigate the ion transport process of ionic conductors by suppressing the polarization effect at the electrode/electrolyte interface. The complex modulus formalism of the polymeric system is defined by the relation
where M′ and M″ are the real and imaginary parts of the complex electric modulus. The frequency dependence of M′ and M″ for all the prepared polymer membrane is shown in Fig. 6a, b, respectively. From the graph, it can be observed that the value of M′ and M″ decreases at low frequencies, which is due to the contribution of interfacial polarization phenomenon and makes a negligible conduction. The presence of long tail at the low-frequency range is due to their large capacitance value associated with the electrodes. This further confirms the non-Debye behavior of the polymer electrolyte [40]. From Fig. 6a, b, it is observed that the values of M′ and M″ increase at the high frequency end, but well-defined dispersion peaks are not observed indicating that the polymer membranes are ionic conductors and this is attributed to the bulk effect of the material [42].
Construction of single-PEM fuel cell
To prove the movement of proton in the polymer membrane, a single fuel cell is constructed following the design of fuel cells constructed by Monisha et al. [45]. The cell consists of bipolar graphite plates with a parallel flow channel area of 7.84 cm2 and mounted on the two base plates, which is made up of acrylic. A silicon gasket is placed between the two graphite plates [45]. The positive electrode (cathode) and negative electrode (anode) are made up of carbon cloth of area ~ 8.41 cm2, which are coated with Pt at a uniform rate of 0.15 mg/cm2 which acts as a catalyst. The highest proton-conducting membrane 1 g of K-carrageenan with 0.5% of NH4SCN is sandwiched between the two electrodes without making a membrane electrolyte assembly (MEA). The single-PEM fuel cell has been assembled with the abovementioned configuration shown in Fig. 7a.
A small electrolyzer operated by a voltage of 3 V is used to produce hydrogen and oxygen gas separately. The hydrogen gas with a flow rate of 10 ml/min and oxygen gas at a rate of 8 ml/min are passed through the single fuel cell. The anode and the cathode reactions for a PEM fuel cell are given below:
The highest-conducting polymer membrane 1 g of K-carrageenan with 0.5% of NH4SCN is placed in a fuel cell kit (Fig. 7b) and a voltage of 502 mV is observed.
Conclusion
The proton-conducting K-carrageenan-based biopolymer electrolyte with different concentrations of NH4SCN has been prepared by solution casting technique. The XRD spectrum confirms the amorphous nature of the prepared polymer electrolyte. The complexation between NH4SCN salt and host polymer matrix has been confirmed by FTIR. From AC impedance analysis, the maximum ionic conductivity of 6.83 × 10−4 Scm−1 was achieved for the membrane doped with 0.5% NH4SCN salt concentration. The fuel cell has been constructed using the above membrane, and its open circuit voltage of 502 mV is observed.
References
Rongxian O, Xie Y, Shen X, Yuan F, Wang H, Wang Q (2012) Solid biopolymer electrolytes based on all-cellulose composites prepared by partially dissolving cellulosic fibers in the ionic liquid 1-butyl-3-methylimidazolium chloride. J Mater Sci 47:5978–5986
Singh R, Polu AR, Bhattacharya B, Rhee H-W, Varlikli C, Singh PK (2016) Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew Sustainable Energy Rev 65:1098–1117
Hayachi A, Noi K, Sakuda A, Tatsumisago M (2012) Nat Commun 3:856
Sahu G, Lin Z, Li JC, Liu ZC, Dudney N, Liang CD (2014) Nat Commun 3:856
Vijayalekshmi V, Khastgir D (2017) Eco-friendly methane sulfonic acid and sodium salt of dodecyl benzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J Membr Sci 523:45–59
Puteh R, Yahya MZA, Ali AMM, Sulaiman MA, Yahya R (2005) Conductivity studies on chitosan-based polymer electrolytes with lithium salts. Indonesian J Phys 16:17–19
Khiar ASA, Puteh R, Arof AK (2006) Conductivity studies of a chitosan-based polymer electrolyte. Physica B 373:23–27
Ramasamy P (2012) A dielectric relaxation study of starch–water and starch–glycerol films. Ionics 18:413–423
Avellaneda CO, Vieira DF, Al-Kahlout A, Heusing S, Leite ER, Pawlicka A, Aegerter MA (2008) All solid state electrochromic devices with gelatin-based electrolyte. Sol Energy Mater Sol Cells 92:228–233
Selvakumar M, Bhat DK (2008) LiClO4 doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J Appl Polym Sci 110:594–602
Pasini Cabello SD, Ochoa NA, Takara EA, Molla S, Compan V (2017) Influence of pectin as a green polymer electrolyte on the transport properties of chitosan-pectin membranes. Carbohydr Polym 157:1759–1768
Koh JCH, Ahmad ZA, Mohamad AA (2012) Bacto agar-based gel polymer electrolyte. Ionics 18:359–364
Guiry MD, Guiry GM (2012) Algae base world-wide electronic publication, National University of Ireland Galway. http://www.algaebase.org/
Liang L, Ni R, Yang S, Mao S (2014) Carrageenan and its applications in drug delivery. Carbohydr Polym 103:1–11
Campo VL, Kawano DF, da Silva DB Jr, Carvalho I (2009) Carrageenans: biological properties, chemical modifications and structural analysis—a review. Carbohydr Polym 77:167–180
Shamsudin IJ, Ahmad A, Hassan NH, Kaddami H (2016) Biopolymer electrolytes based on carboxymethyl–carrageenan and imidazolium ionic liquid. Ionics 22:841–851
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting κ-carrageenan chitosan electrolytes. Funct Mater Lett 1:195–201
Selvasekarapandian S, Hirankumar G, Kawamura J, Kuwata N, Hattori T (2005) 1H solid state NMR studies on the proton conducting polymer electrolytes. Mater Lett 59:2741–2745
Srivastava N, Chandra S (2000) Studies on a new proton conducting polymer system poly(ethylene succinate) + NH4ClO4. Eur Polym J 36:421–433
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting κ carrageenan–chitosan electrolytes. Funct Mater Lett 1:195–201
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi G, Aristatil G, Arun A, Madeswaran S (2016) Proton-conducting I-carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23:2775–2780
Noor NAM, Isa MIN (2015) Ionic conductivity and dielectric properties of CMC doped NH4SCN solid biopolymer electrolytes. Adv Mater Res 1107:230–235
Pascalau V, Popescu V, Popescu GL, Dudescu MC, Borodi G, Dinescu A, Perhait¸ I, Paul M (2012) The alginate/k-carrageenan ratio’s influence on the properties of the cross-linked composite films. J Alloy Compd 536(Supplement1):418–423
Martins JT, Cerqueira MA, Bourbon AI, Pinheiro AC, Bartolomeu Souza WS, Antonio A (2012) Vicente synergistic effects between K-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll 29:280–289
Cerqueira MA, Souza BWS, Simoes J, Teixeira JA, Domingues MR, Coimbra MA (2011) Structural and thermal characterization of galactomannans from non-conventional sources. Carbohydr Polym 83(1):179–185
Feng W, Feng S, Tang K, He X, Jing A, Liang G (2017) A novel composite of collagen-hydroxyapatite/kappa-carrageenan. J Alloy Compd 693:482–489
Daniel-da-Silva A, Lopes A, Gil A, Correia R (2007) Synthesis and characterization of porous k-carrageenan/calcium phosphate nanocomposite scaffolds. J Mater Sci 42:8581–8591
Lima PH, Pereira SV, Rabello RB, Rodriguez-Castellon E, Beppu MM, Chevallier P (2013) Blood protein adsorption on sulfonated chitosan and kappa-carrageenan films. Colloids Surf B Biointerfaces 111:719–725
PradoFern andez J, Rodrıguez JA, azquez V, Tojo E, Andrade JM (2003) Quantitation of k, i and l-carrageenans by mid-infrared spectroscopy and PLS regression. Anal Chim Acta 480:23–37
Selvalakshmi S, Vijaya N, Selvasekarapandian S, Premalatha M (2017) Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J Appl Polym Sci 134. https://doi.org/10.1002/app.44702
Sanchis MJ, Cars M, Culebras M, Gomez CM, Rodriguez S and Torres FG (2017) Molecular dynamics of carrageenan composites reinforced with Cloisite Na+ montmorillonite nanoclayz. https://doi.org/10.1016/j.carbpol.2017.08.012
Rajeswari N, Selvasekarapandian S, Karthikeyan S, Prabu M, Hirankumar G, Nithya H, Sanjeeviraja C (2011) Conductivity and dielectric properties of polyvinyl alcohol–polyvinyl pyrrolidone poly blend film using non-aqueous medium. J Non-Cryst Solids 357:3751–3756
Hashmi SA, Kumar A, Maurya KK, Chandra S (1990) Proton-conducting polymer electrolyte I: the polyethylene oxide + NH4CIO4 system. J Phys D Appl Phys 23:1307–1314
Ye Z, Ma P, Mi T, Li X, Zhang W, Hong X, Chen X, Chen D (2017) Interactions between calcium alginate and carrageenan enhanced mechanical property of a natural composite film for general packaging application. Polym Bull 74:3421–3429
Justina Gaidukevic, Julija Razumiene, Ieva Sakinyte, Susana L.H. Rebelo, Jurgis Barkauskas (2017) Study on the structure and electrocatalytic activity of graphene-based nanocomposite materials containing (SCN)n Carbon 118: 156–167
Latha C, Venkatachalam K (2017) Structural, vibrational, thermal, electrical properties of PVP–PVC blend NH4SCN. Polym Bull 74:3123–3137
Boukamp BA (1986) A package for impedance/admittance analysis. Solid State Ionics 18–19:136–140
Woo HJ, Majid SR, Arof AK (2011) Conduction and thermal properties of a proton conducting polymer electrolyte based on poly (ε-caprolactone). Solid State Ionics 199-200:14–20
Hamsan MH, Shukur MF, Kadir MFZ (2017) The effect of NH4NO3 towards the conductivity enhancement and electrical behavior in methyl cellulose-starch blend based ionic conductors. Ionics 23:1137–1154
Boopathi G, Pugalendhi S, Selvasekarapandian S, Premalatha M, Monisha S, Aristatil G (2016) Development of proton conducting biopolymer membrane based on agar–agar for fuel cell. Ionics 23:2781–2790
Nithya H, Selvasekarapandian S, Christopher Selvin P, Arun Kumar D, Hema M, Kawamura J (2012) Laser raman and conductivity studies of plasticized polymer electrolyte P(ECH-EO):propylenecarbonate:Υ-butyrolactone:LiClO4. J Solid State Electrochem 16:1971–1797
Sikkanthar S, Karthikeyan S, Selvasekarapandian S, Arunkumar D, Nithya H, Kawamura J (2016) Structural, electrical conductivity and transport analysis of PAN–NH4Cl polymer electrolyte system. Ionics 22:1085–1094
Lunkenheimer P, Bobnar V, Pronin AV, Ritus AI, Volkov AA, Loidl A (2002) Origin of apparent colossal dielectric constants. Phys Rev B 66:052105
Anbazhakan K, Selvasekarapandiyan S, Monisha S, Premalatha M, Neelaveni A (2017) Lithium ion conductivity and dielectric properties of P(VdCl-co-AN-co-MMA)-LiCl-EC triblock co-polymer electrolytes. Ionics 23:2663–2668
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Aristatil G, Mani N, Premalatha M, Vinoth Pandi D (2017) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohyd Polym 157:38–47
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christopher Selvin, P., Perumal, P., Selvasekarapandian, S. et al. Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics 24, 3535–3542 (2018). https://doi.org/10.1007/s11581-018-2521-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2521-7