Abstract
Quasi-solid bioelectrolytes based on hydroxyethyl cellulose (HEC) and sodium iodide (NaI) in three different polar aprotic solvent systems, dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and dimethylacetamide (DMA), were fabricated and characterized. FTIR studies revealed active solvent-ion interactions in DMF-based electrolytes in comparison to DMA and DMSO. The effect of the solvent system on the crystallinity of HEC gel electrolytes was more significant at low NaI concentration. In each solvent system, the highest ionic conductivity was achieved at 70 wt% NaI and generally DMF-based electrolytes showed higher conductivity than the other solvents. The availability of multiple complexation sites present in DMF is ascribed to improvement in ion mobility and hence conductivity. Rheological analysis was carried out to elucidate the mechanical properties of the gels. Generally, the mechanical strength of the polymer gels was unaffected by the type of solvent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ever since its introduction in 1970s, polymer electrolyte has been an active field of research. The implementation of solid polymer electrolytes has provided the solutions to pragmatic problems encountered in traditional liquid electrolyte such as leakage and solvent volatility [1]. However, this is often achieved at the expense of ionic conductivity which in turn affects the electrolyte performance in electrochemical devices [2]. This contradiction gave rise to the inception of gel polymer electrolyte (GPE), a unique category of electrolyte which inherits both the conductive properties of liquid and mechanical properties of solid.
Typically, the preparation of GPE involves the dissolution of inorganic salt and polymer in a non-volatile solvent system. The primary function of the polymer is to serve as the matrix of the gel in order to bestow mechanical stability. Addition of inorganic salts introduces charge carriers into the matrix which elevates the ionic conductivity of the electrolyte. Most often, the major composition of the electrolyte is made of the solvent component which is regarded as packets of liquid trapped within the polymer chains. The solvent in fact is a crucial part of the system as it affords the space for charge carrier migration, diminishes crystallinity of the gel, decreases polymer-polymer interaction, and increases the free volume and segmental mobility of the system [3]. Therefore, the choice of solvent employed in a GPE is very salient as it impacts the overall physical, chemical, and electrical properties of the material. In recent literature, much emphasis has been given to explore the effect of polymers and salts on the properties of the electrolyte [1, 4,5,6]. However, studies dedicated to comprehend the effect of solvents on the electrolyte properties have been scarce. Therefore, in this study, we explored the effects of three different polar aprotic solvents on fabrication of GPE based on hydroxyethyl cellulose (HEC).
In accordance to the current pursuit for sustainable materials, polymer electrolytes fabricated from natural polymers, in particular, cellulose derivatives, have obtained special focus recently. Cellulose derivatives are often incorporated into a synthetic polymer system with the purpose of improving the mechanical properties of the materials while at the same time reducing its ecological footprint. Sato et al. found that combining cyanoethylated cellulose, which has a rigid backbone, with cross-linkable methacrylate monomers produces GPE of considerable mechanical strength even at polymer concentration of 7 wt%. The interaction between the highly polar polymer matrixes that included a cellulose derivative with high dielectric constant with the liquid electrolyte aided in retarding electrolyte evaporation in the gel [7]. A similar approach has been done by Nirmale et al. who fabricated GPE based on photo-induced in situ polymerization of PEG-methacrylates along with cellulose triacetate (TCA). The presence of TCA improved ionic conductivity, owing to its ether and carbonyl functional groups [8]. There also have been reports of GPE solely based on cellulose derivatives such as methyl cellulose, carboxymethyl cellulose, hydroxypropyl cellulose, and cyanoethylated hydroxypropyl cellulose [9,10,11,12]. These gel electrolytes attained room temperature conductivities between 10−4 and 10−3 S cm−1 and concurrently exhibited good electrochemical, mechanical, and thermal stability.
HEC is widely recognized as a gelling and thickening agent in cosmetics, pharmaceutical, and paint industry [13]. Recent literatures have also highlighted the prospects of HEC as polymer electrolyte material with good thermal stability and favorable electrochemical performance [14,15,16,17]. According to Zhang et al., a dense HEC membrane sandwiched between two porous PVDF layers helps to avoid micro short-circuits to a large extent which is crucial to improve the safety of the electrochemical device [17]. The attachment of hydroxyethyl group onto the cellulose backbone in HEC imparts organosolubility. This enables the incorporation of various organic solvents for the fabrication of GPE based on the biopolymer. For instance, Li et al. prepared gel membrane by soaking the HEC membrane in organic electrolyte consisting of LiPF6 solution in ethylene carbonate/dimethyl carbonate/ethyl methyl carbonate [18]. The electrolyte showed uptake of organic liquid electrolyte up to 78.3 wt% and good electrochemical performance including high ionic conductivity at room temperature and a high lithium ion transference number. Due to this ability to dissolve in various organic solvents, HEC was utilized as the polymer host in this work.
Another unique aspect in this work is the employment of sodium salt as the charge carrier instead of its widely recognized counterpart, lithium ions. Although lithium salt is efficient in achieving high ionic conductivities, but its long-term availability and cost continue to be major drawbacks [19]. The low-cost, naturally abundant, and non-toxic properties of sodium make it the most practical alternative to lithium [20]. In fact, recent studies by Vondrak et al. and Zurina et al. have reported higher conductivity in sodium-based electrolytes than that in lithium [21, 22]. It has been observed that the mobility of smaller ions is lower than that of cations with larger ions in the gel polymer electrolytes and it is hypothesized that smaller cations tend to be embedded or captured by the polymeric network, and thus their mobility is lowered.
Materials and methods
Materials
Hydroxyethyl cellulose (HEC), dimethylformamide (DMF), dimethylsulfoxide (DMSO), and dimethylacetamide (DMA) were purchased from Merck. Sodium iodide (NaI) salt used was procured from Fisher Chemicals. The materials were used as received.
Preparation of gel polymer electrolytes
Series of gel electrolytes based on three different types of polar aprotic solvents, DMF, DMSO, and DMA, were prepared. In all three systems, the polymer to solvent weight ratio was fixed at 1:15 and required amounts of NaI (10–90 wt%) were added to prepare respective series of GPE. Initially, the salt was dissolved in the respective solvents forming a homogenous solution into which HEC was added. The mixture was then stirred and heated at 70 °C for 6 h.
Characterization
Fourier-transform infrared spectroscopy (FTIR) of the samples was analyzed with a Perkin-Elmer Spectrum 400 spectrometer via the ATR technique. The analysis was done at a spectral range from 650 to 4000 cm−1 with a resolution of 4 cm−1 and scan number of 32.
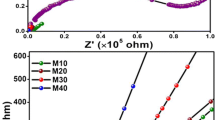
For the electrochemical impedance spectroscopy (EIS) analysis, the impedance data for the samples were measured using a HIOKI 3532-50 LCR Hi-Tester with a frequency range of 50 Hz to 200 kHz at room temperature. The corresponding ionic conductivity, σ, was then calculated by the equation: σ=t/(RbA) where t is thickness of the sample measured, A is the contact surface area, and Rb is the bulk resistance of the sample.
The electrochemical stability window of the GPE was measured using the linear sweep voltammetry (LSV) technique. The LSV was carried out using a SS/electrolyte/SS cell, where SS stands for stainless steel, at a scanning rate of 1 mV s−1 from 0 to 4.0 V.
To confirm the effect of the polar aprotic solvents on the crystallinity of gels, X-ray diffraction (XRD) spectra of the samples were recorded on a PANalytical X’Pert3 MRD diffractometer. The gel samples were uniformly applied on the surface of the sample holder which was then placed in the diffractometer, and the samples were directly scanned at 2θ angles between 5° and 60°.
The rheological tests were performed using an Anton Paar Physica MCR 301 rheometer with a parallel-plate geometry (2.5-cm diameter). Amplitude sweep tests of the gel samples were studied by recording the variations in G′ and G″ modulus as a function of strain ranging from 0.1 to 250% at a measuring gap of 0.075 mm. Operation of the rheometer and analysis of the rheological parameters (G′, G″, tan δ) were carried out using the Rheoplus/32 V3.60 software.
Results and discussion
FTIR analysis
The possible chemical interactions between the salt, polymer, and solvents were studied via FTIR analysis. Owing to the fact that a 1:15 polymer to solvent ratio was used in the electrolyte preparation, the bands attributed to HEC are mostly overshadowed by the intense bands of the solvents. Therefore, the characteristic absorption bands attributed to reactive groups in the solvents with increasing salt concentration are mainly analyzed to observe the sites of chemical interactions.
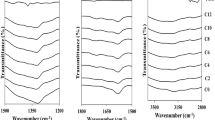
Figure 1 shows the FTIR spectra of DMF-based GPE. The position of absorption bands for C=O stretch, N–(CH3) deformation mode, and O=C–N deformation of DMF molecules is clearly observed at 1661, 1384, and 654 cm−1, respectively [23]. The strongest absorption band in the samples is the one attributed to the most polar group present, C=O stretch of DMF. This band also exhibited a downshift from 1661 cm−1 in blank gels to 1650 cm−1 in sample with 90 wt% NaI. The shift to a lower frequency indicates the interaction between the carbonyl group and the Na+ ions which has a polarizing effect on the electron density of the bond [24]. The O=C–N deformation band does not exhibit any appreciable shift. The effect of the increasing salt concentration on the N–(CH3) deformation bands slightly shifts to a higher wavenumber and such trend was also reported in the FTIR study of ion-solvent interactions in DMA by D.M. Verbovy et al. [24]. It is presumed that this trend is indicative of Na+ cations interaction with the nitrogen atom and positive inductive effect from the methyl group.
As shown in Fig. 2, the FTIR spectra of DMA-based GPEs have similar characteristic bands as DMF-based electrolytes and this is expected as the chemical structures of DMA and DMF are very similar and only differ by the presence of an additional methyl group attached to the carbonyl. Upon addition of salt, the downshift of the C=O stretch is similar to that of DMF but the magnitude of the shift which is from 1624 to 1617 cm−1 is smaller and this maybe an implication of the greater steric hindrance imposed by the larger size of DMA molecules. The N–(CH3) deformation bands also show the same upward shift in frequency from 1394 to 1403 cm−1 as observed in DMF system.
In DMSO-based GPEs, typical absorption bands are observed at 1022, 698, and 667 cm−1 which correspond to S=O stretch and symmetrical and asymmetrical S-C vibrations, respectively (Fig. 3) [25]. However, with increasing amount of salt, only the S=O stretching band shows appreciable shift from 1022 to 1015 cm−1 which can be attributed to cation-sulfonyl association.
Based on these FTIR peak interpretations, the carbonyl and amide groups in the solvents are identified as the main ion complexation sites and Scheme 1 summarizes the possible chemical interactions that are present in the gel matrices.
Crystallinity
The XRD diffractograms (Fig. 4a) of HEC exhibit an intense diffraction peak at around 2θ of 21.5° which indicates the characteristic diffractive peak of a cellulose I structure [26]. As shown in Fig. 4, the intensity of this peak was found to decline in blank HEC gel electrolytes in all three solvent systems. This observation signifies diminished intermolecular hydrogen bonding between the polymer chains due to chemical associations with the solvent molecules. Up to 30 wt% of NaI, DMSO-based GPE shows the lowest crystallinity followed by DMA and DMF and this can be explained in terms of polarity of the sulfonyl group opposite to that of carbonyl group. The sulfonyl group in DMSO is more polar compared to the carbonyl group in DMA and DMF which may give rise to stronger hydrogen bonding of the oxygen atoms with the hydroxyl of HEC, hence reducing their availability for polymer-polymer hydrogen bonding. However, it is interesting to note that beyond 30 wt% of NaI, the diffractograms of DMSO-and DMF-based gels depict similar intensity at 2θ of 21.5°. The small sized Na+ and I− ions are more efficient in disrupting the intermolecular polymer-polymer hydrogen bonding compared to the large solvent molecules. Thus, at high salt concentration, the expected effect of solvent polarity on crystallinity is renounced. In all three solvent systems, gradual incorporation of NaI has resulted in diminishing crystalline peaks. Both DMSO- and DMA-based polymer gels show declined crystallinity up to 90 wt% of NaI, but DMA-based polymer electrolytes show a slight increase in the peak intensity beyond 70 wt% of NaI. This slight increase may be attributed to the occurrence of undissociated salt at higher salt concentration due to occupancy of the complexation sites. The salt aggregates may be absorbed by adjacent polymer chains and thus restricting their segmental motion [27].
Conductivity studies
Figure 5 shows the trend in ionic conductivity at ambient temperature for all three solvent systems. In blank gels, DMSO-based polymer electrolyte recorded the highest value followed by DMA and DMF. This relates with the XRD diffractograms of the blank gels which shows the lowest crystallinity for DMSO. With the introduction of NaI, the ionic conductivity of DMF-based electrolytes emerges to be the highest among all three systems. From the equation, σ = nμe, the value of ionic conductivity is dominated by number of mobile ions (n) and mobility (μ). In this study, the gel electrolytes are formed by the trapping of solvent within the HEC matrix [28, 29]. Thus, the polymer chains behave as a rigid framework in which mobility occurs mainly through the interaction with the active groups of the solvent [30]. As depicted in Scheme 1, in DMF, both the polar oxygen and nitrogen serve as points of migration as opposed to only oxygen in DMSO. Thus, for the same wt% of NaI, DMF-based electrolytes have higher ionic mobility and thus higher ionic conductivity compared to DMSO. Although DMA also possesses both oxygen and nitrogen in its structure, the additional methyl group attached to the carbonyl contributes to increased steric hindrance. In all three solvent systems, the highest ionic conductivity is recorded upon inclusion of 70 wt% of NaI with values of 2.16 × 10−2 S cm−1, 1.24 × 10−2 S cm−1, and 1.12 × 10−2 S cm−1 for DMF, DMA, and DMSO, respectively. The decline in conductivity values beyond 70 wt% of NaI can be the effect of salt agglomeration. As the salt concentration exceeds an optimum value, the chances of ion association to form neutral ion pair increase. Since these ion aggregates do not contribute toward ion conduction, it will lead to a decline in conductivity value [31].
A brief summary of some recent work on sodium ion-based gel polymer electrolyte is listed in Table 1. To the best of our knowledge, sodium ion-based GPE systems utilizing biopolymers are still scarce and from the table, it is evident that the ionic conductivity attained in this work is better than that in most other synthetic polymer GPEs.
Linear sweep voltammetry studies
During fabrication of electrolyte, it is vital to evaluate its electrochemical potential window as this parameter determines the maximum operational voltage of the electrolyte. The potential window, which can be obtained from LSV measurements, decides the applicability of the electrolyte in various electrochemical devices. Figure 6 shows the LSV curve of the highest conducting samples in each solvent system. All three samples have similar decomposition voltage of around 2.8 V. In previous literatures, polymer electrolyte based on cellulose such as carboxymethyl cellulose and sulfonated bacterial cellulose has been reported to have decomposition voltage of 2.5 and 1.9 V respectively [38, 39]. The wider electrochemical window recorded by the HEC gel electrolytes shows that the electrolyte in this study can be employed in devices such as EDLC and batteries.
Rheological analysis
The GPE system is an improvement over previous generations of liquid electrolyte systems whereupon it rectifies the stability issues encountered in the latter. Therefore, alongside superior electrical properties, mechanical stability of the gel is also a significant aspect in the fabrication polymer electrolytes. While mechanical properties have been frequently studied in solid polymer electrolytes, the emphasis on studying the mechanical stability of GPE has been limited. In this work, rheological study has been utilized as a tool to gauge the mechanical properties of the GPE. The highest conducting sample in each solvent system has been subjected to amplitude sweep test. The trend in storage modulus (G′) and loss modulus (G″) with the variation in strain is analyzed to comprehend the viscoelastic properties of the gels.
As shown in Fig. 7, HEC gels with 70 wt% of NaI in DMF, DMA, and DMSO exhibit a sustained linear viscoelastic response up to 100% strain indicating minimal disruption of the network structure. Beyond 100% strain, both storage and loss moduli begin to drop slightly, suggesting a collapse in the gel structure. According to the rheological characterization of polymer gels proposed by Kavanagh and Ross-Murphy, HEC-based polymer gels are categorized as biopolymer physical gels [40]. The term physical gel here is defined as physically cross-linked polymer chains in which the gel formation is governed by non-covalent interactions such as van der Waals, charge transfer, and hydrogen bonding. Beyond the critical strain value, these interactions are disrupted leading to a collapse in the gel structure [41].
Besides the critical strain values, the physical properties of the gel microstructure can be explained in terms of the loss factor, tan δ, which is equals to the ratio of G″ to G′, i.e., the imaginary part to the real part of the deformation energy. When tan δ < 1, G′ dominates over G″, and the gel exhibits a quasi-elastic behavior in which the gel experiences full or partial microstructural regeneration upon deformation. When tan δ = 1, the flow point (“cross-over”) is attained. Beyond the flow point, G″ exceeds G′ (tan δ > 1) and the viscous property of the gel prevails leading to an irreversible microstructural collapse [42]. In all three samples studied, tan δ > 1 when the strain applied was close to 250%. Therefore, it can be concluded that HEC-based gels have similar mechanical strength regardless of the solvent system. This can be also due to the fact that the polymer to solvent weight ratio was similar in all three samples.
Conclusions
FTIR studies confirmed the enhanced interaction between ions and solvents in DMF-based electrolytes compared to DMA- and DMSO-based electrolytes. The crystallinity of HEC gel electrolytes was found to be influenced by the solvent system. At low salt concentration, DMSO-based electrolytes exhibited lower crystallinity compared to DMF and DMA. But, at higher salt concentration, this effect was renounced. The highest ionic conductivity was recorded upon 70 wt% of NaI in each solvent system, with overall maximum value of 2.16 × 10−2 S cm−1 in DMF. The pronounced ionic conductivity in DMF-based system can be attributed to the presence of additional ion complexation sites in this solvent compared to DMA and DMSO. From rheological analysis, it was found that the highest conducting HEC electrolytes in each solvent system showed prominent solid character and the mechanical stabilities of these gels were not affected by the solvent systems.
References
Varshney PK, Gupta S (2011) Natural polymer-based electrolytes for electrochemical devices: a review. Ionics 17(6):479–483. https://doi.org/10.1007/s11581-011-0563-1
Di Noto V, Lavina S, Giffin GA, Negro E, Scrosati B (2011) Polymer electrolytes: present, past and future. Electrochimica Acta 57(supplement C):4–13. https://doi.org/10.1016/j.electacta.2011.08.048
Wu J, Lan Z, Lin J, Huang M, Huang Y, Fan L, Luo G (2015) Electrolytes in dye-sensitized solar cells. Chem Rev 115(5):2136–2173. https://doi.org/10.1021/cr400675m
Long L, Wang S, Xiao M, Meng Y (2016) Polymer electrolytes for lithium polymer batteries. J Mater Chem A 4(26):10038–10069. https://doi.org/10.1039/C6TA02621D
Ngai KS, Ramesh S, Ramesh K, Juan JC (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics 22(8):1259–1279. https://doi.org/10.1007/s11581-016-1756-4
Song JY, Wang YY, Wan CC (1999) Review of gel-type polymer electrolytes for lithium-ion batteries. J Power Sources 77(2):183–197. https://doi.org/10.1016/S0378-7753(98)00193-1
Sato T, Banno K, Maruo T, Nozu R (2005) New design for a safe lithium-ion gel polymer battery. J Power Sources 152(supplement C):264–271. https://doi.org/10.1016/j.jpowsour.2005.03.212
Nirmale TC, Karbhal I, Kalubarme RS, Shelke MV, Varma AJ, Kale BB (2017) Facile synthesis of unique cellulose triacetate based flexible and high performance gel polymer electrolyte for lithium ion batteries. ACS Appl Mater Interfaces 9(40):34773–34782. https://doi.org/10.1021/acsami.7b07020
Ahmad NH, Isa MIN (2016) Characterization of un-plasticized and propylene carbonate plasticized carboxymethyl cellulose doped ammonium chloride solid biopolymer electrolytes. Carbohydr Polym 137:426–432. https://doi.org/10.1016/j.carbpol.2015.10.092
Huang X, Liu Y, Deng J, Yi B, Yu X, Shen P, Tan S (2012) A novel polymer gel electrolyte based on cyanoethylated cellulose for dye-sensitized solar cells. Electrochimica Acta 80:219–226. https://doi.org/10.1016/j.electacta.2012.07.014
Ledwon P, Andrade JR, Lapkowski M, Pawlicka A (2015) Hydroxypropyl cellulose-based gel electrolyte for electrochromic devices. Electrochimica Acta 159:227–233. https://doi.org/10.1016/j.electacta.2015.01.168
Xiao S, Wang F, Yang Y, Chang Z, Wu Y (2014) An environmentally friendly and economic membrane based on cellulose as a gel polymer electrolyte for lithium ion batteries. RSC Adv 4(1):76–81. https://doi.org/10.1039/C3RA46115G
Mudgil D, Barak S, Khatkar BS (2014) Guar gum: processing, properties and food applications—a review. J Food Sci Technol 51(3):409–418. https://doi.org/10.1007/s13197-011-0522-x
Chong MY, Numan A, Liew C-W, Ramesh K, Ramesh S (2017) Comparison of the performance of copper oxide and yttrium oxide nanoparticle based hydroxylethyl cellulose electrolytes for supercapacitors. J Appl Polym Sci 134(13):44636. https://doi.org/10.1002/app.44636
Gupta S, Varshney PK (2017) Effect of plasticizer concentration on structural and electrical properties of hydroxyethyl cellulose (HEC)-based polymer electrolyte. Ionics 23(6):1613–1617. https://doi.org/10.1007/s11581-017-2116-8
Sudhakar YN, Selvakumar M, Bhat DK (2015) Preparation and characterization of phosphoric acid-doped hydroxyethyl cellulose electrolyte for use in supercapacitor. Materials Renewable Sustainable Energy 4(3):10. https://doi.org/10.1007/s40243-015-0051-z
Zhang MY, Li MX, Chang Z, Wang YF, Gao J, Zhu YS, Wu YP, Huang W (2017) A sandwich PVDF/HEC/PVDF gel polymer electrolyte for lithium ion battery. Electrochimica Acta 245(supplement C):752–759. https://doi.org/10.1016/j.electacta.2017.05.154
Li MX, Wang XW, Yang YQ, Chang Z, Wu YP, Holze R (2015) A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. J Membrane Science 476(supplement C):112–118. https://doi.org/10.1016/j.memsci.2014.10.056
Ellis BL, Nazar LF (2012) Sodium and sodium-ion energy storage batteries. Current Opinion Solid State Materials Sci 16(4):168–177. https://doi.org/10.1016/j.cossms.2012.04.002
Dell RM (2000) Batteries: fifty years of materials development. Solid State Ionics 134(1):139–158. https://doi.org/10.1016/S0167-2738(00)00722-0
Osman Z, Md Isa KB, Ahmad A, Othman L (2010) A comparative study of lithium and sodium salts in PAN-based ion conducting polymer electrolytes. Ionics 16(5):431–435. https://doi.org/10.1007/s11581-009-0410-9
Vondrák J, Reiter J, Velická J, Sedlařı́ková M (2004) PMMA-based aprotic gel electrolytes. Solid State Ionics 170(1):79–82. https://doi.org/10.1016/j.ssi.2003.08.060
Zhang B, Zhou Y, Li X, Wang J, Li G, Yun Q, Wang X (2014) Li+−molecule interactions of lithium tetrafluoroborate in propylene carbonate + N,N-dimethylformamide mixtures: an FTIR spectroscopic study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 124(Supplement C):40–45. https://doi.org/10.1016/j.saa.2014.01.001
Verbovy DM, Smagala TG, Brynda MA, Fawcett WR (2006) A FTIR study of ion-solvent interactions in N,N-dimethylacetamide. J Molecular Liquids 129(1):13–17. https://doi.org/10.1016/j.molliq.2006.08.008
Kloss AA, Fawcett WR (1998) ATR-FTIR studies of ionic solvation and ion-pairing in dimethyl sulfoxide solutions of the alkali metal nitrates. J Chem Soc Faraday Trans 94(11):1587–1591. https://doi.org/10.1039/A800427G
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angewandte Chemie 44(22):3358–3303. https://doi.org/10.1002/anie.200460587
He R, Kyu T (2016) Effect of plasticization on ionic conductivity enhancement in relation to glass transition temperature of crosslinked polymer electrolyte membranes. Macromolecules 49(15):5637–5648. https://doi.org/10.1021/acs.macromol.6b00918
Dissanayake MAKL, Thotawatthage CA, Senadeera GKR, Bandara TMWJ, WJMJSR J, Mellander BE (2012) Efficiency enhancement by mixed cation effect in dye-sensitized solar cells with PAN based gel polymer electrolyte. J Photochemistry and Photobiology A: Chemistry 246:29–35. https://doi.org/10.1016/j.jphotochem.2012.06.023
Yusuf SNF, Azzahari AD, Yahya R, Majid SR, Careem MA, Arof AK (2016) From crab shell to solar cell: a gel polymer electrolyte based on N-phthaloylchitosan and its application in dye-sensitized solar cells. RSC Adv 6(33):27714–27724. https://doi.org/10.1039/C6RA04188D
Yusuf SNF, Azzahari AD, Selvanathan V, Yahya R, Careem MA, Arof AK (2017) Improvement of N-phthaloylchitosan based gel polymer electrolyte in dye-sensitized solar cells using a binary salt system. Carbohydrate Polymers 157:938–944. https://doi.org/10.1016/j.carbpol.2016.10.032
Shukur MF, Ibrahim FM, Majid NA, Ithnin R, Kadir MFZ (2013) Electrical analysis of amorphous corn starch-based polymer electrolyte membranes doped with LiI. Phys Scr 88(2):025601. https://doi.org/10.1088/0031-8949/88/02/025601
Yang YQ, Chang Z, Li MX, Wang XW, Wu YP (2015) A sodium ion conducting gel polymer electrolyte. Solid State Ionics 269(supplement C):1–7. https://doi.org/10.1016/j.ssi.2014.11.015
Xue Y (2017) Electrochemical and mechanical properties of sodium-ion conducting cross-linked polymer gel electrolyte, vol 12. doi:https://doi.org/10.20964/2017.11.56
Vanitha D, Bahadur SA, Nallamuthu N, Athimoolam S, Manikandan A (2017) Electrical impedance studies on sodium ion conducting composite blend polymer electrolyte. J Inorg Organomet Polym Mater 27(1):257–265. https://doi.org/10.1007/s10904-016-0468-6
Kumar D, Hashmi SA (2010) Ionic liquid based sodium ion conducting gel polymer electrolytes. Solid State Ionics 181(8):416–423. https://doi.org/10.1016/j.ssi.2010.01.025
Zebardastan N, Khanmirzaei MH, Ramesh S, Ramesh K (2017) Presence of NaI in PEO/PVdF-HFP blend based gel polymer electrolytes for fabrication of dye-sensitized solar cells. Materials Sci Semiconductor Processing 66(supplement C):144–148. https://doi.org/10.1016/j.mssp.2017.04.016
Subban RHY, Arof AK (1996) Sodium iodide added chitosan electrolyte film for polymer batteries. Phys Scr 53(3):382–384. https://doi.org/10.1088/0031-8949/53/3/021
Rani M, Rudhziah S, Ahmad A, Mohamed N (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 6(9):2371–2385. https://doi.org/10.3390/polym6092371
Yue L, Xie Y, Zheng Y, He W, Guo S, Sun Y, Zhang T, Liu S (2017) Sulfonated bacterial cellulose/polyaniline composite membrane for use as gel polymer electrolyte. Composites Sci Technol 145(supplement C):122–131. https://doi.org/10.1016/j.compscitech.2017.04.002
Kavanagh GM, Ross-Murphy SB (1998) Rheological characterisation of polymer gels. Progress Polymer Sci 23(3):533–562. https://doi.org/10.1016/S0079-6700(97)00047-6
Stoppe N, Horn R (2017) How far are rheological parameters from amplitude sweep tests predictable using common physicochemical soil properties? J Phys Conf Ser 790(1). https://doi.org/10.1088/1742-6596/790/1/012032
Mezger TG (2011) The rheology handbook: for users of rotational and oscillatory rheometers. Vincentz Network, Hannover
Funding
This work is supported by the University of Malaya through FG031-17AFR grant. V. Selvanathan is grateful to the Ministry of Higher Education (MOHE), Malaysia, for the MyBrainSc scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Selvanathan, V., Halim, M.N.A., Azzahari, A.D. et al. Effect of polar aprotic solvents on hydroxyethyl cellulose-based gel polymer electrolyte. Ionics 24, 1955–1964 (2018). https://doi.org/10.1007/s11581-018-2455-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2455-0