Abstract
The conducting polymer electrolyte films consisting of polyacrylonitrile (PAN) as the host polymer, lithium triflate (LiCF3SO3) and sodium triflate (NaCF3SO3) as inorganic salts were prepared by the solution-cast technique. The pure PAN film was prepared as a reference. The ionic conductivity for the films is characterized using impedance spectroscopy. The room temperature conductivity for the PAN + 26 wt.% LiCF3SO3 film and the PAN + 24 wt.% NaCF3SO3 film is 3.04 × 10−4 S cm−1 and 7.13 × 10−4 S cm−1, respectively. XRD studies show that the complexation that has occurred in the PAN containing salt films and complexes formed are amorphous. The FTIR spectra results confirmed the complexation has taken place between the salt and the polymer. These results correspond with surface morphology images obtained from SEM analysis. The conductivity–temperature dependence of the highest conducting film from PAN + LiCF3SO3 and PAN + NaCF3SO3 systems follows Arrhenius equation in the temperature range of 303 to 353 K. The PAN containing 24 wt.% LiCF3SO3 film has a higher ionic conductivity and lower activation energy compared to the PAN containing 26 wt.%LiCF3SO3 film. These results can be explained based on the Lewis acidity of the alkali ions, i.e., the interaction between Li+ ion and the nitrogen atom of PAN is stronger than that of Na+ ion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer electrolytes have received much attention due to their potential applications in rechargeable batteries and other electrochemical devices [1–7]. Most research activities on polymer electrolytes have been concentrated on PEO-based electrolyte systems during the last few decades following the initial discovery of Wright [8] that PEO formed crystalline complexes with alkali metal salts. The pioneering work of Armand and his collaborators [9] then led to the development of polymer-based electrolyte for battery applications. Compared to PEO-based electrolytes, the PAN-based lithium salt complexes possess many advantages, such as higher conductivity and good mechanical properties at room temperature. Although several reports on PEO-based polymer electrolytes can be found in the literature [10–12], to our knowledge, no systematic conductivity study on PEO containing sodium triflate has been reported.
Ionic mobility and charge carrier concentration are two important factors which influence the conductivity of the electrolyte. It has been observed by Vondrák et al. [13] that the mobility of smaller ions Li+ and/or Mg2+ is lower than that of cations with larger ions Na+ and/or Zn2+ in the gel polymer electrolytes. They noted that the resistivity of a gel with a smaller cation is higher than that of a gel containing a larger cation. Hence, the mobility of Li+ is lower than that of Na+ and the same relation is found between salts containing Mg2+ and Zn2+, respectively. The conductivity data obtained by them also supported the hypothesis that smaller cations are embedded or captured by the polymeric network, and their mobility is lowered. The conductivity of charge carriers present in an electrolyte generally depends upon the concentration of salt containing the mobile species as well as on the extent up to which the salt is dissociated.
The aim of this work is to investigate the effect of lithium triflate (LiCF3SO3) and sodium triflate (NaCF3SO3) on ionic conductivity, surface morphology, complexation and the interactions with the polymer PAN by using impedance spectroscopy, X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM).
Experimental methods
Sample preparation
Polyacrylonitrile (PAN), with molecular weight of 150,000 g/mol, lithium triflate (LiCF3SO3), sodium triflate (NaCF3SO3), and dimethylformamide (DMF) were obtained from Aldrich. PAN was dissolved in DMF and the mixture was stirred at 60 °C until the solution turned into a clear and homogeneous. LiCF3SO3 and NaCF3SO3 salts were added accordingly. The mixtures were continuously stirred for several hours. After complete dissolution, the solutions were cast in petri dishes and left to dry under vacuum at 50 °C for 48 h until the films were formed. Further drying was achieved for 24 h at 80 °C to remove the residual solvent. The DMF residue in the films estimated from thermogravimetric analysis (TGA) was less than 5 wt.%. The films were then kept in a desiccator for further drying until the characterizations are to be carried out.
Characterization techniques
Impedance spectroscopy measurements were used to determine the conductivity of the films. The films were cut into a round shape that fit the size of the electrodes. The films were then sandwiched between the two stainless steel blocking electrodes with a diameter of 2 cm. A HIOKI 3532 LCR bridge that has been interfaced with a computer was used to perform the impedance measurement for each polymer electrolyte film in the frequency range of 50 Hz to 1 MHz. From the impedance plots obtained, the bulk resistance, R b of each sample was determined and hence the conductivity (σ) of the samples was then calculated using σ = t/R b A; where t is the sample thickness (cm), A the effective contact area of the electrode and the sample (cm2), and R b is the bulk resistance (Ω). The conductivity–temperature dependence studies were carried out in the temperature range from 303 to 353 K.
To study the phase structure and complexation of the conducting polymer electrolyte films, XRD measurement was carried out using a PAN Analytical Expert Pro MPD in the range of 2θ from 10° to 80°.
Fourier-transform infrared (FTIR) spectra exhibited in this work were taken using a MAGNA-IR550 Spectrophotometer-Series II in the wavenumber region between 400 and 4,000 cm−1. The films used in this work were cut into suitable sizes and placed in the specimen holder of the spectrophotometer. In the present work, the FTIR spectrum of pure PAN film was also taken to serve as reference. The resolution of the spectrophotometer was 1 cm−1.
The surface morphology of the films was observed by SEM using SEC Mini-SEM-Bruker EDS System.
Results and discussion
The ionic conductivity versus salt content and the conductivity versus frequency plots for the PAN + NaCF3SO3 system and the PAN + LiCF3SO3 system at room temperature are presented in Fig. 1a and b, respectively. It can be observed that the conductivity of pure PAN film increases two orders of magnitude when 6 wt.% of NaCF3SO3 salt was added and continues to do so until it reached the highest conductivity, i.e., 7.13 × 10−4 S cm−1 at 24 wt.% NaCF3SO3. Therefore, 24 wt.% NaCF3SO3 is the conductivity optimizing concentration. The conductivity for PAN + LiCF3SO3 system is abruptly increases when more than 10 wt.% of LiCF3SO3 was added. The highest ionic conductivity was achieved at 26 wt.% LiCF3SO3 content and its value was 3.04 × 10−4 S cm−1. The conductivity of this film is further increased to ∼10−3 S cm−1 with the addition of plasticizer, ethylene carbonate (EC). This work has been published elsewhere [14]. Abraham et al. [15] have prepared PAN polymer electrolyte films with EC and PC as plasticizers containing LiPF6/AsF6/LiN(SO2CF3)2 and they obtained the conductivity value in order of 10−4–10−3 S cm−1 at room temperature. These results are in good agreement with the works that are described and reported by other researchers [16–18].
a The ionic conductivity versus salt content for the PAN + NaCF3SO3 system at room temperature. The inset figure shows the conductivity versus frequency plot for the film containing (a) 24 wt.% (b) 28 wt.% (c) 30 wt.% (d) 26 wt.% (e) 22 wt.% (f) 20 wt.% (g) 18 wt.% (h) 16 wt.% (i) 14 wt.% (j) 10 wt.% and (k) 6 wt.% salt b The ionic conductivity versus salt content for the PAN + LiCF3SO3 system at room temperature. The inset figure shows the conductivity versus frequency plot for the film containing (a) 26 wt.% (b) 28 wt.% (c) 30 wt.% (d) 24 wt.% (e) 22 wt.% (f) 20 wt.% (g) 18 wt.% (h) 6 wt.% and (i) 14 wt.% salt
Generally, ionic conductivity of polymer electrolytes depends on the charge carrier concentration, n and carrier mobility, μ as described by relation σ = nqμ, where q representing the charge of mobile carrier. The increase in the ionic conductivity with increasing salt concentrations can be related to the increase in the number of mobile ion in the conducting polymer electrolyte films. The decrease in conductivity value at higher salt concentration is observed and can be explained by aggregation of the ions, leading to the formation of ion cluster, thus decreasing the number of mobile ions and hence the mobility [19]. The inset figures in Fig. 1 show the variation in conductivity with frequency for both polymer electrolyte systems. The bulk conductivity is very low at low frequencies, <1 kHz. This can be attributed to the accumulation of mobile ions at the electrode–electrolyte interface at low frequencies and hence there are less mobile ions in the bulk material contribute to conductivity.
To analyze the mechanism of ionic conduction, the conductivity–temperature dependence of the highest conducting films from the PAN + NaCF3SO3 system and the PAN + LiCF3SO3 system were investigated at temperature range from 303 K to temperature below the glass transition temperature, T g of pure PAN film, 353 K. From thermal analysis, pure PAN film exhibited a distinct T g at 85 °C (308 K). The conductivity–temperature dependence plots of these films are represented in Fig. 2a and b, respectively. The plots show that the conductivity increases as the temperature increases and obeys Arrhenius rule, \( \sigma \left( {\text{T}} \right) = {\sigma_{\text{o}}}{ \exp }\left( { - {E_{\text{a}}}/{\text{RT}}} \right) \), where σ o is the conductivity pre-exponential factor and E a is the activation energy for conduction [20]. It is also understood that the increase in conductivity with temperature can be linked to the decrease in viscosity and hence increased chain flexibility [21]. The activation energy, E a, can be evaluated from the slope of the plots [21]. The E a for the PAN + 24 wt.% NaCF3SO3 film and PAN + 26 wt.% LiCF3SO3 film have been calculated to be 0.23 eV and 0.28 eV, respectively. It can be observed that PAN + 24 wt.% NaCF3SO3 film has a higher ionic conductivity and lower activation energy compared to PAN + 26 wt.%LiCF3SO3 film. This result can be explained based on the Lewis acidity of the alkali ions, i.e., the strength of the interaction of cations with the Lewis base of the polymer electrolyte [22]. The interaction between Li+ ion and the nitrogen atom of PAN is stronger than that of Na+ ion. Thus, Li+ ion transfer requires higher activation energy than Na+ ion in polymer electrolytes. These results agree well with the works reported by Sagane et al. and Abe et al. [22–24].
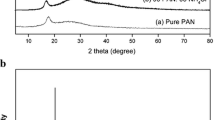
Figure 3 represents the XRD patterns of pure PAN film, LiCF3SO3, NaCF3SO3 and the films with the highest ionic conductivity from PAN + LiCF3SO3 and PAN + NaCF3SO3 systems. XRD patterns of LiCF3SO3 exhibited the diffraction peaks at 2θ = 16.7°, 19.8°, 20.3°, 22.5°, 24.6°, 25.5°, 33.0°, 33.6°, and 41.7° as shown in Fig. 3b while the diffraction peak of NaCF3SO3 can be found at 2θ = 23.7°, 32.9°, 34.8°, 42.7°, 48.7°, 53.5° and 56.20° as shown in Fig. 3c. The XRD patterns of pure PAN film and films containing salts were found to be amorphous showing no diffraction peaks over the diffraction angle, 2θ of 10° to 80° as observed in Fig. 3a, d, and e. It can be observed that most of the peaks pertaining to pure LiCF3SO3 and NaCF3SO3 are absent in the polymer electrolyte films and indicates the complete dissolution of the salt in the polymer matrix PAN. Thus, the XRD studies confirm that complexation has occurred in the polymer matrices and the complex formed are amorphous. Berthier et al. [25] established that ionic conductivity in polymer electrolytes is associated with the amorphous phase of the studied samples.
In order to investigate the complex formation in the polymer matrices, FTIR studies have been carried out. The FTIR spectra in the wavenumber range from 2,000 cm−1 to 4,000 cm−1 of pure PAN, PAN + LiCF3SO3 system, and PAN + NaCF3SO3 system are shown in Fig. 4. The nitrile band, C≡N, assigned to stretching band in the FTIR spectrum appeared at 2,247 cm−1 for pure PAN. The nitrile band is displaced towards the lower frequency around 2,244 cm−1 due to inductive effect created by the interaction between the nitrogen atom in C≡N with Li+ and Na+ ions from the salts. It also can be observed that the intensity of this band is reduced with increases of salts concentration. This shows that the complexation has occurred between the polymer, PAN and the salts. Similar FTIR spectra for pure PAN and PAN containing EC, PC, and LiClO4 have been observed by Rajendran and his co-workers [26].
Figure 5 shows the SEM micrographs of (a) pure PAN (b) PAN + 26 wt.% LiCF3SO3, and (c) PAN + 24 wt.%NaCF3SO3. It can be seen that the surface morphology of the pure PAN film is smooth and homogenous. However, when LiCF3SO3 and NaCF3SO3 salts were added the surface becomes rough and uneven. It also can be observed that the pores appear in the salted films as shown in Fig. 3b and c. This reveals that LiCF3SO3 and NaCF3SO3 salts strongly interact with polymer host as observed in the FTIR spectra.
Conclusions
The ion-conducting PAN polymer electrolyte films containing LiCF3SO3 and NaCF3SO3 salts have been prepared and studied. The PAN film containing 24 wt.% NaCF3SO3 has a higher ionic conductivity and lower activation energy compared to the PAN film containing 26 wt.% LiCF3SO3 film. The results indicate that the interaction between Li+ ion and the nitrogen atom of PAN is stronger than that of Na+ ion. The plots of conductivity–temperature dependence follow Arrhenius equation in the temperature range of 303 to 353 K. XRD studies show that the complexation has occurred and complexes formed are amorphous. These results correspond with FTIR spectra and SEM analysis.
References
Le Nest JP, Gandini A (1990) In: Scrosati B (ed) Proc. 2nd International Symposium on Polymer Electrolytes. Elsevier, Amsterdam, p 129
Scrosati B (1993) In: Scrosati B (ed) Applications of electroactive polymers. Chapman and Hall, London, p 251
Hooper A, Gauthier M, Belanger A (1990) In: Linford RG (ed) Electrochemical science and technology of polymers, vol 2. Elsevier, London, p 375
Murata K, Izuchi S, Yoshihisa Y (2000) Electrochim Acta 45:1501–1508
Wang C, Xia Y, Koumoto K, Sakai T (2002) J Electrochem Soc 149:A967–A972
Chen T, Chen-Yang YW (2004) Recent development of application of polymer electrolytes on polymer lithium secondary batteries. Huaxue 62(4):445–454
Hayamizu K, Aihara Y (2004) Electrochimica Acta 49:3397–3402
Fenton BE, Parker JM, Wright PW (1973) Polymer 14:589
Armand B (1979) In: Vashisthta P et al (eds) Fast ion transport in solids. Elsevier, North-Holland
Bandara LRAK, Dissayanake MAKL, Mellander BE (1998) Electrochimica Acta 43:1447–1451
Kumar B, Rodrigues SJ, Koka S (2002) Electrochimica Acta 47:4125–4131
Wang Y-J, Pan Y, Wang L, Pang M-J, Chen L (2005) Mater Lett 59:3021–3026
Vondrák J, Reiter J, Velicka J, Sedlaříkova M (2004) Solid State Ion 170:79–82
Osman Z, Md Isa KB, Ahmad A (2009) In: Arof AK, Majid SR, Teo LP, Kufian MZ (eds) Proc. of National Workshop on Functional Materials (NWFM). (ISBN 978-967-5148-43-9), pp 1-5
Abraham KM, Choe HS, Pasquariello DM (1998) Electrochim Acta 43:2399–2412
Chen-Yang YW, Chen HC, Lin FJ, Chen CC (2002) Solid State Ion 150:327–335
Yoon HK, Chung WS, Jo NJ (2004) Electrochim Acta 50:289–293
Huang B, Wang Z, Li G, Huang H, Xue R, Chen L, Wang F (1996) Solid State Ion 85:79–84
Ramya CS, Selvasekarapandian S, Savitha T, Hirankumar G (2007) Physica B 393:2672–2677
Souquet JL, Levy M, Duclot M (1994) Solid State Ion 70–71:337–345
Rajendran S, Sivakumar M, Subadevi R (2004) Mater Lett 58:641–649
Sagane F, Abe T, Iriyama Y, Ogumi Z (2005) J Power Sources 146:749–752
Abe T, Fukuda H, Iriyama Y, Ogumi Z (2004) J Electrochem Soc 151(8):A1120–A1123
Abe T, Ohtsuka M, Iriyama Y, Ogumi Z (2004) J Electrochem Soc 151(11):A1153–A1950
Berthier C, Gorecki W, Minier M, Armand MB, Chabagno JM, Rigaud P (1983) Solid State Ion 11:91–95
Rajendran S, Kannan R, Mahendran O (2001) Mater Lett 48:331–335
Acknowledgements
The authors would like to thank the University of Malaya and Academy of Sciences Malaysia for the grants and scholarship awarded.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osman, Z., Md Isa, K.B., Ahmad, A. et al. A comparative study of lithium and sodium salts in PAN-based ion conducting polymer electrolytes. Ionics 16, 431–435 (2010). https://doi.org/10.1007/s11581-009-0410-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0410-9