Abstract
Agar as a natural polymer is used to prepare quasi-solid-state polymer electrolytes (QSPEs). Two different iodide salts namely sodium iodide (NaI) and potassium iodide (KI) are incorporated. To enhance the ionic conductivity of the QSPE system, 1-methyl-3-propylimidazolium iodide (MPII) ionic liquid is added. The highest ionic conductivity of 1.48 × 10−3 S cm−1 was achieved after addition of 50 wt.% of KI and 3.0 g of MPII ionic liquid. QSPEs are studied for temperature-dependent ionic conductivity behavior. QSPEs are studied for structural properties using Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD). The structural studies revealed that the complexation between agar polymer, iodide salts, and MPII ionic liquid has occurred. QSPEs are sandwiched between counter and working electrodes to fabricated DSSC and analyzed under sun simulator. The highest efficiency of 2.16% is achieved with incorporation of 3.0 g MPII ionic liquid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dye-sensitized solar cells (DSSCs) draw the researchers’ attention due to some advantages such as low cost, competitive energy conversion efficiency, simple structure, flexible, and low toxicity [1]. At the same time, the improvement of DSSC technology must balance with the manufacturing cost to be cost-effective compared to other conventional energy resources [2]. Electrolyte is one of the important parts in DSSC which normally can be in form of liquid, solid, gel, or other forms like quasi-solid. Liquid electrolytes may face several problems such as leakages, shape inflexibility, and electrochemical instability. Furthermore, there are several investigations on applications of polymer electrolytes in electrochemical devices such as dye-sensitized solar cells [3–7], lithium-ion batteries [8, 9], supercapacitors [10, 11], and fuel cells [12, 13]. Moreover, due to excellent contacting, low vapor pressure, and pore filling in the quasi-solid-state polymer electrolytes, they can be good alternative to liquid as electrolytes in DSSCs [14–16].
Bacteriological agar as the host polymer is mixture of agaropectin and agarose polysaccharides in which agarose is a neutral charge while agaropectine is heavily modified with acidic groups of sulfates and pyruvates [17, 18].
One method to increase the ionic conductivity is by plasticizing the polymer with organic solvents such as glycerol which was used in the preparation of the quasi-solid-state polymer electrolytes in this work.
In this work, ionic conductivity and structural properties of QSPEs were performed using electrochemical impedance spectroscopy (EIS), Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD). Dye-sensitized solar cells (DSSCs) were fabricated by sandwiching QSPEs between counter and working electrodes.
Experimental
Materials
Agar was purchased from BioLab. Iodine pearl, sodium iodide (NaI), and potassium iodide (KI) were purchased from Friendemann Schmidt. 1-methyl-3-propylimidazolium iodide (MPII) ionic liquid, glycerol (purity ≥99.5%), and Triton X-100 were purchased from Sigma-Aldrich. TiO2 P90 and P25 were purchased from Aeroxide. Carbowax were purchased from Supelco Analytical.
Preparation of quasi-solid-state polymer electrolytes
Quasi-solid-state polymer electrolytes were prepared by stirring 1 g of agar, 5 ml of glycerol as a solvent, and appropriate amounts of iodine (10 M percentage of NaI and KI), sodium iodide (NaI), and potassium iodide (KI), according to Table 1, were added and stirred at 100 °C to homogenously dissolve the chemicals and become gelatinized. For the system incorporated with ionic liquid, 1-methyl-3-propylimidazolium iodide (MPII) ionic liquid was added. Then, the prepared samples were left to cool down to room temperature.
Dye-sensitized solar cell fabrication
In this work, by coating two layers of TiO2, the photo-anode electrode was prepared. For the first layer, a uniform thin layer of TiO2 (P90) was spin-coated on FTO where 0.5 g of TiO2 (P90) was first grounded for about 30 min in an agate mortar followed by addition of 2 ml of HNO3 (pH = 1). The solution was spin-coated at 1000 rpm for 2 s and then spin-coated at 2350 rpm for 60 s in order to get a more uniform thin layer with better adhesion. The first coated layer was sintered in the oven at 450 °C for 30 min.
In second layer, 0.5 g of TiO2 (P25) was grounded for about 30 min with 2 ml of the HNO3 (pH = 1) in an agate mortar. Afterwards, 0.1 g of carbowax and one drop of Triton X-100 were added. Doctor blade method was used to prepare the second layer and sintered in the oven at 450 °C for 30 min. The photo-anode electrode was immersed in N719 dye for 24 h. Moreover, the Pt solution was coated on the FTO glass to prepare the counter electrode. The quasi-solid-state polymer electrolytes were sandwiched between two photo-anode and counter electrodes to fabricate DSSCs and characterized under 1 sun simulator.
Characterization methods
Electrochemical impedance spectroscopy
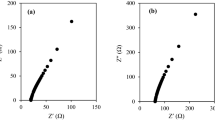
Electrochemical impedance spectroscopy (EIS) was studied using HIOKI 3532-50 LCR Hi-Tester (frequency ∼ 50 Hz to 1 MHz) to measure the ionic conductivity of the quasi-solid-state polymer electrolyte systems. The ionic conductivity values were calculated using the equation bellow
where σ is the ionic conductivity (S cm−1), L is the thickness of the sample (cm), A is the surface area of the stainless-steel blocking electrodes (cm2), and Rb is the bulk resistance (Ω) which can be obtained from Cole-Cole plot.
Temperature-dependent ionic conductivity study was carried out with temperature range from 30 to 100 °C.
Structural studies
The interaction in chemical complexes and structural properties were analyzed using Fourier transform infrared spectroscopy (FTIR), Thermo Scientific Nicolet iSIO Smart ITR with wavenumbers between 4000 and 600 cm−1. X-ray patterns were recorded using XRD-Siemens D 5000 diffractometer under 40 kV and 40 mA with Cu Kα radiation at a wavelength of 1.5406 Å with 2θ ranging from 5 to 80°.
Dye-sensitized solar cell
The DSSCs were fabricated by sandwiching the QSPEs between counter and working electrodes (FTO/TiO2/Dye/QSPE/Pt/FTO). J-V characteristics of DSSCs were obtained using Metrohm Autolab potentiostat (PGSTAT128N) with Newport LCS-100 Series Sun simulator under the illumination of 100 (mW cm−2).
Results and discussion
Electrochemical impedance spectroscopy
Ionic conductivity
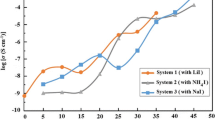
Quasi-solid-state polymer electrolytes were prepared using NaI, KI, and MPII to find a sample with the highest ionic conductivity for the application in DSSC. In the first two systems, the agar is incorporated with only NaI and KI salt. Table 1 shows the designations and ionic conductivity values for systems with NaI and KI iodide salts. It was observed that with the increment of NaI and KI, the ionic conductivity of the QSPEs is increased which can be spotted in Fig. 1. The highest achieved ionic conductivity in the first system (agar/NaI) was 1.36 × 10−4 S cm−1 at room temperature with designation of ANa-5 (50 wt.% NaI). In first system (agar/NaI), there is a drop in ionic conductivity value after addition of 20 wt.% of NaI which can be due to aggregation and accumulation in the mixture. In second system, the sample AK-5 (50 wt.% KI) had the highest ionic conductivity of 1.75 × 10−4 S cm−1 at room temperature. After addition of NaI and KI, it is revealed that the ion transport mechanism is relatively influenced by the salts with ion carriers of Na+ and K+ [19–22]. The difference of ionic conductivity between NaI system and KI system is due to lattice energy. The higher ionic conductivity achievement in KI system is due to lower lattice energy of KI (649 KJ/mol) compared with NaI (704 KJ/mol) [23]. Moreover, lower lattice energy in KI results in easier solvation of K+ in polymer matrix which results in higher number of K+ ionic carriers and higher mobility as well as ionic conductivity.
In the first two systems without ionic liquid, AK-5 shows the highest ionic conductivity which is used for third system incorporated with MPII ionic liquid (Agar/KI/MPII). MPII ionic liquid was added with amounts of 0.1 up to 3.0 g. The designation and ionic conductivity values for third system are demonstrated in Table 2. The EIS results show the ionic conductivity in third system increases with the increase of the MPII ionic liquid. The highest ionic conductivity of 1.48 × 10−3 S cm−1 was achieved after the addition of 3.0 g of MPII ionic liquid (AKP-5). Figure 2 exhibits the variation of ionic conductivity with the addition of MPII ionic liquid. The figure shows the increment of ionic liquid with the addition of the MPII content.
Temperature-dependent ionic conductivity
The temperature-dependent ionic conductivity was studied with the temperature range of 30 to 100 °C. Figure 3 shows the temperature-dependent ionic conductivity results for samples AKP-1, AKP-2, AKP-3, AKP-4, and AKP-5 in third system. Figure exhibits that the ionic conductivity of QSPEs was increased with the increment of the temperature due to ion hopping to the neighboring vacancies.
Fourier transform infrared spectroscopy (FTIR)
Figure 4 exhibits the FTIR spectra of pure agar, AK-5, AKP-3, AKP-4, and AKP-5 at wavenumbers between 4000 and 600 cm−1. The band assignments are listed in Table 3.
FTIR spectra in Fig. 4 indicate that the peak in quasi-solid-state polymer electrolyte (AK-5) with 3301 cm−1 shifts to higher wavenumbers of 3310, 3323, and 3331 cm−1 in AKP-3, AKP-4, and AKP-5, respectively, after addition of MPII ionic liquid. The shifts show that the complexation between pure agar, KI, and MPII ionic liquid has occurred indicating that the shift is attributed to O–H deformation and miscibility of polymer, KI, and MPII ionic liquid [24]. Furthermore, this can be explained as a result of hydrogen bonding interaction between K+ cations in KI and anions (I−) in MPII ionic liquid [25]. The FTIR results further indicate that the QSPEs become more amorphous after incorporation of MPII ionic liquid.
X-ray diffraction
Figure 5 demonstrates the XRD patterns of pure agar, AK-5, AKP-3, AKP-4, and AKP-5. The XRD results in pure agar show a peak at 2θ = 13° and a broad hunch of 2θ = 11–17°. The existence of KI salt in the QSPEs resulted in appearance of a new peak that shifts to a higher degree at 2θ = 23°. The shifts in XRD results can further confirm that the complexation between agar and potassium iodide has occurred. After addition of the MPII ionic liquid, the broad peaks of the QSPEs appeared slightly at higher ranges with broader hunches of 2θ = 18–37°, 2θ = 18–38°, and 2θ = 18–38° for samples AKP-3, AKP-4, and AKP-5, respectively. The XRD results show that the broadening is increased resulting in more amorphous nature of QSPEs. This can be an evidence of miscibility of agar polymer, KI, and MPII ionic liquid.
Dye-sensitized solar cell
The energy conversion efficiency can be calculated using equation
where η is the energy conversion efficiency, Pin is the incident light power, Jsc, Voc, and FF are the short circuit current density (mA cm−2), the open circuit potential (V), and fill factor (%), respectively.
DSSC parameters are listed in the Table 4. The results show that the energy conversion efficiency of the DSSC is increasing with the addition of MPII ionic liquid. The efficiency values for AKP-1, AKP-2, AKP-3, AKP-4, and AKP-5 are 0.89, 0.95, 1.09, and 2.16%, respectively, which obtained from J-V results. Figure 6 shows the J-V graph for the highest achieved efficiency (2.16%) with AKP-5 sample. In this work, the results show the highest energy conversion efficiency of 2.16% among DSSCs which demonstrates improvement of the efficiency after using agar as natural polymer with the incorporation of MPII ionic liquid. Moreover, this work is showing significant enhancement of efficiency compared with some recent works on DSSCs using natural polymers: Khanmirzaei et al. (2015), using pure rice starch as the natural polymer, achieved efficiencies of 0.78 and 2.09% [5, 14]; Yang et al. (2015), achieved efficiency of 1.73% using agarose [26]; and Buraidah et al. (2016), with the highest efficiency of 1.13% with incorporation of chitosan natural polymer [27].
Conclusion
The quasi-solid-state polymer electrolytes were prepared. Two iodide salts of NaI and KI were used for two first systems. The QSPE with 50 wt.% potassium iodide was incorporated with MPII ionic liquid for the third system. The highest ionic conductivity of 1.48 × 10−3 Scm−1 was achieved with the addition of 3.0 g MPII (AKP-5). DSSCs were fabricated with sandwiching QSPEs between counter and working electrodes. AKP-5 sample with 3.0 g MPII ionic liquid showed the highest energy conversion efficiency of 2.16%.
References
Grätzel M (2006) The advent of mesoscopic injection solar cells. Prog Photovolt Res Appl 14(5):429–442
El Chaar L, Lamont LA, El Zein N (2011) Review of photovoltaic technologies. Renew Sust Energ Rev 15(5):2165–2175
Aziz MF, Buraidah MH, Careem MA, Arof AK (2015) PVA based gel polymer electrolytes with mixed iodide salts (K + I- and Bu4N + I-) for dye-sensitized solar cell application. Electrochim Acta 182:217–223
Khanmirzaei MH, Ramesh S, Ramesh K (2015) Hydroxypropyl cellulose based non-volatile gel polymer electrolytes for dye-sensitized solar cell applications using 1-methyl-3-propylimidazolium iodide ionic liquid. Sci Rep 5:18056
Khanmirzaei MH, Ramesh S, Ramesh K (2015) Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater Des 85:833–837
Lee JH, Park CH, Jung JP, Kim JH (2015) Worm-like mesoporous TiO2 thin films templated using comb copolymer for dye-sensitized solar cells with polymer electrolyte. J Power Sources 298:14–22
Wei W, Song D, Kang YS (2015) Stepwise cosensitization for high efficiency dye-sensitized solar cells utilizing solid-state polymer electrolytes. Mater Lett 161:435–438
He R, Echeverri M, Ward D, Zhu Y, Kyu T (2016) Highly conductive solvent-free polymer electrolyte membrane for lithium-ion batteries: effect of prepolymer molecular weight. J Membr Sci 498:208–217
Kuo P-L, Tsao C-H, Hsu C-H, Chen S-T, Hsu H-M (2016) A new strategy for preparing oligomeric ionic liquid gel polymer electrolytes for high-performance and nonflammable lithium ion batteries. J Membr Sci 499:462–469
Sivaraman P, Shashidhara K, Thakur AP, Samui AB, Bhattacharyya AR (2015) Nanocomposite solid polymer electrolytes based on polyethylene oxide, modified nanoclay, and tetraethylammonium tetrafluoroborate for application in solid-state supercapacitor. Polym Eng Sci 55(7):1536–1545
Yang L, Hu J, Lei G, Liu H (2014) Ionic liquid-gelled polyvinylidene fluoride/polyvinyl acetate polymer electrolyte for solid supercapacitor. Chem Eng J 258:320–326
Patru A, Rabis A, Temmel SE, Kotz R, Schmidt TJ (2016) Pt/IrO2 TiO2 cathode catalyst for low temperature polymer electrolyte fuel cell application in MEAs, performance and stability issues. Catal Today 262:161–169
Sasikala S, Selvaganesh SV, Sahu AK, Carbone A, Passalacqua E (2016) Block co-polymer templated mesoporous carbon-Nafion hybrid membranes for polymer electrolyte fuel cells under reduced relative humidity. J Membr Sci 499:503–514
Khanmirzaei MH, Ramesh S, Ramesh K (2015) Effect of different iodide salts on ionic conductivity and structural and thermal behavior of rice-starch-based polymer electrolytes for dye-sensitized solar cell application. Ionics 21(8):2383–2391
Ng HM, Ramesh S, Ramesh K (2015) Efficiency improvement by incorporating 1-methyl-3-propylimidazolium iodide ionic liquid in gel polymer electrolytes for dye-sensitized solar cells. Electrochim Acta 175:169–175
Rahman MYA, Ahmad A, Umar AA, Taslim R, Su’ait MS, Salleh MM (2014) Polymer electrolyte for photoelectrochemical cell and dye-sensitized solar cell: a brief review. Ionics 20(9):1201–1205
Leones RFS, Rodrigues LC, Marrucho IM, Esperanca JMSS, Pawlicka A, Silva MM (2012) Investigation of polymer electrolyte based on agar and ionic liquids. Express Polym Lett 6(12):1007–1016
Koh JH, Ahmad Z, Mohamad A (2012) Bacto agar-based gel polymer electrolyte. Ionics 18(4):359–364
Rajendran S, Babu R, Usha Rani M (2011) Effect of complexing salt on conductivity of PVC/PEO polymer blend electrolytes. Bull Mater Sci 34(7):1525–1530
Tambelli CC, Bloise AC, Rosário AV, Pereira EC, Magon CJ, Donoso JP (2002) Characterisation of PEO–Al2O3 composite polymer electrolytes. Electrochim Acta 47(11):1677–1682
Pitawala HMJC, Dissanayake MAKL, Seneviratne VA (2007) Combined effect of Al2O3 nano-fillers and EC plasticizer on ionic conductivity enhancement in the solid polymer electrolyte (PEO)9LiTf. Solid State Ionics 178(13–14):885–888
Klongkan S, Pumchusak J (2015) Effects of Nano alumina and plasticizers on morphology, ionic conductivity, thermal and mechanical properties of PEO-LiCF3SO3 solid polymer electrolyte. Electrochim Acta 161:171–176
Kaya S, Kaya C (2015) A simple method for the calculation of lattice energies of inorganic ionic crystals based on the chemical hardness. Inorg Chem 54(17):8207–8213
Freitas FS, JND F, Ito BI, M-AD P, Nogueira AF (2009) Electrochemical and structural characterization of polymer gel electrolytes based on a PEO copolymer and an imidazolium-based ionic liquid for dye-sensitized solar cells. Acs Appl Mater Inter 1(12):2870–2877
Campbell JLE, Johnson KE (1993) Speciation of the proton in ambient-temperature molten-salts. Inorg Chem 32(18):3809–3815
Yang Y, Gao J, Yi P, Cui J, Guo X (2015) The influence of Co3O4 concentration on quasi-solid state dye-sensitized solar cells with polymer electrolyte. Solid State Ionics 279:1–5
Buraidah MH, Teo LP, Yong CMA, Shah S, Arof AK (2016) Performance of polymer electrolyte based on chitosan blended with poly(ethylene oxide) for plasmonic dye-sensitized solar cell. Opt Mater 57:202–211
Acknowledgements
This work was supported by the Fundamental Research Grant Scheme (FP012-2015A) from Ministry of Education, Malaysia, University of Malaya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nadia, S.R., Khanmirzaei, M.H., Ramesh, S. et al. Quasi-solid-state agar-based polymer electrolytes for dye-sensitized solar cell applications using imidazolium-based ionic liquid. Ionics 23, 1585–1590 (2017). https://doi.org/10.1007/s11581-016-1946-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1946-0